Overview of peripheral arteriovenous malformations: From diagnosis to treatment methods
Yuchen Shen, Lixin Su, Deming Wang, Xindong Fan
Vascular Anomaly Center,Department of Interventional Therapy,Shanghai Ninth People's Hospital,Shanghai Jiao Tong University School of Medicine,No.639 Zhi Zao Ju Rd, 200011, Shanghai, China
Keywords:
ABSTRACT Based on the latest classification by the International Society for the Study of Vascular Anomalies in 2018,vascular malformations (VMs) can be categorized into simple, combined VMs of major named vessels, and VMs associated with other anomalies.Simple VMs include lymphatic, venous, capillary, and arteriovenous malformations(AVMs).AVMs represent disorders of direct arteriovenous shunts caused by the absence of a capillary bed between the involved arteries and veins.This abnormal vascular communication causes arterial blood to accumulate in the venous vessels,thus resulting in venous hypertension and characteristic clinical manifestations,such as pulsation, tremors, and elevated temperature.AVMs can occur sporadically or as manifestations of syndromic lesions and are considered among the most complex and challenging VMs.The diagnosis and treatment of AVMs can vary depending on the lesion location and associated clinical symptoms,thus complicating their management.Herein, we discuss peripheral AVMs in terms of their clinical manifestations, imaging examinations, and staging systems to provide a comprehensive reference for the treatment, evaluation methods, and follow-up procedures for this vascular anomaly.
1.Etiology
Arteriovenous malformations(AVMs)are malformed vascular masses with a high flow velocity formed by a direct artery–vein connection within the arteriovenous system.1They are currently regarded as a congenital disease that may be caused by vascular dysplasia during early pregnancy.2AVMs exhibit slow progression with accelerated development during puberty or pregnancy.3Furthermore, as AVMs progress,patients may experience cutaneous and mucosal ischemic ulcers, uncontrolled acute bleeding, and even life-threatening events such as congestive heart failure due to a prolonged high reflux status.4However,the specific pathogenesis of AVMs is yet to be elucidated.Previous studies have primarily identified AVMs as sporadic lesions.Meanwhile,familial AVMs are rarely investigated, and the available results indicate different gene mutation types and clinical manifestations.5Additionally,partial somatic mutations and abnormal signaling pathways that can potentially induce endothelial cell dysfunction have been observed in sporadic AVMs.6Researchers have suggested that MAP2K1 gene mutations may be related to sporadic AVM onset,1along with the implication of RASA1,EPHB4,ENG,BMP9,ACVRL1,SMAD4,and BMPR2 gene mutations in AVM-related disorders.5Moreover, researchers investigating the molecular mechanisms of AVMs have proposed rapamycin or trimetinib as potential target drugs to treat AVMs7,8;however,more investigations are required to support this strategy.
2.Clinical presentation and staging
Although AVM lesions typically occur at birth, most do not present clinical symptoms until childhood and are aggravated by hormone-level changes associated with puberty, pregnancy, hormone treatment, or external factors, such as surgery and trauma.9Furthermore, the clinical manifestations of AVMs differ depending on the malformation site.Based on the primary region of AVM lesions,we propose an anatomical classification of peripheral AVMs to assist clinicians in diagnosing vascular disorders more systematically(Fig.1).10

2.1.Soft-tissue AVMs
The classic clinical symptoms of AVMs located in soft tissues (soft tissue AVMs) include skin erythema, soft tissue mass or swelling, pain,and superficial vein dilatation.Physical examination may reveal the swelling of ill-defined soft tissue with normal color and texture of the surface skin and mucosa as well as dilated capillaries or color changes to dark red/pink.11Additionally, the lesions and surrounding areas may present tortuous and dilated veins in addition to large pulsating veins.The local skin temperature increases,accompanied by persistent palpable tremors and blowing murmurs.12Moreover,when patients with head and neck AVMs turn their heads toward the healthy side, external jugular vein dilatation can be observed on the affected side.10Other indicators of patients with AVMs include tortuous and dilated outflow veins,as well as relatively typical local manifestations upon physical examination,including bleeding, ulcers, and infections caused by skin and mucosal ischemia,particularly in patients with acral AVMs.13In the later stages of this disease,patients may exhibit decompensated presentations,such as congestive heart failure involving palpitations, shortness of breath, and chest tightness symptoms,owing to long-term increased venous return.14This form of decompensated manifestation is typically observed in patients with AVMs in the head,neck,and trunk regions,whereas it occurs in the extremities of patients with extensive and progressive AVMs.
2.2.AVMs in the bone
AVMs can emerge in the bone tissue as lesions in the primary central bone or soft tissue of adjacent bones.Intraosseous AVMs were previously known as central hemangiomas of the jaw because they are typically observed in the upper and lower jaws.15Incidental intraosseous AVMs have been detected in various bones, including the sphenoid bones,zygomatic bones, vertebrae, ulna, radius, humerus, femur, clavicle,scapula,and ribs.10In addition to the classic clinical symptoms of AVMs,pathological fractures can develop in AVMs of long bones.16Intraosseous AVMs can penetrate the bone and spread to adjacent soft tissue.In this case,clinical treatment is typically focused only on soft-tissue lesions and not on intraosseous AVMs, thus resulting in treatment failure.17Furthermore, AVMs of the jaw present with specific clinical manifestations owing to the complex anatomical structures of the oral and maxillofacial regions.In these cases, the main symptoms are recurrent and coupled with a small amount of spontaneous or acute bleeding,which are difficult to manage.In children, acute hemorrhage primarily occurs during mixed dentition,particularly at approximately 10 years of age.10This symptom is primarily caused by the extraction of loose teeth,alternating deciduous and permanent teeth, or surgery after misdiagnosis.Hemorrhage can occur after the completion of jaw and tooth development.Moreover, most patients experience repeated periodontal bleeding as an early indicator of acute bleeding, whereas some exhibit acute massive bleeding as the first symptom, which is typically accompanied by tooth loosening at the bleeding site.18Additionally, AVMs of the jaw mainly arise in the molar or premolar areas, with most cases occurring concurrently with root resorption.With regard to lesions originating in the mandible, patients occasionally present numbness in the mandibular region.These lesions may be confined to the jaw or surrounding soft tissues.15Finally, children with AVMs during the growth and development period should be closely monitored until the period ends.
2.3.Visceral AVMs
Visceral AVMs are relatively rare and have been sporadically reported in the kidneys, lungs, gastrointestinal tract, pelvic cavity, and other organs.19The clinical symptoms of visceral AVMs are relatively insidious and distinct from those of soft-tissue AVMs.In visceral AVMs, some patients are asymptomatic or present only nonspecific general symptoms(e.g., swelling, pain, throbbing, and bleeding), which are difficult to differentially diagnose and are determined by the location and size of the lesion.Typical symptoms of congenital renal AVMs include renovascular hypertension, pain, hematuria, abdominal or ventral murmurs, and cardiopulmonary overload.20Furthermore,gastrointestinal AVMs can cause gastrointestinal bleeding, melena, hemorrhoids, and other conditions.21Pelvic AVMs, which are more easily overlooked owing to their deep location and low incidence,can manifest as asymptomatic macrovascular lesions or other abnormalities,such as abdominal pain,dysuria,frequent urination, hematuria, foot drop, exertional dyspnea, or recurrent abortion.22Moreover, asymptomatic pelvic AVMs in young female patients may cause life-threatening massive bleeding during normal labor or Cesarean sections.Most pulmonary AVMs have no clinical manifestations and only a few demonstrate decreased oxygen saturation.23Thus, the clinical manifestations of visceral AVMs are not highly specific, thus rendering their diagnosis and evaluation dependent on auxiliary examinations.
2.4.Clinical staging of AVMs
Currently, the clinical staging of AVMs is mainly based on Schobinger's method,which was popularized by Mulliken et al.24Based on the clinical symptoms, AVMs can be classified into the following stages:Stage I,which features bluish stain,warmth,and arteriovenous shunting,as revealed via Doppler scanning;Stage II,whose features are similar to those of Stage I but with additional features of enlargement, pulsations,thrill and bruit, and tortuous/tense veins; Stage III (destruction stage),whose features are the same as those of Stage II but with additional features of dystrophic skin changes, i.e., ulceration, bleeding, persistent pain, or tissue necrosis; and Stage IV, whose features are the same as those of Stage III but with an additional congestive cardiac failure.
3.Imaging examination of AVMs
Imaging examination is important in the diagnosis, hemodynamic evaluation,and follow-up of AVMs.Color Doppler flow imaging(CDFI),the preferred screening method,can be used to objectively evaluate the velocity,flow,and other hemodynamic parameters of AVMs.CDFI allows one to analyze these parameters to determine the blood flow status of AVM lesions,feeding arteries,and draining veins.In B-mode ultrasound examinations, AVMs are typically observed as ill-defined lesions with dilatation of the arterial and venous systems.In some patients, tissue thickening with soft tissue edema or fatty infiltration may be apparent.CDFI examination further reveals local high-flow and blood-rich lesions,and the vascular density is typically >5 vessels/cm2.Other important diagnostic manifestations include increased systolic velocity and diastolic flow in feeding arteries, arterialization of draining veins, and highly turbulent flows.25In certain difficult cases,CDFI can assist in the localization of the puncture site.
Contrast-enhanced computed tomography (CT) can provide ample information for diagnosing AVMs, including better visualization of the lesion location, blood flow status, and involvement of adjacent tissues.The basic manifestations of AVMs on contrast-enhanced CT are abnormal vascular masses, dilated and enhanced outflow veins, and tortuous feeding arteries in the arterial phase,which are crucial for the diagnosis of AVMs.26In bone AVMs, the imaging findings included bone destruction, perforation, and pathological fractures.In mandibular AVMs, a wider mandibular nerve canal was observed on the affected side than on the contralateral side.16AVM lesions may exhibit an abnormal mixed signal shadow on magnetic resonance imaging (MRI), an equally low signal shadow on T1-weighted imaging,and increased signal intensity on T2-weighted imaging,accompanied by a flow void.After the injection of contrast media, the AVM lesions showed considerable heterogeneous enhancement.
Compared with other auxiliary examinations, digital subtraction angiography (DSA) can be employed to intuitively and dynamically determine the hemodynamic characteristics of the lesions.In particular,it provides greater intuitiveness and clarity than other methods for examining arteriovenous fistulas, feeding arteries, and draining veins.The characteristic findings related to AVMs based on DSA include clumped or nodular vascular malformations, enlarged and increased feeding arteries, and early and dilated draining veins.Additionally,increased blood flow velocity and flow in a malformed vascular mass may result in thicker single or multiple feeding arteries in the vascular mass.27Moreover,the source of the feeding arteries is related to the location of the malformed vascular mass.Furthermore, the draining veins of the malformed vascular mass are thickened,tortuous,and visualized almost simultaneously with the malformed vascular mass in the arterial phase.The Cho28and Yakes29classification methods are widely recognized and applied systems based on DSA and contrast-enhanced cross-sectional imaging of AVMs(Tables 1 and 2).However,DSA is currently performed simultaneously with interventional embolization because of its invasive nature and is rarely used solely for preoperative evaluation and diagnosis.
4.Diagnosis of AVMs
A definite diagnosis of AVMs can be easily achieved after a comprehensive evaluation of the patient's clinical manifestations and auxiliary examination data.However, the key challenge is to avoid misdiagnoses and missed diagnoses.Although DSA is considered the “gold standard”for AVM diagnosis, it presents a few limitations in clinical practice.For example, in cases involving a rapid arteriovenous shunt, the contrast agent flows prematurely into the refluxing vein via a fistula but does not fill the lesion area.Therefore, a malformed vascular mass cannot be clearly visualized in the venous phase of angiography.In such cases,angiography may not accurately reflect the lesion status, which is more prominent in bone AVMs.Additionally,arteriovenous shunts may occur in some neoplastic diseases.30Thus, the misdiagnosis of neoplastic diseases as AVMs based on DSA findings alone may delay appropriate treatment.Consequently, AVMs should be diagnosed after a comprehensive evaluation of the clinical manifestations and auxiliary examination data of patients.25Furthermore,practitioners should be cognizant of the fact that among the different auxiliary examination methods,enhanced CT offers clear advantages in displaying the lesion extent,blood flow status of the lesion, feeding arteries and reflux veins, relationship between the lesion and adjacent tissues,and bone status.31For patients with suspected AVMs, preoperative enhanced CT and clinical manifestations can be combined to establish a clinical diagnosis, followed by DSA for reconfirmation and evaluation before interventionalembolization.Moreover, timely pathological examination should be performed for clinically suspected cases while effectively controlling bleeding to avoid inaccurate diagnosis or treatment.

Table 1 Cho Classification for peripheral arteriovenous malformations (AVMs).
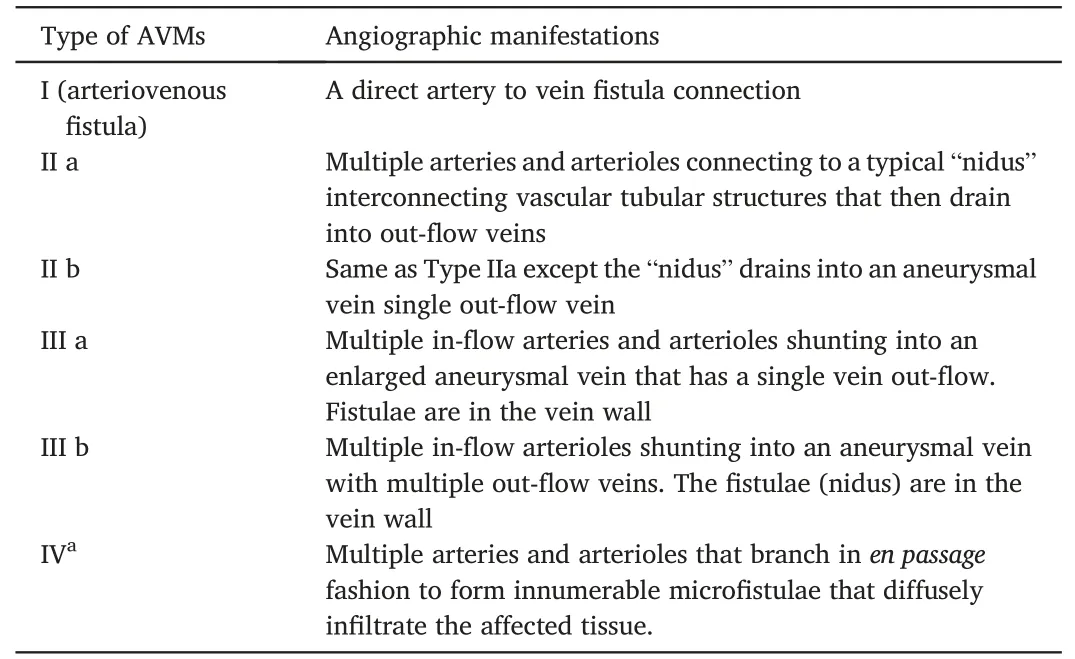
Table 2 Yakes Classification for peripheral arteriovenous malformations (AVMs).
5.Treatment of AVMs
The key treatment strategy for AVMs is the elimination or relief of venous hypertension.Based on this strategy, the primary treatment modality is interventional embolization supplemented with laser and surgical techniques.32Surgery is an auxiliary method used only when a single lesion is superficial or limited, or when debulking is required because the lesion has become fibrotic after sufficient embolization.33Surgical amputation is vital in the treatment of advanced limb AVMs.Laser therapy is principally employed in patients with static or superficial AVMs,or only superficial skin color changes after adequate embolization,thus indicating its use as an auxiliary method for interventional embolization to improve the color of the skin or mucosa.34
Indications for the interventional embolization of AVMs depend on the lesion size, location,and symptom severity.In patients with asymptomatic focal AVMs involving the trunk and limbs,enhanced CT or MRI is recommended to determine the extent and vascular structure of the malformation at the initial diagnosis.After the preliminary diagnosis,regular clinical follow-ups are required at 3,6,and 12 months to confirm the safety of the treatment;however,annual follow-ups can be performed for quiescent lesions.12,35Meanwhile, for patients with AVMs at the advanced, destruction, or decompensation stages, interventional embolization should be actively administered.Moreover, AVM embolization has no absolute contraindications,whereas its relative contraindications depend on the embolization method and material used.Insufficient professional knowledge and related experience in AVM treatment are the most important relative contraindications.However, considering that this discussion is specific to AVM treatment,we focus on the selection of the embolization material and technique.Each embolization material possesses its own unique advantages and disadvantages and should be selected based on the hemodynamic characteristics of the specific lesion and the therapist's experience.
The technical aspects pertaining to the interventional embolization of AVMs include the strategy to determine the location of the actual abnormal vascular mass, the approach for the deformed vascular mass,and the type of embolic material to be used to destroy the deformed vascular mass and obstruct abnormal communication.
5.1.Localization of AVM lesions
Most AVMs are characterized by a malformed vascular mass(“nidus”)between the feeding artery and draining vein.The nidus includes scattered networks of dense, tortuous, and ill-differentiated vessels with extremely low vascular resistance that exhibit invasive recruitment of collateral vessels and promote vascularization.36Although arterial occlusion methods (e.g., surgical ligation, coil embolization, and covered stents) may yield certain short-term effects, they cannot obstruct or destroy the nidus.Furthermore,their long-term efficacy is unsatisfactory.The collateral vasculature of AVMs continues to proliferate because of the negative-pressure suction effect at the low-resistance vascular bed of the nidus, which further stimulates the rapid progression of the lesion.Therefore, the main goal of AVM treatment is to eliminate the nidus,thereby increasing distal capillary blood flow and reducing the tendency for vascular arterialization in the venous system.37Additionally, preoperative imaging and intraoperative angiography, along with multilevel and angle analyses, can provide sufficient information regarding the nidus location.
Approach for accessing AVM lesions
AVMs can be accessed using transarterial, transvenous, and direct puncture approaches.Among them, the transarterial approach is the most direct.For Yakes type IV AVMs, the transarterial approach is the best option, as it facilitates superselective embolization to enable the diffusion,penetration,and destruction of the deformed vascular mass.38In relation to Yakes type I AVMs(arteriovenous fistulas), the fistula can be accessed directly through the artery, whereas some AVMs can be accessed through the artery via the fistula that connects to the venous end.In the case of embolization materials,mechanical agents(e.g.,coils)may offer a curative effect.39
Although the transarterial approach is the most direct method,most patients cannot undergo treatment using this approach due to conditions such as extreme tortuosity,spasms,or numerous feeding arteries.In such cases,embolization and destruction of the nidus can be achieved using a transvenous approach.However,using liquid embolic agents during the intravenous approach can be challenging because of the effect of blood flow direction.Nevertheless, coil-assisted embolization and the application of polymeric embolic agents can regulate blood flow and reduce the risk of ectopic embolization caused by the outflow of embolic agents.31
Direct percutaneous puncture is another technique that can be used solely or in combination with other methods for accessing the nidus.In clinical practice,the direct puncture of the nidus and the dilation of the reflux vein are typically more direct and effective than other approaches.40
5.2.Selection of embolic agent
Various embolic agents, each with its unique advantages, risks, and technical features,can be used for AVM treatment.The clinical treatment outcomes from using these agents depend on several important factors,including operator experience, familiarity with the embolic agent, and awareness regarding their subtle variations in different vascular beds.
Embolic agents such as N-butyl cyanoacrylate (NBCA), polyvinyl alcohol particle embolic agents(polyvinyl alcohol,PVA),and Onyx liquid embolization agents cannot destroy endothelial cells in deformed vascular masses.41Moreover, the regeneration of abnormal lumen and the recanalization of lesions may occur even after adequate embolization.Currently, absolute ethanol is the only liquid embolic agent that can potentially cure AVMs.The injection of absolute ethanol causes protein denaturation and direct and extensive damage to the endothelial cells,thus nullifying their endocrine function.42These changes promote thrombosis in the nidus.Although the use of absolute ethanol as an embolizing agent is controversial, it is widely used to treat AVMs.43,44Nevertheless,the use of absolute ethanol is associated with a higher risk of complications,including nerve damage,skin ulcers,and fatal primary cardiopulmonary failure.45The recommended maximum single dose of absolute ethanol is less than 1 mL/kg to avoid hemolysis, acute kidney injury, and cardiopulmonary function problems.42Moreover, the radiolucent nature of absolute ethanol presents a certain risk to the treatment,and the addition of contrast agents may reduce its concentration and efficacy.46Therefore, the embolization treatment of high-flow AVMs with absolute ethanol should only be performed by doctors with extensive experience and a clear understanding of its risk characteristics.Additionally, the absolute ethanol embolization of AVMs must be performed under general anesthesia to increase treatment safety,along with a close monitoring of vital signs such as the invasive arterial pressure,blood oxygen saturation,and heart rate.47Furthermore,absolute ethanol should never be injected into normal arteries because it may cause the necrosis of the nerves,muscles,and connective tissues supplied by those arteries.As an auxiliary embolization method prior to absolute ethanol injection,coil application to the draining vein can be adopted to reduce the flow velocity of the lesion and the amount of absolute ethanol as well as to avoid complications.
Particulate embolic agents, including PVA and microspheres, are highly effective for managing acute bleeding episodes and preventing embolization before the surgical resection of giant AVMs.48The diameter of particulate embolic agents ranges from 150 to 1200 μm and is a crucial parameter for AVM treatment.An exceedingly large diameter prevents the complete penetration of the lesion nidus.Furthermore, the affected region is susceptible to recanalization and recurrence,despite immediate angiographic improvement.Conversely, an excessively small diameter fails to achieve complete occlusion of the malformed vessel mass,which is accompanied by the potential risk of untargeted embolization via abnormal shunts.Moreover, the direct administration of particulate embolic agents in AVM lesions with considerable direct arteriovenous communication should be avoided to prevent ectopic embolization.
Polymeric embolic agents include NBCA and Onyx.Onyx is a nonviscous liquid embolic agent comprising vinyl alcohol, dimethyl sulfoxide,and tantalum powder.It can overcome the disadvantages of NBCA adhesion and is routinely used to treat AVMs in certain treatment centers.49Based on the angioarchitectural characteristics of AVMs, their access routes entail transarterial superselective delivery via the axial microcatheter system, the direct puncture technique, and retrograde transvenous delivery.Although polymeric embolic agents offer therapeutic value for AVM embolization, they cannot destroy vascular endothelial cells, and their potential recurrence rate is relatively high.31
Mechanical embolizations,such as occluders and coils,do not induce any direct chemical damage to the AVM nidus; however, the utility of these devices should not be disregarded.In cases where AVM blood flow is rapid and the buffer insufficient, a physical embolization device can provide mechanical filling to reduce the flow velocity, thus facilitating further fluid embolization and reducing the required dose of a liquid embolic agent for complete AVM closure.50Furthermore, mechanical embolization can be used as an auxiliary method to control the flow rate of typical AVM-draining veins, thereby reducing the likelihood of non-targeted embolization caused by liquid embolic agents during transarterial or direct puncture delivery.51Additionally, during the absolute ethanol treatment of the AVM nidus and refluxing vein,adequate coil embolization can reduce the flow velocity of the lesion and the amount of absolute ethanol,thus improving the treatment efficiency and decreasing the risk of systemic complications.In AVM embolization,coils and covered stents should not be used to obstruct the feeding artery because they are ineffective in reducing venous hypertension and can promote collateral circulation and lesion development.52
5.2.Evaluation and follow-up of AVMs
Owing to the low incidence of AVMs,varied treatment methods,and scarcity of unified standards,evaluating therapeutic methods for AVMs is difficult.Clinical diagnosis of AVMs is based on medical history,physical examination, and enhanced CT and MRI analyses.Furthermore, DSA,which is an invasive examination method, is necessary for suspected cases of recurrence or progression.
Patients with AVMs are at high risk of recurrence.AVM recurrence may result from the recanalization of the embolization site or the continued dilatation of the focal vessels.31Moreover, the time to recurrence cannot be estimated easily,including for cases of successful treatment.Therefore,the follow-up time for patients with AVMs should be 2,4, 6, and 12 months after the final treatment.Additionally, DSA examination should be performed after one year,followed by an annual routine re-examination.However, these time points should be recalculated if a relapse occurs after treatment.
6.Conclusions
The natural course and symptoms of AVMs depend significantly on the location and extent of the lesions,and their correct diagnosis requires a comprehensive analysis of the clinical manifestations and auxiliary examinations of the patients.Currently, AVM treatment involves a multidisciplinary comprehensive approach and is primarily based on interventional embolization,supported by other methods.The extensive use of various treatment methods combined with the technical experience and professional knowledge of clinical specialists are crucial for ensuring the efficacy of clinical treatment for AVMs.Moreover, multicenter prospective clinical trials can provide useful evidence for investigating and improving existing treatment strategies and reducing risks.Further research delineating the etiology and mechanism of AVMs will allow one to better understand their development from a molecular biology perspective and provide a reference for targeted drug therapy.
Author contributions
Xindong Fan: Conceptualization, Methodology; Yuchen Shen.: Data curation, Writing, Original draft preparation; Lixin Su: Visualization,Investigation; Deming Wang: Supervision, Validation, Writing- Reviewing and Editing.
Funding
This work was supported by the Transverse Research Project of Shanghai Ninth People's Hospital(No.JYHX2022007).
Declaration of competing interest
Xindong Fan is an editorial board member for Journal of Interventional Medicine,he was not involved in the editorial review or decision to publish this article.All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We appreciate all the medical workers from the Department of Interventional Therapy,Shanghai Ninth People's Hospital,Shanghai Jiao Tong University School of Medicine.We were grateful to Prof.Wayne F.Yakes,MD.,from The Yakes Vascular Malformation Center,Englewood,Colorado, the United States, for imparting the ethanol embolization of arteriovenous malformations to us.
 Journal of Interventional Medicine2023年4期
Journal of Interventional Medicine2023年4期
- Journal of Interventional Medicine的其它文章
- Mechanisms and therapeutic strategies to combat the recurrence and progression of hepatocellular carcinoma after thermal ablation
- Embolization of brain arteriovenous malformations with squid co-polymer embolic material: Initial experience
- A novel cerebrovascular drug-coated balloon catheter for treating symptomatic intracranial atherosclerotic stenosis lesions:Study protocol for a prospective, multicenter, single-arm, target-value clinical trial
- Combination of transarterial radioembolization with atezolizumab and bevacizumab for intermediate and advanced staged hepatocellular carcinoma: A preliminary report of safety and feasibility
- Argon-helium cryoablation treatment of undifferentiated pleomorphic sarcoma of the thyroid: A case report and literature review
- “Guidezilla” extension catheter combined with balloon technique for treating pulmonary artery stenosis caused by Takayasu arteritis
