Benzydamine hydrochloride ameliorates ethanol-induced inflammation in RAW 264.7 macrophages by stabilizing redox homeostasis
Tiasha Dasgupta,Venkatraman Manickam
Department of Biomedical Sciences, School of Biosciences and Technology, Vellore Institute of Technology, Vellore 632014, Tamil Nadu, India
ABSTRACT Objective:To evaluate the protective effect of benzydamine hydrochloride against ethanol-induced oxidative stress and inflammation in RAW 264.7 macrophages.Methods:RAW 264.7 macrophages were treated with ethanol (100 mM) and benzydamine hydrochloride (7.5 µM).The imflammatory status was confirmed by measuring pro-(TNF-α and IL-6) and anti-inflammatory (IL-10) cytokines through ELISA and RT-PCR assays.Reactive oxygen species generation and mitochondrial membrane potential were investigated to study the protective role of benzydamine hydrochloride against ethanol-induced oxidative stress.Apoptosis detection was also investigated using flow cytometry and acridine orange/ethidium bromide staining.Results:Benzydamine hydrochloride significantly decreased the secretion of TNF-α and IL-6,as well as the generation of reactive oxygen species inside the cells,thereby stabilizing the mitochondrial membrane potential and reducing DNA fragmentation.The ethanolinduced cellular necrosis was also reversed by the administration of benzydamine hydrochloride.Conclusions:Benzydamine hydrochloride ameliorates ethanolinduced cell apoptosis and inflammation in RAW macrophages.
KEYWORDS: Alcohol;Benzydamine hydrochloride;Inflammation;Oxidative stress;Apoptosis
1.Introduction
Alcohol abuse is common yet a complicated condition that affects more or less every other organ in our body and is associated with diverse pathological events.Almost 2 billion individuals drink alcohol worldwide,and more than 75 million have been identified as being at risk for alcohol-associated liver disease[1].Chronic alcohol use disorder has distinct types of abusive behaviors such as binge drinking[2],excessive consumption,and addiction[3].These events induce transitory immunosuppression associated with the development of chronic inflammation which is linked to an increase in the production of pro-inflammatory cytokines like TNF-α,IL-6,IL-1β,as well as inducible nitric oxide (iNOS) and cyclooxygenase-2 (COX-2)viathe activation of transcription factor nuclear factor-κB (NF-κB)[4].
Notably,pro-inflammatory mediators produced during chronic alcohol exposure sustain the inflammatory state through depletion of antioxidant systems and enhancing ethanol metabolism through cytochrome P450 2E1 (CYP2E1),which encourages the production of reactive oxygen species (ROS) in the cytosol,as well as in mitochondria,therefore eventually leading to oxidative stress,inflammation,and DNA damage[5].Furthermore,inflammatory cytokine-induced ROS production promotes mitochondrial membrane depolarization that initiates mitochondrial-independent pathways of apoptosis[6].At sites of tissue damage,macrophages exposed to ethanol or its metabolites such as acetaldehyde,generate inflammatory mediators and ROS which are the key players in the pathogenesis of alcohol-induced degenerative condition[7].Therefore,regulating pro-inflammatory mediators and ROS levels is important to protect against ethanol-induced inflammatory diseases.
Studies to date established benzydamine hydrochloride (an indazole derivative) as a non-steroidal anti-inflammatory drug(NSAID) having antibacterial,analgesic,and anesthetic properties[8].Intriguingly,both locally and systemically,it could alleviate symptoms related to inflammatory conditions such as mouth ulcers[8],mucositis[9],pericoronitis[10],tonsillopharyngitis[11],oral lichen planus[12],and with post-operative sore throats[13].Moreover,benzydamine hydrochloride exhibits an anti-inflammatory effect by inhibiting TNF-α production in LPS-treated RAW 264.7 macrophages[14].It is proposed that this drug due to its basic nature could accumulate in the inflamed tissue which is more acidic[15].Thus,benzydamine hydrochloride could be used to prevent persistent alcohol and its metabolite,acetaldehyde-induced oxidative stress,and inflammation concerned with alcohol use pathogenesis,paving the way for novel anti-inflammatory therapies among alcohol patients.In the current study,we assessed the protective effect of benzydamine hydrochloride against ethanol-induced inflammation and oxidative stress in RAW macrophages.
2.Materials and methods
2.1.Chemicals and reagents
Mouse macrophage cell line (RAW 264.7) was sourced from National Centre for Cell Sciences (NCCS,Pune,India).Benzydamine hydrochloride,TRIzol,Rhodamine 123,DAPI,and MTT were purchased from Sigma-Aldrich (India).Molecular biology grade ethanol,DCFH-DA dye,cell culture materials including Dulbecco’s Modified Eagle’s Medium (DMEM),fetal bovine serum (FBS),and dimethyl sulfoxide (DMSO),were obtained from Hi-Media laboratories,Mumbai,India.The annexin V-FITC kit for apoptosis was purchased from Invitrogen (Bangalore,India).cDNA synthesis kit and SYBR-green kit for RT-PCR were obtained from Takara,Japan.All cell culture plastic materials were obtained from Tarson,India.
2.2.Cell culture and cell viability assay
RAW 264.7 macrophages were grown in DMEM supplemented with 10% FBS in a humidified incubator at 37 ℃,5% CO2.Cells were extracted and studies were carried out after they achieved 80%confluency.
For the cytotoxicity assay,RAW cells were seeded with a cell density of 1×104per well supplemented with complete media in a 96-well plate and kept overnight in a humidified incubator.The next day,cells were treated with ethanol (molecular biology grade ethanol) at different concentrations ranging from 50 mM to 600 mM for 24 h.After the incubation period,the media were removed and 20 µL MTT solution (5 mg/mL) was added into the wells and further incubated for 4 h at 37 ℃ in a dark condition.After incubation,100µL DMSO was added and absorbance measurement was taken at 570 nm by a microplate reader (Bio-Tek,USA) to quantify the viable cells as described before[16].
Furthermore,the therapeutic effect of benzydamine hydrochloride on ethanol-stimulated cells at different concentrations ranging from 2.5 µM to 50 µM was determined as mentioned above.
2.3.Measurement of pro-inflammatory and antiinflammatory cytokines
The abovementioned protocol was followed and cell supernatant was collected to quantify the amount of TNF-α,IL-6,and IL-10 using an ELISA assay kit according to the manufacturer’s protocol(Sigma-Aldrich).The absorbance was then measured by an ELISA reader (Bio-Rad,CA) at 450 nm.
2.4.Nuclear extract preparation
The manufacturer’s instructions for the nuclear extraction kit(Cayman Chemicals,Bangalore,India) were followed when creating the nuclear extracts from RAW macrophages.To stop additional protein changes,the cells were briefly homogenized in ice-cold PBS containing phosphatase inhibitors.The homogenates were centrifuged at 300 ×gfor 5 min.The pellets were then cleaned with detergent and resuspended in the hypotonic buffer.The cytoplasmic fractions were then produced by centrifuging at 14 000 ×gfor 30 s.The nuclear proteins were then solubilized in the lysis solution containing proteasome inhibitors after the cell nuclei had been lysed by the nuclear extraction buffer.
2.5.DNA-binding activity of NF-κB
Using a TransAM NF-κB p65 test kit (Active Motif),a quick ELISAbased method,the binding of NF-κB to DNA was assessed in nuclear extracts.The consensus binding site for NF-κB (5’-GGGACTTTCC-3’)is an oligonucleotide coated on multiwell plates for this test.To enable NF-κB DNA binding,nuclear proteins (20 mg) were added to each well and incubated for 1 h.The NF-κB complex coupled to the oligonucleotide was then discovered by utilizing an antibody that is specific for the NF-κB p65 subunit.The colorimetric measurement is based on a secondary antibody linked to horseradish peroxidase.The absorbance was taken in an ELISA reader (Bio-Rad,CA) at 450 nm.
2.6.Intercellular ROS generation and quantification using flow cytometry
2,7-dichlorofluorescein diacetate (DCFH-DA) was used to detect the fluorescence expression of intracellular ROS[17].Briefly,RAW 264.7 cells were seeded into a 6-well plate and kept overnight for attachment in a humidified incubator.Cells were treated with 100 mM ethanol for 24 h.Then the medium was removed and treated with 7.5 µM of benzydamine hydrochloride for another 24 h.After treatment,the medium was discarded and washed with phosphatebuffered saline (PBS) twice and incubated with DCFH-DA dye for 15 min in the dark[17].The intercellular ROS accumulation was observed under a confocal microscope (EVOS M5000 Imaging System) at the emission wavelength of 530 nm and the excitation wavelength of 485 nm.
For quantifying the ROS level,the abovementioned protocol was followed to stimulate and treat the cells.After that,the cells were trypsinized and collected followed by treatment with 20 µM DCFHDA dye for 30 min in the dark.The intercellular ROS level was measured using flow cytometry and CytExpert software was used to interpret the data (CytoFLEX,Beckman Coulter,USA).
2.7.Mitochondrial membrane potential (MMP) study using Rhodamine-123 dye
Briefly,cells were seeded in a 6-well plate at a cell density of 1×105per well and kept overnight in a humidified incubator.The above mentioned protocol was followed to stimulate and treat the cells.After incubation,the cells were washed with PBS twice and incubated with Rhodamine 123 at a concentration of 5 µg/mL for 20 min[18].The MMP was measured using flow cytometry (CytoFLEX,Beckman Coulter,USA) at the excitation wavelength of 488 nm.
2.8.DNA fragmentation analysis by DAPI staining
Cells were seeded in a 6-well plate followed by stimulation with ethanol and treatment with benzydamine hydrochloride.After treatment,cells were washed with PBS twice fixed with 4%paraformaldehyde,and incubated with 1 µg/mL DAPI for 10 min.The cells were visualized under a fluorescence microscope (EVOS M5000 Imaging System) at the excitation and emission wavelengths of 358 and 460 nm,respectively[19].
2.9.Apoptosis study using Annexin V/PI apoptosis kit
The percentage of apoptotic cells was detected using an apoptosis kit according to the manufacturer’s protocol.RAW 264.7 cells were stimulated with ethanol for 24 h,followed by benzydamine hydrochloride treatment at the indicated dose for 24 h.Afterward,the cells were harvested and washed twice using 1× PBS and resuspended in 100 µL Annexin V binding buffer and incubated with 5 µL of Annexin V-FITC and 1 µL PI for 15 min at room temperature.The cells were then suspended in 400 µL annexin binding buffer.The percentage of apoptotic cells was analyzed using a flow cytometer (CytoFLEX,Beckman Coulter,USA) at 518 nm for Annexin-V and 620 nm for PI.
2.10.Apoptosis study using acridine orange/ethidium bromide (AO/EB) double staining
To identify the morphological alterations induced by apoptosis,EB and AO dual staining were utilized.The nuclei are stained red and green by the nucleic acid dyes EB and AO,respectively.Cells were treated as mentioned above and stained with 50 µL (1∶1)[20] of AO/EB and viewed under a fluorescence microscope (Olympus inverted fluorescence microscope).
2.11.mRNA isolation and RT-PCR analysis
RAW macrophage cells were stimulated and treated with benzydamine hydrochloride as mentioned above.After the desired time mRNA was isolated using TRIzol.RNA was quantified using Nanodrop (BioSpectrometer®,Eppendorf).Using PrimeScript™RT Reagent Kit (Perfect Real Time),the RNA was converted to cDNA,followed by amplification using Real-Time PCR master mix SYBR®Premix Ex Taq™II (Tli RNase Plus) using appropriate forward and reverse primers (Table 1).For internal standard glyceraldehyde-3-phosphate dehydrogenase (GADPH) was used and the findings were calculated using the 2-ΔΔCtmethod,and the final results were expressed as the fold change in comparison to control.
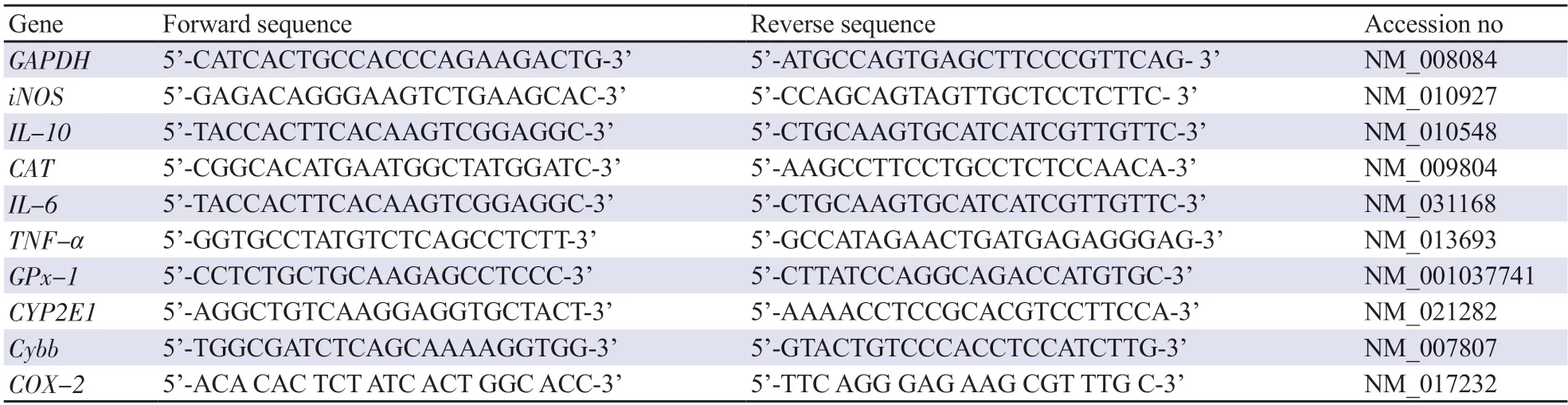
Table 1.Sequences for primers used for qRT-PCR.
2.12.Statistical analysis
For statistical analysis,GraphPad Prism 8.0 was used and all experiments were repeated at least three times.Data is represented as mean±SD.One-way analysis of variance with Bonferroni’s multiple comparisons was used to evaluate group differences.P< 0.05 was considered to be significant.
3.Results
3.1.Benzydamine hydrochloride ameliorates ethanolinduced toxicity to RAW 264.7 macrophages
The viability of RAW 264.7 macrophages treated with different concentrations (50-600 mM) of ethanol for 24 h was evaluated using an MTT assay.With the increasing concentration of ethanol,the viability of cells was decreased in a dose-dependent manner as shown in Figure 1A.Ethanol at the concentration of 100 mM showed relatively minimal toxicity having a survival rate of (63.16 ±1.05)% and the inflammatory status at 100 mM was confirmed using TNF-α ELISA (Supplementary Figure 1);thus 100 mM ethanol with moderate toxicity and increased TNF-α concentration was applied for subsequent experiments.
To study the therapeutic effect of benzydamine hydrochloride,ethanol (100 mM)-stimulated cells were treated for 24 h with different concentrations of benzydamine hydrochloride (2.5-50 µM),and cell viability was measured by an MTT assay.As manifested in Figure 1B,this drug at the concentration of 7.5 µM showed an improvement in cell viability (P< 0.001).Based on the cytoprotective ability,the concentration of 7.5 µM was used to carry out subsequent experiments.
3.2.Benzydamine hydrochloride modulates the production of pro-inflammatory and anti-inflammatory cytokines and reduces NF-κB nuclear binding activity in ethanolstimulated macrophages
To confirm whether benzydamine hydrochloride reduces inflammatory response in ethanol-stimulated RAW264.7 macrophages,the cells were treated with the drug (7.5 µM) for 24 h.After ethanol (100 mM for 24 h) stimulation,cytokine production was detected using ELISA and the expression was studied using RT-PCR.Administration of 7.5 µM benzydamine hydrochloride significantly reduced ethanol-induced TNF-α and IL-6 production while increasing anti-inflammatory cytokine IL-10 (P< 0.05),as shown in Figure 2A.Consistent with the above results,theTNF-αandIL-6mRNA expression was significantly downregulated by benzydamine hydrochloride,andIL-10mRNA expression was upregulated in macrophages (P< 0.05) (Figure 2B).Moreover,benzydamine hydrochloride at a dose of 7.5 µM reduced ethanolinduced NF-κB activity in macrophages (P< 0.05) (Figure 2C).These results suggest that benzydamine hydrochloride can modulate the inflammatory mediators to control the inflammatory response by inhibiting NF-κB in ethanol-induced macrophages.
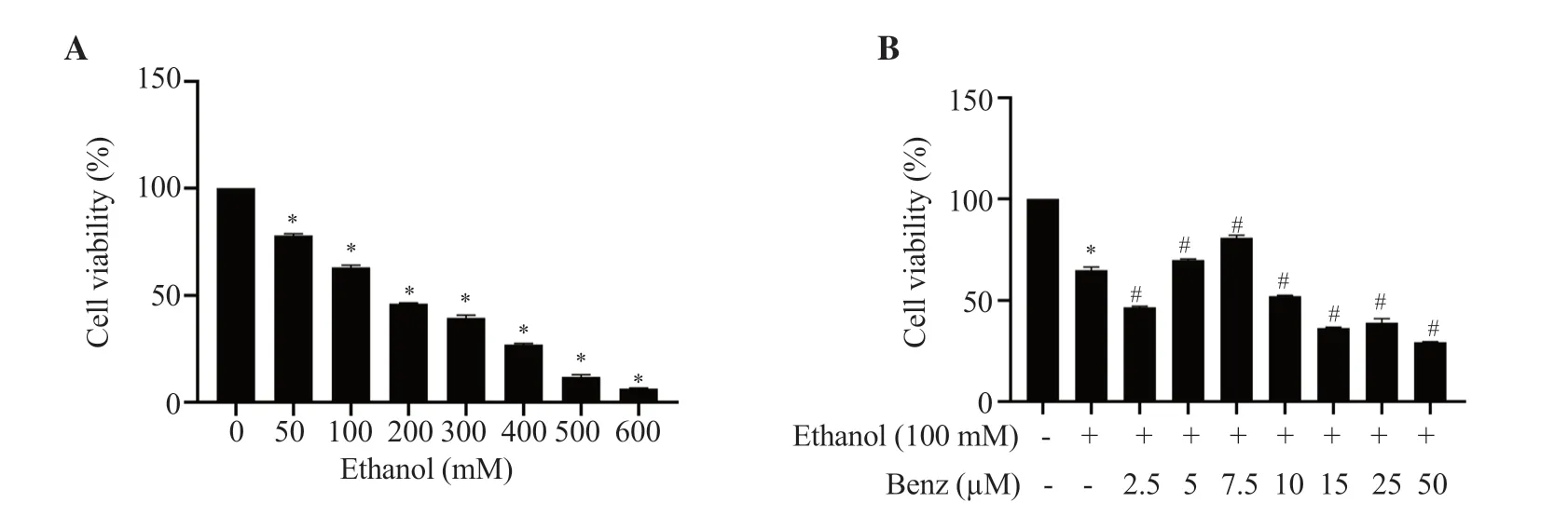
Figure 1.Effects of (A) ethanol and (B) benzydamine hydrochloride on the viability of RAW 264.7 cells.Data are presented as mean ± SD (n=6).*P < 0.001 vs.the control group;#P < 0.001 vs.the ethanol-treated group.Benz: benzydamine hydrochloride.
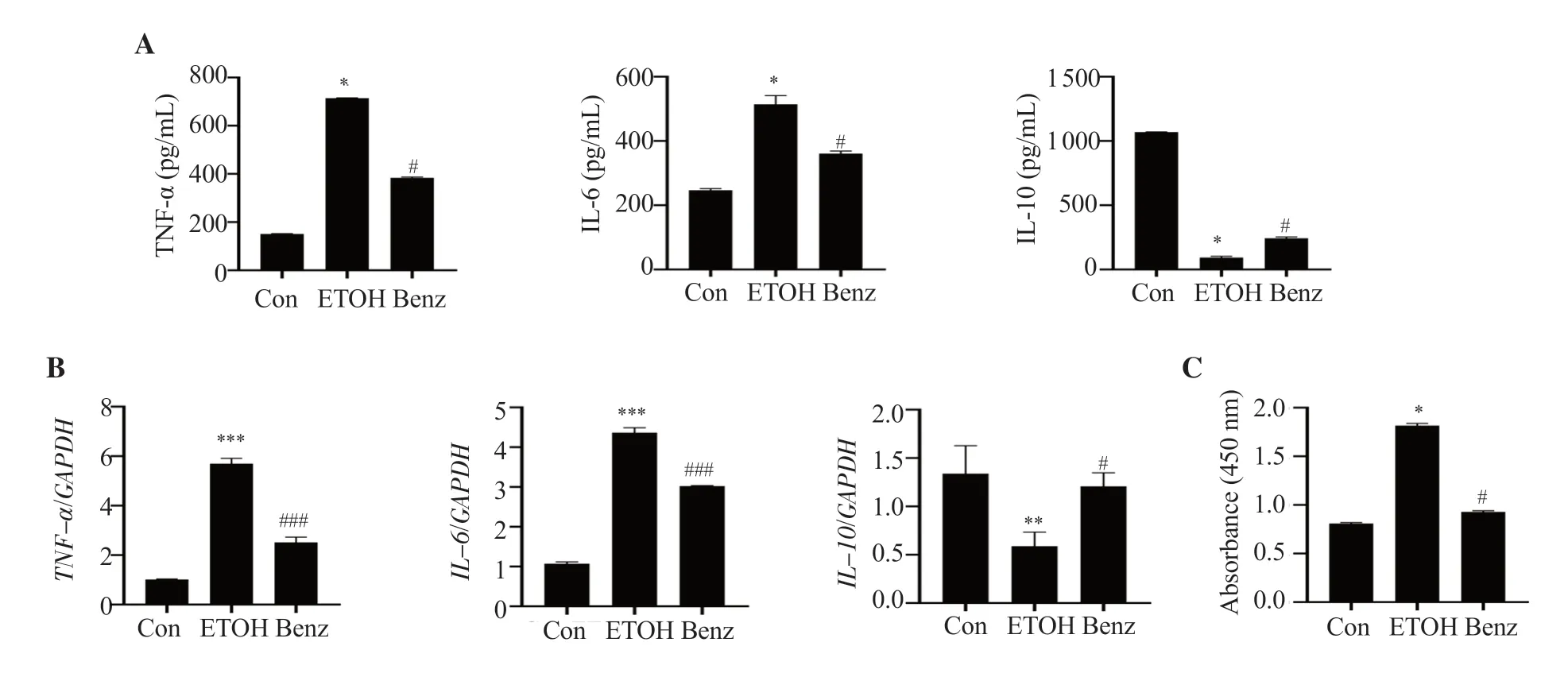
Figure 2.Benzydamine hydrochloride reduces ethanol-induced pro-and anti-inflammatory cytokines in RAW 264.7 macrophages.(A) The levels of TNF-α,IL-6 and IL-10 were determined by ELISA.The results are expressed as mean ± SD (n=6).*P < 0.05 vs.the control group; #P < 0.05 vs.the ethanol-treated group.(B) mRNA expression of pro-and anti-inflammatory cytokines.Data are expressed as mean ± SD (n=3).**P < 0.01,***P < 0.001 vs.the control group;#P < 0.05,###P < 0.001 vs.the ethanol-treated group.(C) Effect of benzydamine hydrochloride on NF-κB DNA binding activity in ethanol-induced RAW 264.7 macrophages.Data are expressed as mean ± SD (n=6).*P < 0.05 vs.the control group;#P < 0.05 vs.the ethanol-treated group.Con: control;ETOH: ethanol.
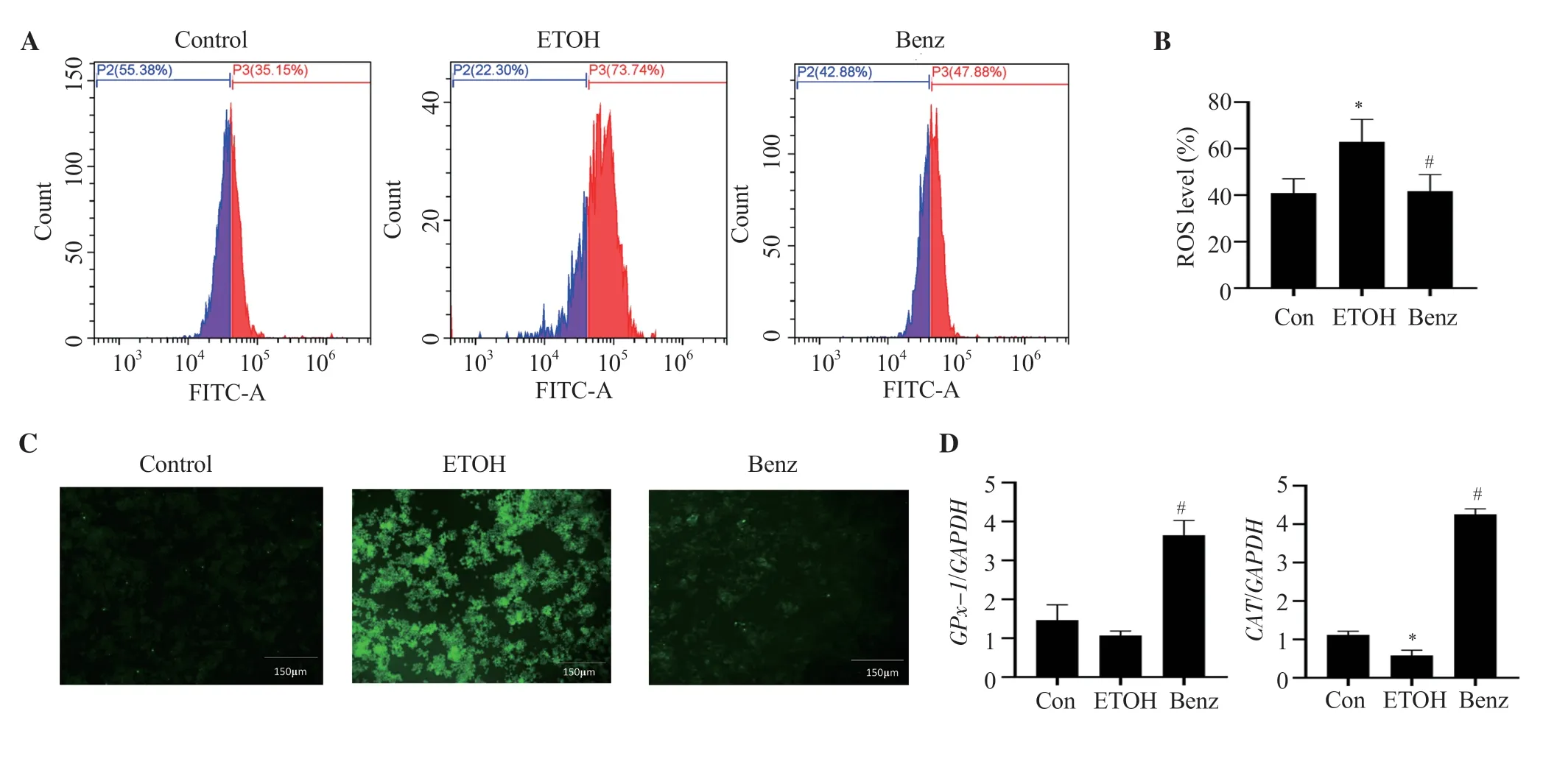
Figure 3.Benzydamine hydrochloride reverses the production of reactive oxygen species (ROS) on ethanol-induced RAW 264.7 macrophages.(A) Effect of benzydamine hydrochloride (7.5 μM) on ROS generation and accumulation in ethanol-induced RAW 264.7 macrophage cells by flow cytometry.(B)Quantitative analysis of intercellular ROS in ethanol-induced RAW 264.7 macrophages.The results are expressed as mean ± SD (n=3).*P < 0.05 vs.the control group;#P < 0.05 vs.the ethanol-treated group.(C) Effect of benzydamine hydrochloride on ROS generation by florescence microscopy.The images were taken at 10× magnification,scale bar: 150 μm.(D) Effect of benzydamine hydrochloride on the gene expression of antioxidative enzymes.The results are expressed as mean ± SD (n=3).*P < 0.05 vs.the control group;#P < 0.05 vs.the ethanol-treated group.
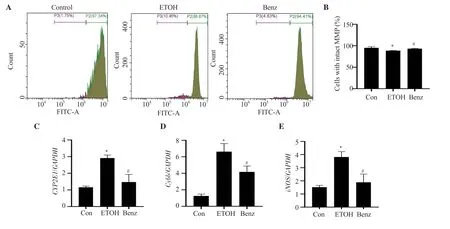
Figure 4.Benzydamine hydrochloride restores ethanol-induced mitochondrial membrane potential (MMP) and regulates the gene expression of CYP2E1,Cybb and iNOS in RAW 264.7 macrophages.(A) Effect of benzydamine hydrochloride (7.5 μM) on MMP in ethanol-induced RAW 264.7 macrophage cells by flow cytometry.(B) Quantitative analysis of cells with intact MMP in ethanol-induced RAW 264.7 macrophages after treatment with benzydamine hydrochloride.The results are expressed as mean ± SD (n=3).*P < 0.05 vs.the control group;#P < 0.05 vs.the ethanol-treated group.(C-E) Effect of benzydamine hydrochloride on the gene expression of CYP2E1,Cybb and iNOS.The results are expressed as mean ± SD (n=3).*P < 0.05 vs.the control group;#P < 0.05 vs.ethanol-treated group.
3.3.Benzydamine hydrochloride reduces intercellular ROS generation and normalizes antioxidative gene expression in ethanol-induced RAW 264.7 macrophages
We observed ROS production was increased significantly after ethanol stimulation.However,benzydamine hydrochloride treatment significantly decreased the ROS level in RAW 264.7 cells,demonstrating its inhibitory effect on cellular ROS generation as represented in Figure 3A and 3B (P< 0.05).The inhibitory effect of benzydamine hydrochloride on cellular ROS generation was also confirmed through a fluorescence microscope.As displayed in Figure 3C,the intensity of green fluorescence increased in ethanol-stimulated cells,which was reduced by benzydamine hydrochloride.
To assess the potential antioxidative activity of benzydamine hydrochloride in ethanol-induced RAW 264.7 cells,the gene expression of two downstream major antioxidative enzymesGPx-1andCATwas determined by quantitative PCR.Under ethanol-stimulated conditions,the expression of two genes was decreased compared to control cells,whereas at the concentration of 7.5 µM,benzydamine hydrochloride increased the expression ofGPx-1andCATsignificantly (P< 0.05)(Figure 3D).
3.4.Benzydamine hydrochloride inhibits CYP2E1 gene expression, and increases MMP in ethanol-induced RAW 264.7 macrophages
MMP was decreased significantly by ethanol stimulation (P<0.05).However,benzydamine hydrochloride markedly reversed ethanol-induced reduced MMP in RAW 264.7 macrophages (Figure 4A-B).Interestingly,the mRNA expression ofCYP2E1,a crucial ethanol metabolizing enzyme that generates ROS,was noticeably upregulated in the ethanol-treated group,which was ameliorated in the benzydamine hydrochloride-treated group (P< 0.05),as shown in Figure 4C.Moreover,Cybbwas increased by ethanol but decreased significantly by benzydamine hydrochloride (P< 0.05) in RAW 264.7 macrophages (Figure 4D).Benzydamine hydrochloride at the concentration of 7.5 µM also decreasediNOSgene expression significantly in ethanol-stimulated cells (P< 0.05) (Figure 4E).
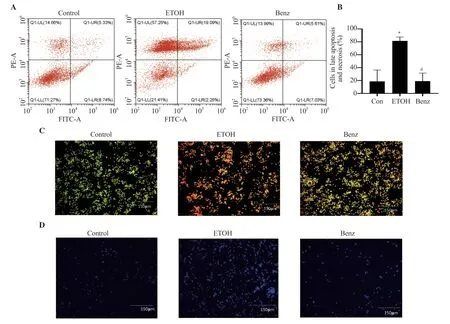
Figure 5.Effect of benzydamine hydrochloride on ethanol-induced apoptosis and necrosis of RAW 264.7 macrophages.(A) Effect of benzydamine hydrochloride (7.5 μM) on late apoptosis and necrosis in ethanol-induced RAW 264.7 cells by flow cytometry.(B) Quantitative analysis of late apoptotic and necrotic cells in ethanol-induced RAW 264.7 macrophages.(C-D) RAW 264.7 macrophages stained with acridine orange/ethidium bromide and DAPI,respectively.The images were taken at 10× magnification,scale bar: 150 μm.The results are expressed as mean ± SD (n=3).*P < 0.05 vs.the control group;#P < 0.05 vs.the ethanol-treated group.
3.5.Benzydamine hydrochloride ameliorates ethanolinduced apoptosis and necrosis in RAW 264.7 macrophages
The apoptotic effect of benzydamine hydrochloride was analyzed by staining the cells with annexin V-FITC and PI using flow cytometry.Ethanol induced approximately 80% of combined late apoptotic and necrotic cell mass,whereas benzydamine hydrochloride treatment reduced late apoptotic and necrotic cells(P< 0.05) (Figure 5A-B).Additionally,the above result was validated with AO/EB staining.The ethanol-treated group showed apoptotic and necrotic bodies,however,benzydamine hydrochloride treatment inhibited ethanol-induced cell death (Figure 5C).For DNA condensation and fragmentation,DAPI staining shows that the ethanol-treated group (Figure 5D) showed an increase in DNA fragmentation compared to the control group,which was reversed by benzydamine hydrochloride.
4.Discussion
Ethanol consumption is known to disturb the innate immune system and disrupt metabolic homeostasis which in turn leads to development of various diseases including cancer.According to a World Health Organization (WHO) report from 2018,alcohol misuse was responsible for 3 million fatalities (5.3% of all deaths)worldwide in 2016.Alcohol intake harms a variety of organs and systems.Because alcohol is primarily processed by liver cells,which exhibit high quantities of two important alcohol oxidizing enzymes,alcohol dehydrogenase and CYP2E1,the liver is a prime target for its harmful effects.However,alcohol also affects other organs such as the brain,intestines,pancreas,lungs,and immune system[7,21-23].The current investigation is done to verify whether benzydamine hydrochloride can have any protective effects on the macrophages exposed to ethanol.
In various experimental models,ethanol aggravates cytokine production.For instance,it has been noted that ethanol increases the levels of the inflammatory mediators IL-6 and TNF-α in the liver of rats[24].Previous studies demonstrated the anti-inflammatory properties of benzydamine hydrochloride in macrophages when stimulated with LPS by regulating pro-inflammatory cytokine TNF-α[25].In the present study,benzydamine hydrochloride significantly reduced ethanol-induced IL-6 and TNF-α generation.NF-κB,a major transcription factor that helps in controlling DNA transcription,induces the production of major pro-inflammatory mediators like IL-1β,IL-6,and TNF-α and promotes cell survival,upon activated primarily by the primary inflammatory cytokine TNF-α[26].Usually,the activated NF-κB is translocated into the nucleus,promoting the gene transcription of the pro-inflammatory cytokines and carrying forward the process of inflammation[27].In our study,ethanol stimulation promoted active NF-κB translocation into the nucleus,which was abrogated by benzydamine hydrochloride treatment.
Enhanced oxidative stress has been linked to ethanol metabolism.An imbalance between the generation of free radicals and the antioxidant system is referred to as oxidative stress[28].In our study,ethanol-activated macrophages showed a substantial rise in cellular ROS generation in response to ethanol.Superoxide dismutase(SOD),CAT and glutathione peroxidase (GPx) activities were found to be impaired in ethanol-induced oxidative stress[29].The crucial enzyme system known as CAT is capable of neutralizing ROS and its by-products.While chronic alcohol use raises CAT enzyme activity,however,intermittent alcohol intake sessions lower it[30].In our study,the expression of the antioxidant enzyme CAT showed a similar pattern as reported previously and it showed a 0.5-fold decrease in the ethanol group.However,benzydamine hydrochloride treatment restored the activity of the enzyme and shielded RAW macrophage cells from ethanol-induced oxidative damage.The GPx uses glutathione (GSH) to degrade organic hydroperoxides and H2O2and produce glutathione disulfide.A flavoprotein called glutathione reductase regenerates GSH and supplies reducing power for a variety of linked thiol transferase and peroxidase enzymes[31].The expression ofGPx-1in our findings showed a gradual decrease in ethanol-stimulated cells which was reversed in the benzydamine hydrochloride-treated group.These findings suggest the potential protective role of benzydamine hydrochloride against oxidative stress in ethanol-stimulated RAW 264.7 macrophages.
Additionally,CYP2E1,the primary protein of alcohol metabolism,converts ethanol to acetaldehyde,generating ROS such as hydrogen peroxide (H2O2) that can harm the tissue[32].Along with being a very poisonous substance,acetaldehyde can also cause the generation of ROS,which includes reactive molecules and free radicals formed from molecular oxygen[5].Acetaldehyde concentrations enhance the expression and activity of nicotinamide adenine dinucleotide phosphate oxidase 2 (NOX-2),which is essential for the production of acetaldehyde-induced mitochondrial ROS[33].In RAW 264.7 macrophages,ethanol boosted the expression of theCybbgene,which codes for NOX-2.Treatment with benzydamine hydrochloride significantly decreased the elevated gene expression ofCybb.Additionally,oxidant levels can be possibly raised by copper/ironcatalyzed Fenton-Weiss-Haber reactions of H2O2,cellular activation of NADPH oxidase/xanthine oxidase (NOX/XOX),nitric oxide synthase (NOS),and mitochondrial oxidative phosphorylation[34].The precise mechanism of toxic actions of ethanol-induced oxidative stress is not well defined.Alcohol consumption causes oxidative damage to mitochondria and disruptions in cellular processes.According to a theory by Haorah and colleagues,the activation of NOX/XOX and inducible NOS (iNOS) by acetaldehyde causes the production of ROS and nitric oxide (NO) in human neurons during ethanol metabolismviaalcohol dehydrogenase and CYP2E1[35].It was demonstrated that mice overexpressingCYP2E1through knock-inCYP2E1developed greater liver damage and hepatic steatosis after ethanol consumption.Thus the ability of CYP2E1 to oxidize ethanol contributes to the generation of reactive molecules which promotes the production of ROS and anionic radicals[36].Our experimental data showed that benzydamine hydrochloride significantly reduced ethanol-inducedCYP2E1gene expression.A previous report revealed that ethanol consumption plays a vital role in disrupting mitochondrial membrane integrity of RAW macrophages thus resulting in decreased MMP and increased ROS production[32].In our investigation,ethanol significantly reduced MMP which was reversed by benzydamine hydrochloride treatment.These results provide experimental evidence for the antioxidant activity of benzydamine hydrochloride against ethanol-stimulated RAW macrophages.Another important inflammatory factor that is triggered by alcohol treatment along with iNOS is COX-2[37].A significant increase in the expression of COX-2 was observed after ethanol stimulation.However,benzydamine hydrochloride used in this current study did not significantly decrease ethanol-inducedCOX-2gene expression (Supplementary Figure 2),confirming the negative role of benzydamine hydrochloride in COX-2 inhibition as described in a previous study[38].
In ethanol-induced inflammation and cellular damage,cell apoptosis and necrosis are some of the major detrimental consequences[39].Based on previous reports,ethanol consumption damaged mitochondrial integrity which in turn produced a large amount of ROS and induced apoptosis[39].In our study,macrophages demonstrated typical chromatin condensation and nuclear lysis in response to ethanol stimulation,indicating that cells undergo apoptosis.This result is also confirmed by a rise in cell death in flow cytometric analysis.Benzydamine hydrochloride therapy prevented cell apoptosis and eventually improved aberrant nuclear morphology.Cell necrosis and apoptosis were noticed after ethanol treatment by AO/EB staining,which were ameliorated by benzydamine hydrochloride.These preliminary findings demonstrate that benzydamine hydrochloride effectively inhibits ethanolinduced cell death and reduces inflammation in RAW macrophages.However,additionalin vivoand other cell line investigations are required to validate its applicability in ethanol-stimulated conditions.In the future,the expression of proteins involved in the inflammatory pathway under the ethanol-stimulated condition should be studied utilizing Western blot analysis to elucidate the protective mechanism of the drug.
In conclusion,our study demonstrates the ameliorative effect of benzydamine hydrochloride against ethanol-induced inflammationin vitroby lowering ROS-induced oxidative stress,preventing cell apoptosis,and modulating inflammatory markers.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgments
The authors would like to extend heartful gratitude to Prof.(Dr.)Tamizhselvi R (VIT,Vellore) for her expert guidance in framing the manuscript and also VIT management for providing the seed fund(SG2021 0255) to carry out this research.
Funding
This study was supported by Indian Council of Medical Research(ICMR),the Government of India agency research grant (F.N.5/9/1328/2020-Nut).
Data availability statement
The data supporting the findings of this study are available from the corresponding authors upon request.
Authors’contributions
TD contributed to the conception and design,acquisition of data,drafting of the article,and final approval of the manuscript.VM contributed to the analysis of data,critical revision of the manuscript content,and final approval of the manuscript.
 Asian Pacific Journal of Tropical Biomedicine2024年2期
Asian Pacific Journal of Tropical Biomedicine2024年2期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Catalpa bignonioides extract improves exercise performance through regulation of growth and metabolism in skeletal muscles
- Icariin plus curcumol enhances autophagy through the mTOR pathway and promotes cathepsin B-mediated pyroptosis of prostate cancer cells
- Hydrangea serrata extract exerts tumor inhibitory activity against hepatocellular carcinoma HepG2 cells via inducing p27/CDK2-mediated cell cycle arrest and apoptosis
- NUDT5 promotes the growth,metastasis,and Warburg effect of IDH wild-type glioblastoma multiforme cells by upregulating TRIM47
