Oxidation of Benzothiophenes Using Tert-amyl Hydroperoxide*
ZHOU Xinrui(周新锐), GAI Hongtao (盖洪涛), WANG Jing(王静), ZHANG Shanshan (张珊珊), YANG Jinzong(杨锦宗) and ZHANG Shufen(张淑芬)
Oxidation of Benzothiophenes Using-amyl Hydroperoxide*
ZHOU Xinrui(周新锐)**, GAI Hongtao (盖洪涛), WANG Jing(王静), ZHANG Shanshan (张珊珊), YANG Jinzong(杨锦宗) and ZHANG Shufen(张淑芬)
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116012, China
Homogeneous oxidation using an oil-soluble oxidant,-amyl hydroperoxide (TAHP), for ultra-deep desulfurization was performed under mild conditions in the presence of molybdenum oxide catalysts. Dibenzothiophene (DBT), benzothiophene (BT) and 4, 6-dimethyl-dibenzothiophene (DMDBT), which are the refractory sulfur compounds for hydrodesulfurization (HDS), were employed as model substrates for a simulated diesel fuel. Activity of molybdenum oxide supported on a macroporous weak acidic resin was investigated. The mass conversion of DBT reached near 100% at 90°C and a TAHP/DBT molar ratio of 3 with 1% of molybdenum oxide supported onAmberlite IRC-748 resin for 1 h. It was further found that the activities of the model substrates decreased in the order of DMDBT>DBT>BT. In the flow oxidation using TAHP as the oxidant, mass conversion of DBT increased remarkably from 61.3% to 98.5% when dropping the weight hourly space velocity (WHSV) from 40h-1to 10 h-1. A series of experiments dealt with selectivity of this oxidation using TAHP revealed that the model unsaturated compounds,.. 4, 6, 8-trimethyl-2-nonylene, and 2-methylnaphthalene did not affect the oxidation of DBT. Carbazole had nearly no effect on the conversion of DBT using TAHP, but had some influence on the one using-butyl hydroperoxide (TBHP). The mass conversion of DBT decreased remarkably from 75.2% to 3.6% when the content of carbazole increased from 0 to 500 μg·g-1. In the flow oxidation using TAHP as the oxidant, the concentration of DBT in model fuels was reduced from 500 µg·g-1to 7.2 µg·g-1at WHSV of 10 h-1, and then reduced to 3.8 µg·g-1by adsorption of Al2O3.
desulfurization, benzothiophene,-amyl hydroperoxide
1 INTRODUCTION
Sulfur oxides emitted from the combustion of fuel are one of the key precursors of acid rain. With an environmental concern, regulations and legislations of many countries call for reducing the sulfur content of fuel drastically. Conventional hydrodesulfurization (HDS) is facing a major operational and economic challenge to meet these new sticker regulations and legislations [1-3]. There is an urgent necessary to develop new approaches to deep desulfurization of fuels.
Oxidative desulfurization (ODS) has been considered as one of promising alternative method for ultra-deep desulfurization because it can be conducted under very mild conditions [4, 5]. Various studies on the ODS process have been reported, wherein peroxyacids were known as the most positive oxidants [6, 7]. However, they involved water in the reaction system, which results in the biphasic problems of the mass transfer and oil phase recovery. Wang. [8] reported that dibenzothiophene (DBT) could be oxidized to DBT sulfone by an oil-soluble oxidant,-butyl hydroperoxide (TBHP), in the presence of Mo(VI)/Al2O3catalyst. Similarly, Stanger and Angelici [9]published that the oxidation of DBT can be carried outusing TBHP in the presence of silica catalyst, and also Chica. [10] found that the ODS of sulfur compounds using TBHP can be conducted in the presence of different metal-containing catalysts.However, most of these studies were focused on the catalyst researches based on the oxidation of DBT using TBHP; the reaction period is still greatly concerned for developing an industrially feasible process.
Recently, we tried to use the derivative of TBHP,-amyl hydroperoxide (TAHP), as oil-soluble oxidant to perform ODS with a simulated diesel fuel. The results indicate that TAHP presents higher activity than TBHP [11]. On the other hand, the yield of TAHP is much higher than the yield of TBHP in their syntheses [12], because TAHP is more oil-soluble than TBHP and easier to separate from the two-phase system. In addition, TAHP owns a wide industrial supplement from C5by-products; this is a great advantage for it to be applied in large-scale.
In the present study,derivatives of DBT, benzothiophene (BT) and 4, 6-dimethyl-dibenzothiophene (DMDBT), were employed as models of aromatic sulfur compounds. Activity and selectivity of TAHP for ODS were investigated in detail with both batch reaction and flow reaction.
2 EXPERIMENTAL
2.1 Materials
DBT (analytical-grade reagent, AR) and BT (AR) were obtained from Acros. DMDBT (AR) was obtained from Sigma-Aldrich;-hexadecane (chromatographic-grade reagent) was obtained from Merck-Schuchardt. Decalin (AR); cyclohexene (chemical pure,CP), 2-methylnaphthalene,-amyl hydroxide (AR), hydrogen peroxide (mass concentration: 30%, AR), sulfuric acid (AR), ammonium heptamolybdate ((NH4)6Mo7O24·4H2O, AR), carbazole (AR), macroporous cationic exchange resins of weak acid series, Amberlite IRC-748 (A748) and Amberlite IRC-84 (A84), were purchased from Beijing Chemicals Co.;4, 6, 8-trimethyl-2-nonylene (AR)was provided by the Daqing Petrochemical Factory; Al2O3[chromatographic-grade reagent, 75-150 μm (100-200 mesh)] was purchased from ShanghaiLudu Chemical Reagent Factory.
2.2 Instruments
The reaction products were identified andquantified by Infrared Spectroscopy (IR, Nicolet-20DXB), Gas Chromatography-Mass Spectrometry (GC-MS, HP6890GC/5793MS),and Gas Chromatography-FlameIonization Detector (GC-FID, HP6890). The GC was equipped with a HP-5 capillary column (30 m×0.32 mm×0.25µm),using highly purified nitrogen (≥99.9995%, mass concentration) as the carrying gas. The temperature of HP-5 column was set at 140°C, and then increased at the rate of 10°C·min-1to 270°C. The limit of accuracy of the analysis of DBT was 1 μg·g-1, confirmed by the standard reference.
2.3 Synthesis of TAHP and TBHP
TAHP was prepared with-amyl alcoholfollowing the method published by Milas and Surgenor [13] with some improvement [14]. The colorless liquid TAHP was obtained in 68% (by mass) yield. The data supported the observation that TAHP structure agreed well with the Ref. [15].
TBHP was synthesized with-butyl alcohol by the same method as TAHP in 53% (by mass) yield. The data supported the observation that TBHP structure agreed well with the Ref. [12].
2.4 Preparation of the molybdenum oxide catalyst supported on resin
The supported catalysts were prepared with resin A748 or A84 by immersing in aqueous (NH4)6Mo7O24·4H2O solution [16]. The mass contents of molybdenum oxide were found respectively to be 14.3% and 10.3% in comparison with the blank hydrogenised resins.
2.5 Typical procedure
Typical flow procedure: the oxidative desulfurization was conducted in a fixed bed stainless steel flow reactor (i.d. 5 mm, length 20 cm) packed with 5 g of the catalysts Mo(VI)/A748 or Mo(VI)/A84 beads respectively diluted with 10 g of blank hydrogenised A748 or A84 resin, combined with an adsorption column packed with 5 g of Al2O3adsorbent. The reactor was dried by a flow of nitrogen at 80°C for 30 min, and the feed containing 500 μg·g-1of DBT in decalin was introduced, a defined TAHP with a molar ratio of TAHP/DBT of 3 and 500 μg·g-1of-hexadecane as internal standard for GC analysis at 90°C with a WHSV of 10-40 h-1by a syringe pump.
Typical batch procedure:to a 100ml three-neck glass flask with a reflux column, thermometer and magnetic stirrer, added 0.5 g of the catalyst,0.1817g of TAHP and 500μg·g-1DBT decalin solution until 50 g.The mixture was stirred continuously at 90°C for 1 h.

Figure 1 Structure of A84 and A748
The compositions ofthe reaction productswere analyzed byGC-FID. The peak corresponding to model compounds was identified by its retention time compared with references and the sulfur content was examined by the internal standard method, while the peak of corresponding sulfone was identified by GC-MS (HP6890/5973MS).
3 RESULTS AND DISCUSSION
The influence of oxidation conditions on the conversion of DBT was investigated in the previous study; the optimum conditions obtained were at 90°C with an O/S molar ratio of 3 for 1 h [11], and the initial concentration of DBT in decalin was set to 500μg·g-1, which were adopted in this study except special case. It should be mentioned that the O/S symbol is defined here as the molar ratio of TAHP and sulfur compound, and the conversion of model compound is calculated with the GC data, which is the percentage of the initial concentration minus the concentration in product to the initial concentration.
3.1 Activity of catalyst of molybdenum oxide supported on the resin


Figure 2 Effect of the catalyst supporter [90°C and an O/S molar ratio of 3 with 1% (by mass)of catalyst(Mo(VI)/A748, Mo(VI)/A84 or molybdenum oxide unsupported) for 1 h]◇ MoO3/A784;■ MoO3/A84;▲ MoO3
Figure 3 IR spectra IR spectrums of Mo(VI)/A84 (above) and Mo(VI)/A748 (below)

Figure 4 Conversions of DBT using TAHP and TBHP with time [90°C andan O/S molar ratio of 3 with 1% (by mass) Mo(VI)/A748]▲ TAHP;◆ TBHP
3.2 Comparison of reactivity between TAHP and TBHP
Oxidative reactions of DBT using TAHP and TBHP freshly prepared by the same waywere performed with the batch reaction. As shown in the Fig. 4, after 1 h of reaction time, mass conversation of DBT using TAHP reached to near 100%, whereas the one using TBHP merely reached to75.2% (by mass).
These results indicated that activity of TAHP was significantly higher than TBHP. The reason is probably the hyperconjugation of more methyl groups of TBHP, which increases the electron density of peroxide bond and decreases its oxidative activity at the same time (Fig. 5).
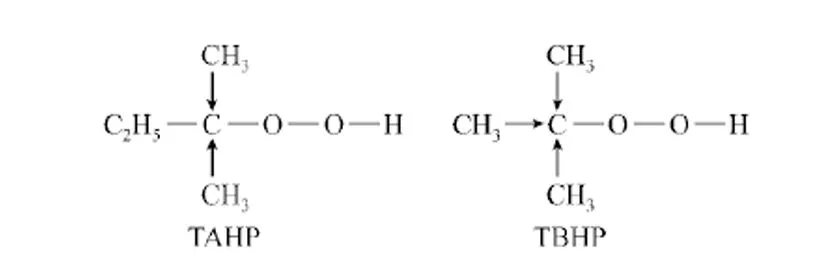
Figure 5 Structures of TAHP and TBHP
3.3 Reactivity of benzothiophene derivatives
Besides DBT, two more aromatic sulfur compounds, BT and DMDBT, were employed as model compounds to investigate their reactivity in the new oxidative system; therein, DMDBT was declaimed as the most refractory sulfur compound for HDS. Oxidation of 500 μg·g-1of each DBT, BT and DMDBT in decalin using TAHP was examined at 90°C and an O/S molar ratio of 3 with 1% (by mass) of Mo(VI)/A748 for 0.5 h. The GC-FID chromatograms show that three model compounds diminish at different degree (Fig. 6). The mass conversions of DMDBT, DBT and BT were 93.5%, 80.2% and 46.7%. The reactivity of these model substrates decreased in the order of DMDBT>DBT>BT, reversing the order in HDS. This indicates that the two methyl groups at 4 and 6 positions of DMDBT as electron-donating groups make the aromatic rings’ electron density increase; therefore, DMDBT is easier to accept the oxidant attacking.

Figure 6 GC-FID Spectrum of BT, DBT and DMDBT before and after oxidation
[90°C and an O/S molar ratio of 3 with 1% (by mass) Mo(VI)/A748 and a 500 μg·g-1of each BT, DBT and DMDBT in decalin for 0.5 h]
3.4 Selectivity of the catalytic oxidation in the presence of other active compounds
Actual fuel contain large quantities of alkenes and aromatics, therefore, there is a need to identify whether these alkenes and aromatics could be oxidized by a side reaction during the present ODS process. 4,6,8-trimethyl-2-nonylene and 2-methylnaphthalene were selected as the model compounds of the branched internal alkenesand two-ringaromatics respectively. The oxidation of these model compounds and DBT in decalin was examined in the TAHP/Mo(VI)/A748 system. The results analyzed by GC-FID demonstratedthat no concentrationchanges of these model compounds werefound in comparison with the internal standard, and no new peaks were observed in the GC spectrum.Moreover, the activities of the short and non-branched alkenes (cyclohexene) were also examined for achieving the basic information about the TAHP activity and selectivity; even cyclohexene was a very improbable component ofa middle and heavy distillate. The result of the oxidation of cyclohexene in the TAHP/Mo(VI) system at 90°C was positive according to the several new peaks appeared in the higher retention time of GC spectrum. The mass conversion of cyclohexene reached to 14.5% after oxidation (Table 1) and the conversion of DBT decreased compared with no model unsaturated compounds.It indicates that the DBT oxidation could be retardedby the short and non-branched alkenes as claimed in the Ref. [17], but not influenced by the branched alkenes ortwo-ring aromatics under the present process because their nucleophilic attacks to the TAHP/Mo(VI) complex are resisted by the steric hindrance.

Table 1 Oxidation of the model unsaturated hydrocarbons
Note: 90°C andan O/S molar ratio of 3 with 1% (by mass) Mo(VI)/A748.15% of the initial mass concentration of the model compounds (each 5%) and 1% of the initial mass concentration of DBT in decalin.
Figure 7 depicts that by using TBHP as the oxidant, the mass conversion of DBT decreases from 75.2% to 3.6% as the content of carbazole increases from 0 μg·g-1to 500 μg·g-1, whereas by using TAHP as the oxidant, the mass conversion of DBT decreases from 100% to 98.6%, just slightly affected by carbazole.

Figure 7 Effect of carbazole [90°C and an O/S molar ratio of 3 with 1% (by mass) Mo(VI)/A748 for 1 h]□ TAHP;■ TBHP
3.5 Reuse of Mo(VI)/A748 during the oxidation
Oxidation of DBT was performed in 10 consecutive batch reactions at 90°C and an O/S molar ratio of 3 with1% (by mass) of Mo(VI)/A748 for 1 h in decalin, where the catalyst was reused after simply alcohol washing and vacuum drying. The results showed that the catalysts’ activities did not decrease (Table 2), but the mass of catalysts seemed slightlyreduced probably due to the losswith moving and leaching.

Figure 8 Mechanism of DBT oxidation
3.6 Mechanism of the oxidation using TAHP
The oxidation of DBT is considered to be a consecutive nucleophilic addition,during which DBT first reacts with a hydroperoxide molecule to produce DBT sulfoxide 1, and then the DBTsulfoxide further reacts with another hydroperoxide molecule toform DBT sulfone 2(Fig.8). Sulfoxide is detected first in the samplesat the very early beginning of oxidation using TAHP by TLC and GC-FID. Afterwards, the peak of DBT sulfoxide disappears with reaction time. This phenomenon is in agreementwith Gilman’s early observation that the rate of oxidation to the sulfoxide with acetyl peroxide is several hundred times greater than the subsequent oxidation of the sulfoxide to the sulfone [18], because of the lower nucleophilicity and the more steric inhibition of the sulfur atom in the sulfoxide than in the DBT molecule. The coordination of hydroperoxide to Mo-O bond is prompted by molybdenum oxidedispersed on macroporous polyacrylic cationic exchange resin because of increase in the electrophilicity of peroxy oxygen. Moreover, according to the activities of TAHP, TBHP and cyclohexanone peroxide (CYHPO) in the previous study [19], as well as the detailed comparison of TAHP and TBHP in this study, it is found that the structure of the R-group has a strong effect on the activity of the hydroperoxide. The reason can be attributed to the difference in electron density of the peroxy bond, and that alower value of the electron density of peroxy bondleads to higher activity of oxidant.According to donation of the methyl group, the order of peroxy bond electron density decreases in the order of TBHP>TAHP>CYHPO, which is just reversing the order of the activity of alkyl oil-soluble hydroperoxide: CYHPO>TAHP>TBHP.
3.7 Catalytic oxidation of DBT with a flow process
The oxidative desulfurizations of DBT were examined under various weight hourly space velocity (WHSV) in the fixed bed stainless steel flow reactor and the adsorption column described above. The results show that the conversion of DBT increases remarkably with the WHSV decreasing; at 10 h-1of WHSV the mass conversion of DBT reached to 98.5% (Fig. 9). The product at WHSV of 10 h-1was further treated by the adsorption with Al2O3, which is an effective adsorbent for the removal of sulfone [20, 21]. The data from oxidation- adsorption were summarized inTable 3. The concentration of DBT in model fuel was reduced from 500 µg·g-1to 7.2 µg·g-1, and then reduced to 3.8 µg·g-1by adsorption. DBT sulfone (DBTO2) was removed almost completely by adsorption. The lowest sulfur content (DBT and DBTO2) in the model fuel treated was less than 4.8 µg·g-1after a complete oxidation- adsorption process.

Figure 9 Desulfurization of DBT under various WHSV
[90°C and an O/S molar ratio of 3 with 1% (by mass) Mo(VI)/A748]

Table 2 Results of reuse of Mo(VI)/A748 catalyst

Table 3 Sulfur compound concentrationduring oxidation/adsorption (µg·g-1)
Note: 90°C, 10 h-1of WHSV and an O/S molar ratio of 3 with 1% (by mass) Mo(VI)/A748.
4 CONCLUSIONS
Catalytic oxidation of DBT using a novel oil-soluble oxidant, TAHP, was further investigated for ultra-deep desulfurization. The results show that the weak acidic resin can significantly increase the catalytic activity of Mo(VI) oxide. The mass conversion of DBT reachesnear 100% with 1% (by mass) of Mo (VI)/A748 at 90°C and an O/S molar ratio of 3 for 1 h. TAHP shows higher activity than TBHP. Adding the active compounds in the oxidation of DBT, the conversion of DBT using TAHP has less influence than using TBHP. The activities of the model substrates decrease in the order of DMDBT>DBT>BT. In the flow oxidation using TAHP, the concentration of DBT in model fuel was reduced from 500 µg·g-1to 7.2 µg·g-1at WHSV of 10 h-1, and then reduced to 3.8 µg·g-1by adsorption of Al2O3,and the lowest sulfur content (DBT and DBTO2) in the model fuel treated was less than 4.8 µg·g-1.
1 Song, C.S., “An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel”,, 86, 211-263 (2003).
2 Ma, X.L., Zhou, A.N., Song, C.S., “A novel method for oxidative desulfurization of liquid hydrocarbon fuels based on catalytic oxidation using molecular oxygen coupled with selective adsorption”,, 123, 276-284 (2007).
3 Li, J.Y.,Zhou,X.R.,Zhao,D.F.,“Benzothiophene and its derivatives”,, 68(13), w055(2005).
4 Chen, L.J., Guo, S.H., Zhao, D.S., “Oxidative desulfurization of simulated gasoline over metal oxide-loaded molecular sieve”,...., 15 (4), 520-523 (2007).
5 Kong, L.Y., Li, G., Wang, X.S., “Mild oxidation of thiophene over TS–1/H2O2”,, 93–95, 341–345 (2004).
6 Chen, L.J., Guo, S.H., Zhao, D.S., “Oxidation of thiophenes over silica gel in hydrogen peroxide/formic acid system”,...., 14 (6), 835-838 (2006).
7 Znnikos, F.,Lois, E., Stournas, S., “Desulfurization of petroleum fractions by oxidation and solvent extraction”,., 42(1), 35-45(1995).
8 Wang, D.,Qian, E.W., Amano, H., Okata, K.,Ishihara, A.,Kabe, T., “Oxidative desulfurization of fuel oil (I) Oxidation of dibenzothiophenes using-butyl hydroperoxide”,..,253(1), 91-99(2003).
9 Stanger, K.J.,Angelici, R.J., “Silica-catalyzed-butyl hydroperoxide oxidation of dibenzothiophene and its 4,6-dimethyl derivative: A route tolow-sulfur petroleum feedstocks”,,20(5), 1757-1760(2006).
10 Chica,A.,Corma, A.,Dómine, M.E.,“Catalytic oxidative desulfurization (ODS) of diesel fuel on a continuous fixed-bed reactor”,.,242(2), 299-308(2006).
11 Zhou, X.R.,Li,J.Y., Zhao, D.F.,Zhao, C.X., “Oxidative desulfurization of dibenzothiophene using-amyl hydroperoxide”,..., 34(4), 506-508(2006).
12 Milas, N.A., Surgenor, D.M., “Studies in organic peroxides (VIII)-butyl hydroperoxide and di--butyl peroxide”,...., 68(2), 205-208 (1946).
13 Milas, N.A., Surgenor, D.M., “Studies in organic peroxides (X)-amyl hydroperoxide and di--amyl peroxide”,...., 68(4), 643-644(1946).
14 Zhou,X.R., Li,J.Y., Zhao,D.F.,Wang,L., “Improvement in synthetic method of-amyl hydroperoxide”,,28(2), 109-112(2006).
15 Olah,G.A., Parker, D.G.,Yoneda, N., Pelizza,F., “Oxyfunctionalization of hydrocarbons (1) Protolytic cleavage-rearrangement reactions of tertiary alkyl hydroperoxides with magic acid”,....,98(8), 2245-2250(1976).
16 Kotov,S.V., Boneva,S., Kolev, T., “Some molybdenum-containing chelating ion-exchange resins/polyampholites as catalysts for the epoxidation of alkenes by organic hydroperoxides”,, 154(1), 121-129 (2000).
17 Mitchell, M.J., Finney, S.N., “New molybdenum catalysts for alkyl olefin epoxidation: Their implications for the mechanism of oxygen atom transfer”,...., 123,862-869 (2001).
18 Gilman,H.,Esmay,D.L., “The oxidation of dibenzothiophene and phenoxathiin with hydrogen peroxide”,...., 74(8), 2021-2024(1952).
19 Zhou,X.R.,Zhao, C.X., Yang,J.Z., Zhang,S.F., “Catalytic oxidation of dibenzothiophene using cyclohexanone peroxide”,,21(1), 7-10(2007).
20 Larrubia, M.A., Ramirez, J., Busca, G.A., “An FT-IR study of the adsorption of indole, carbazole,benzothiophene,dibenzothiophene and 4,6-dibenzothiophene over solid adsorbents and catalysts”,..,224(1), 167-168 (2002).
21 Zhang, Y.W., Shen, J., Yang, L.N., “Research on catalytic oxidation and adsorption desulfurization over HPWA/SiO2-Al2O3”,,34 (2), 97-100 (2005).
2008-05-07,
2008-11-11.
Program for Changjiang Scholars and Innovative Research Team in University.
** To whom correspondence should be addressed. E-mail: xinrui@dlut.edu.cn
——别克WILDCAT EV CONCEPT
 Chinese Journal of Chemical Engineering2009年2期
Chinese Journal of Chemical Engineering2009年2期
- Chinese Journal of Chemical Engineering的其它文章
- Design and Performance Analysis of Micro Proton Exchange Membrane Fuel Cells*
- Isolation of Cordyceps ophioglossoides L2 from Fruit Body and Optimization of Fermentation Conditions for Its Mycelial Growth*
- Efficient and Comprehensive Utilization of Hemicellulose in the Corn Stover*
- Simulating Surface Aeration Systems at Different Scale of Mixing Time*
- Kinetics of Reaction-Crystallization of Struvite in the Continuous Draft Tube Magma Type Crystallizers—Influence of Different Internal Hydrodynamics
- Preparation and Characterization of Tungsten-substituted Molybdophosphoric Acids and Catalytic Cyclodehydration of 1,4-Butanediol to Tetrahydrofuran*
