Methanol Synthesis from CO2 Hydrogenation with a Cu/Zn/Al/Zr Fibrous Catalyst*
AN Xin (安欣), ZUO Yizan (左宜赞), ZHANG Qiang (张强) and WANG Jinfu (王金福)**
Methanol Synthesis from CO2Hydrogenation with a Cu/Zn/Al/Zr Fibrous Catalyst*
AN Xin (安欣), ZUO Yizan (左宜赞), ZHANG Qiang (张强) and WANG Jinfu (王金福)**
Beijing Key Laboratory of Green Chemical Reaction Engineering and Technology, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
A highly active Cu/Zn/Al/Zr fibrous catalyst was developed for methanol synthesis from CO2hydrogenation. Various factors that affect the activity of the catalyst, including the reaction temperature, pressure and space velocity, were investigated. The kinetic parameters in Graaf’s kinetic model for methanol synthesis were obtained. A quasi-stable economical process for CO2hydrogenation through CO circulation was simulated and higher methanol yield was obtained.
kinetics, CO2hydrogenation, methanol, Cu/Zn/Al/Zr catalyst
1 Introduction
The greenhouse effect is a threat to the living environment of mankind. The transformation of CO2into useful chemicals,.. methanol, is an attractive way to protect the global environment since CO2is an important greenhouse gas and methanol itself is a useful raw chemical and solvent [1, 2]. For methanol synthesis, Cu/Zn based catalysts were always used [3-16]. Various catalysts, including Cu/Zn/Al, Cu/Zn/Cr, Cu/Zn/Zr, Cu/Zn/Ga, Cu/Zn/Ge. [7-11, 14, 15], were developed for methanol synthesis. It is believed that Cu adsorbs CO2and Zn adsorbs H2, and the reaction takes place on the surface of the catalyst. Thus, a catalyst with good Cu/Zn dispersion is a key factor for high yield and selectivity for methanol synthesis. Among many catalysts, the Cu/Zn/Al catalyst has been commonly used in various studies, and a lot of modified Cu/Zn/Al based catalysts were reported recently [8, 11, 15, 17]. Using the phase separation effect of nanoparticles on a catalyst surface, a fibrous Cu/Zn/Al/Zr catalyst that is active for methanol production from CO2hydrogenation was prepared by our group recently [16]. A 5% Zr addition led to a methanol space time yield 80% higher than that on the commercial catalyst that is the present catalyst of choice in China.
However, for CO2hydrogenation, there is a competing process between the reverse water shift reaction and methanol synthesis reaction. The reverse water shift reaction reaches thermodynamic equilibrium fast, while the methanol synthesis reaction is much slower. Although the catalyst showed a high activity and high methanol yield, CO, which was the product of the reverse water shift reaction, was present in the final product. From the viewpoint of the utility of the carbon source, no CO should be produced during methanol synthesis process because CO can be recycled into the reactor for methanol synthesis. To understand the concept further, the first step is to understand the catalytic behavior and develop the kinetic model. There have been some kinetic studies of methanol synthesis from CO2hydrogenation. The catalysts were Cu/Zn/Al, Pt-Ca/C, or Cu/Zr based, which showed lower activity than the novel fibrous Cu/Zn/Al/Zr catalyst [3-6, 13, 17]. Kinetics data for the Cu/Zn/Al/Zr catalyst on CO2hydrogenation to methanol had not been reported, and the kinetic model was developed in this work as a basis for us to understand the catalytic behavior and design a process of CO2hydrogenation.
In this work, the fibrous Cu/Zn/Al/Zr catalyst was used in CO2hydrogenation to methanol at various temperature, pressure and space velocity. Base on the regression parameters fitted to the assumed Graaf’s kinetic model, a CO2hydrogenation process that CO cycle in the system was simulated, the space time yield of methanol increased, which was helpful for scaling up this process in future.
2 Experimental
The fibrous Cu/Zn/Al/Zr catalyst was prepared by a novel co-precipitation procedure reported recently [17]. Typically, the solution of Cu(NO3)2· 6H2O, Zn(NO3)2· 6H2O, Al(NO3)3· 6H2O and ZrOCl2with a concentration of 0.6 mol·L-1were mixed with a ratio of 6︰3︰0.5︰0.5, then the mixture was precipitated with the solution Na2CO3at 353 K with strong stirring for 1 h. After filtration and washing, the catalyst was dried at 120°C for 12 h and calcined at 350°C for 4 h to give the Cu/Zn/Al/Zr catalyst. The prepared catalyst was ground into powder and mixed with SiO2, and load into the fixed bed reactor.
The catalytic reaction on the Cu/Zn/Al/Zr catalyst was carried out in a fixed bed reactor. The reactor is with a diameter of 12 mm and a length of 500 mm. The catalyst is packed at the middle section (about 100 mm) of the reactor, where the temperature was uniform. All of the gas, including the hydrogen, nitrogen, and carbon dioxide, is of purity above 99.999%. Thus, other impurity in industrial, such as CO, H2S, COS, was neglected. Before reaction, the catalyst was reduced with a 5% H2/95% N2mixture at atmospheric pressure by raising the temperature slowly to the reaction temperature over 10 h. Then the reduction gas was switched to the reaction gas and the pressure was raised to the reaction pressure to start the reaction. The first sample of the effluent was taken 2 h after steady reaction conditions were established, and then samples were taken every 30 min for online analysis of the effluent composition. The reaction temperature was controlled by furnace heating and the pressure was controlled by the feeding rate.
The structure of the catalysts was studied by using Transmission Electron Microscope (TEM) and N2adsorption method. The morphology of the Cu/Zn/Al/Zr catalyst was characterized by a JEM 2010 high resolution scanning electron microscope (SEM) operated at 120.0 kV. The BET surface area was obtained with a high resolution BET equipment described in Li[18].
The reaction equipment is connected on-line to a GC 7890II gas chromatograph provided with a thermal conductivity detector (TCD), a Porapak T (5 m) column parallel connected with a TDX-01 (3 m) column. All detector and columns are placed in the oven and operate within the 0-150°C range. A Porapak T column was used for separation of MeOH and H2O, and a TDX-01 column was used for separation of H2, CO, and CO2. CO2conversion, MeOH yield and CO yield are defined as follows:
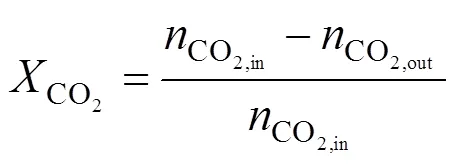

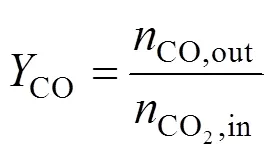
Space time yield of MeOH,MeOH, which describes the amount of MeOH, CO production on per gram catalyst per second, is defined and calculated using the following equation:

(kg·g-1)/22.4 (L·mol-1) (4)
3 Methanol synthesis from CO2 hydrogenation
A fibrous Cu/Zn/Al/Zr catalyst (12︰6︰1︰1) was used to catalyze methanol synthesis in the fixed bed. As shown in Fig. 1, the Cu/Zn/Al/Zr catalyst existed as regular one-dimensional nanomaterial agglomerates. In the fibrous Cu/Zn/Al/Zr catalyst, the effect of Zr incorporation on the metallic function used for methanol synthesis were two aspects, the first is the phase separation effect [16, 19], which was attributed from fibrous agglomerate morphology and the slow rate for Cu/Zn sintering, The second factor for the high activity of a Cu/Zn/Al/Zr catalyst is related to the effect of ion doping and valence compensation. Zr4+dissolved in the ZnO crystal causes the formation of positive ion defects on the surface of Cu-ZnO. These defects can adsorb Cu+and form and stabilize more active sites, Cu0-Cu+-O-Zn2+, on the catalyst surface[16]. Thus, a high performance of methanol catalyst that with a BET surface area of 70.9 m2·g-1was obtained. This was used in the CO2hydrogenation.

Figure 1 TEM images of the fibrous catalyst
If the space velocity was above 900 ml·(g cat)-1·h-1in the present reactor, the external diffusion was not shown. Furthermore, the size of the catalyst particles should be less than 0.35 mm to avoid internal diffusion, which similar to previous reports [20-23]. In the present studies, the space velocities in the kinetic experiments were all above 1000 ml·(g cat)-1·h-1, and the particle size of the Cu/Zn/Al/Zr catalyst was less than 0.05 mm. The thermodynamics of methanol synthesis reactions were also studied similar to the previous reports [24]. The equilibrium constants at different temperatures were calculated using van Hoff equation as below:
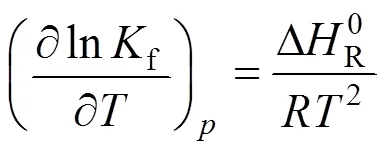
These were used to calculate the equilibrium conversions and yields for given initial conditions. In the following parts, various factors, including reaction temperature, pressure, and space velocity have a large effect on methanol synthesis.
3.1 The effect of reaction temperature
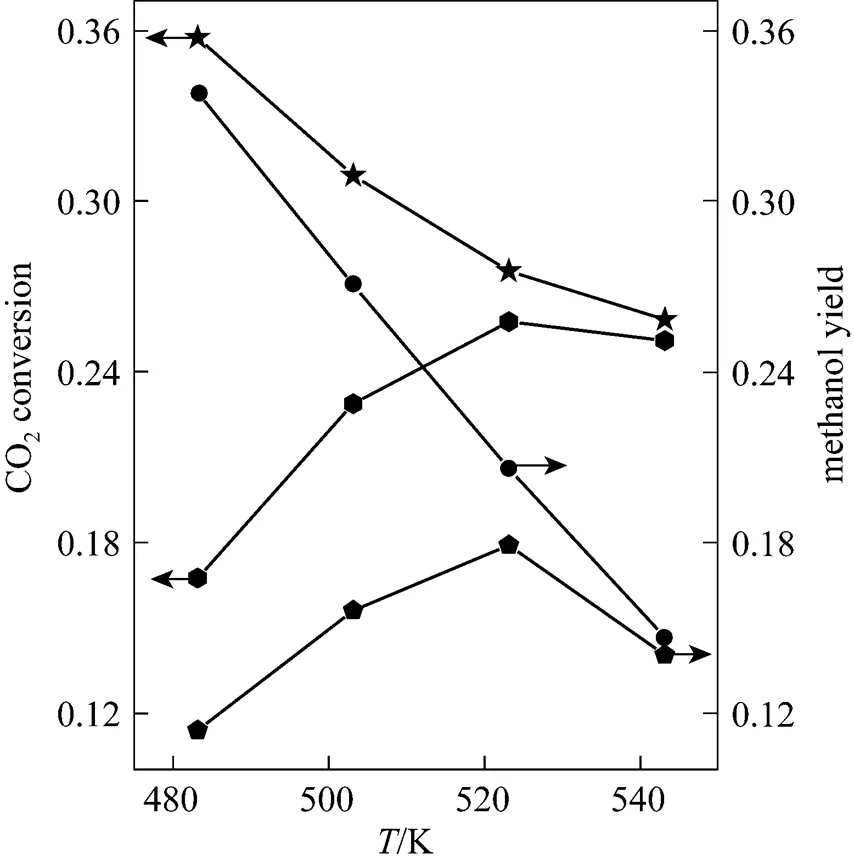
CO2hydrogenation takes place at temperatures from 483 to 543 K. The relationship between reaction temperature and CO2conversion or methanol yield is shown in Fig. 2. Methanol synthesis and the reverse water shift reaction occurred in the reactor. When the reaction temperature was higher, the reaction rate increased, and more CO2was converted into methanol when the system is far from thermodynamic equilibrium. At 523 K, CO2conversion can reach 0.258. However, the CO2conversion was decreased to 0.251 when the temperature was 543 K because of thermodynamic equilibrium. With the increase of temperature, the CO2conversion reached to thermodynamic gradually. Moreover, methanol yield, which is the lower line in Fig. 2, showed trends similar to CO2conversion. It was noticed that only a fraction of CO2was converted to methanol, while the rest was converted to CO. Methanol yield increased from 11.4% at 483 K to 17.9% at 523 K, which is 36% higher than the yield at 483 K. However, although methanol synthesis is an exothermic process and the temperature increasing further is not favorable, but because the reverse water shift reaction is endothermic, which resulted in that the methanol yield was decreased. We can also see that methanol synthesis was more sensitive than the reverse water shift reaction with respect to the reaction temperature. The fibrous catalyst has its highest methanol yield at 523 K at a space velocity of 6000 ml·(g cat)-1·h-1and a pressure of 5 MPa.


3.2 The effect of pressure
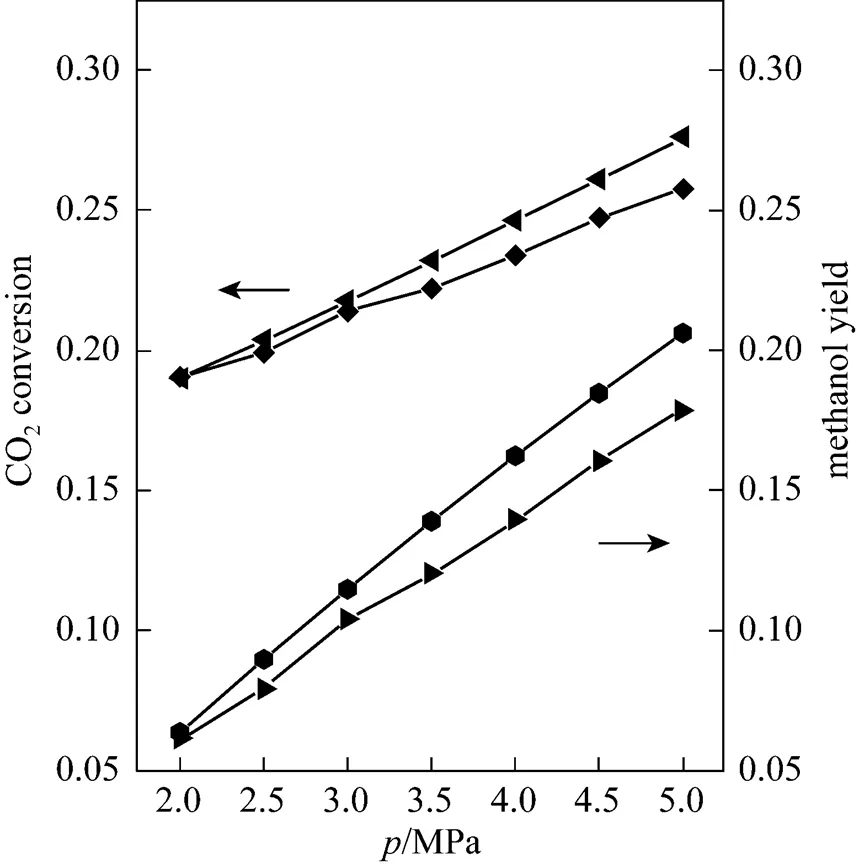
From the thermodynamics, a high pressure is beneficial for methanol production from CO2. On the fibrous Cu/Zn/Al/Zr catalyst, the relationship between the pressure and CO2conversion and methanol yield is shown in Fig. 3. At 2.0 MPa, CO2conversion was just 0.190. When the pressure was increased to 5.0 MPa, CO2conversion was increased to 0.258. When the pressure increased, the difference between the experimental results and thermodynamic equilibrium was also increased. Furthermore, the yield of methanol also showed similar trends: methanol yield was 0.061 and 0.179 at pressures of 2.0 and 5.0 MPa, respectively. It was also observed that the methanol yield increased faster than the CO2conversion which meant that methanol synthesis was more sensitive than the reverse water shift reaction with respect to the reaction pressure. This is in agreement with many other reports. A high pressure was also effective for the Cu/Zn/Al/Zr catalyst in our case. Meanwhile, the flow rate through the reactor was also increased. Too high a reaction pressure has a much higher requirement for the material of the facility and also poses a safety problem.
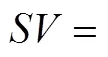

3.3 The effect of the space velocity
The space velocity, which is a parameter that reflects the reactor efficiency, was also tested with a H2/CO2ratio of 3 and a pressure of 5.0 MPa. Space velocities from 1000 to 10000 ml·(g cat)-1·h-1were used to test the catalytic behavior. At the space velocity of 1000 ml·(g cat)-1·h-1, CO2conversion was about 0.262. When it was increased to 10000 ml·(g cat)-1·h-1, the CO2conversion was decreased to 0.232. A decreasing trend was shown in CO2conversion and methanol yield. But it was noticed that the CO2conversion decrease was 11.4%, while the methanol yield was decreased 31.0% from a space velocity of 1000 ml·(g cat)-1·h-1to 10000 ml·(g cat)-1·h-1. With a higher space velocity, more reactant was introduced into the reactor and the residence time was shorter, and CO2conversion decreased. Here, the reverse water shift reaction was much faster than the methanol synthesisreaction, and the higher space velocity had less effect on the reverse water shift reaction than methanol synthesis, which caused methanol yield to decrease more.
4 Kinetics for methanol synthesis from CO2 hydrogenation
4.1 Kinetic modeling
Various kinetic models have been proposed for this process. Natta derived a model based on the ZnO/Cr2O3catalyst of the high pressure process, which has now been almost completely abandoned owing to the present favor of the low pressure process [25]. Bakemeier. noted an important discrepancy between their experimental observations on ZnO/Cr2O3and Natta’s kinetics, particularly in the case of CO2rich feeds [26]. For this reason, a CO2dependency was introduced into the equation using a Langmuir type isotherm. Leonov. [27] were the first to model methanol synthesis kinetics over a Cu/ZnO/Al2O3catalyst. Their model again assumed CO to be the source of carbon in methanol and did not account for the influence of CO2in the feed. Klier. no longer considered CO to be the only carbon source, but still considered it the most important source of carbon in methanol [28]. Villa. realized that a thorough modeling of the methanol synthesis system should also include a description of the water gas shift reaction [29]. Graaf. considered both the hydrogenation of CO and CO2and the water gas shift reaction [30, 31]. Inspired by the work of Herman. [32], Graaf. proposed a dual site mechanism, with CO and CO2adsorbing on an s1 type site and H2and water adsorbing on a site s2. The formation of methanol from CO and CO2occurs through successive hydrogenations, while the water gas shift reaction proceeds along a formate route. Assuming adsorption and desorption to be in equilibrium and taking every elementary step in each of the three overall reactions in turn as rate determining, Graaf. examined 48 possible models and used statistical discrimination to get the final set of kinetic equations, which are used below.
In our case, the fibrous Cu/Zn/Al/Zr catalyst showed high activity for producing methanol from CO2hydrogenation. Thus, there are three independent reactions present in methanol synthesis from CO2, namely,Methanol synthesis from CO:
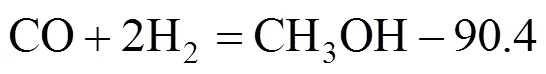
Reverse water gas shift:

Methanol synthesis from CO2:
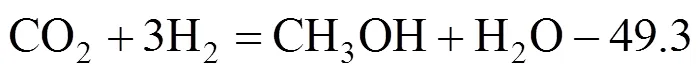
Since the time for reaction (7) to reach thermodynamic equilibrium is very short, it is valid to believe that even for CO2hydrogenation, the CO can be converted into CO2and adsorb on the Cu site, so the kinetics is the same for CO hydrogenation and CO2hydrogenation. Among the above models, Graaf’s model [30, 31] gave a better fit and was chosen in our case. The kinetic rate equations for (6)-(8) are as follows:
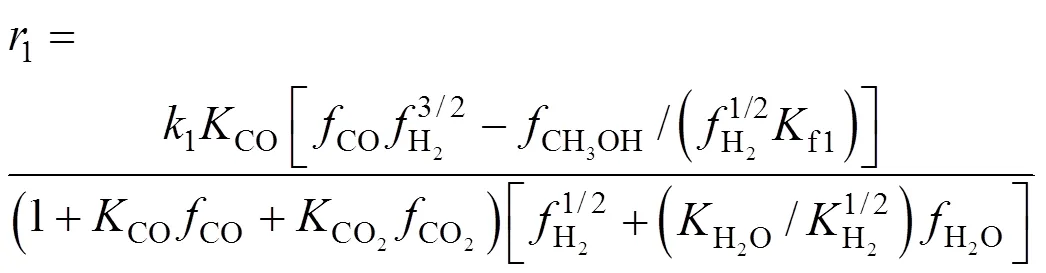
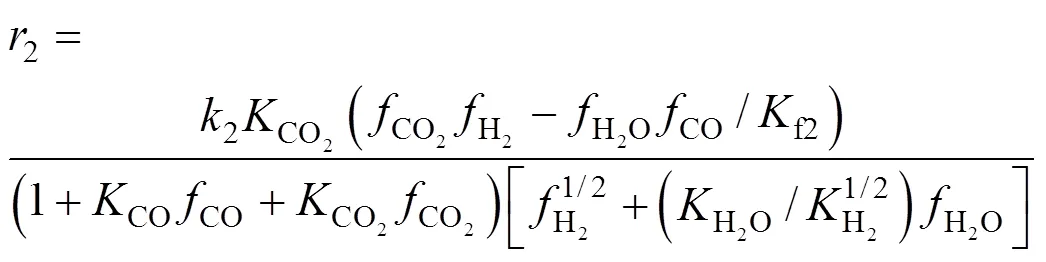

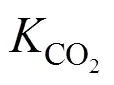
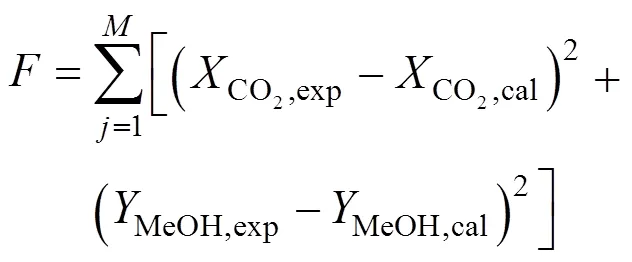
whereandwas obtained by integration of the reaction rate. The initial value of each parameter was estimated and the simplex method was used as the algorithms to realize the optimization. It was converged when the residual was less than 10-9. The best fit kinetic parameters are listed in Table 1. Now a model for methanol synthesis on Cu/Zn/Al/Zr catalyst was obtained and it can be used predicted the CO2conversions and methanol yields. The prediction value can be compared with the experimental results as shown in Fig. 5. The fit is good, with an average deviation of about 5%, and can be predicted the methanol synthesis as follows.

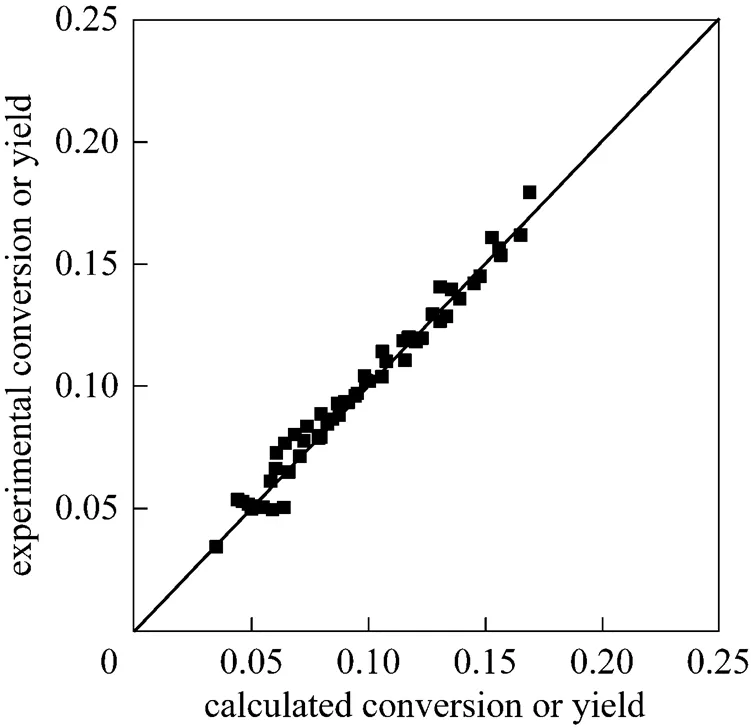
Figure 5 Comparison of model and experimental results
Table1 Regression parameters of the kinetic model

ParameterAiBi or KCO8.3965×10-111.1827×105 1.7214×10-108.1287×104 4.3676×10-121.1508×105 k14.0638×10-6 k29.0421×108 k31.5188×10-33

4.2 Simulation of the CO cycle process with the empirical kinetic model
Once the Cu/Zn/Al/Zr catalytic behavior has been quantified, a process, which contains the methanol synthesis in a fixed bed reactor can be simulated with the above kinetic model. When CO was added into the feeding gas, the reactant ratio was changed, and then the reaction rate also changed. This can be predicted using the Graaf’s model with the regression parameters. Take an example, 3% CO2was replaced by CO in the feed gas to the reactor, the calculation result was shown in Fig. 6. It can be seen that both the methanol yield increased. With temperature higher, the increase is more obviously. At 533 K, then the yield of methanol was increase from 0.145 to 0.182 (Fig. 6). With CO addition in the reactant, higher CO2and H2concentration can be obtained, then more CO2and H2molecular will absorb on the catalyst surface and yield of the methanol increased.
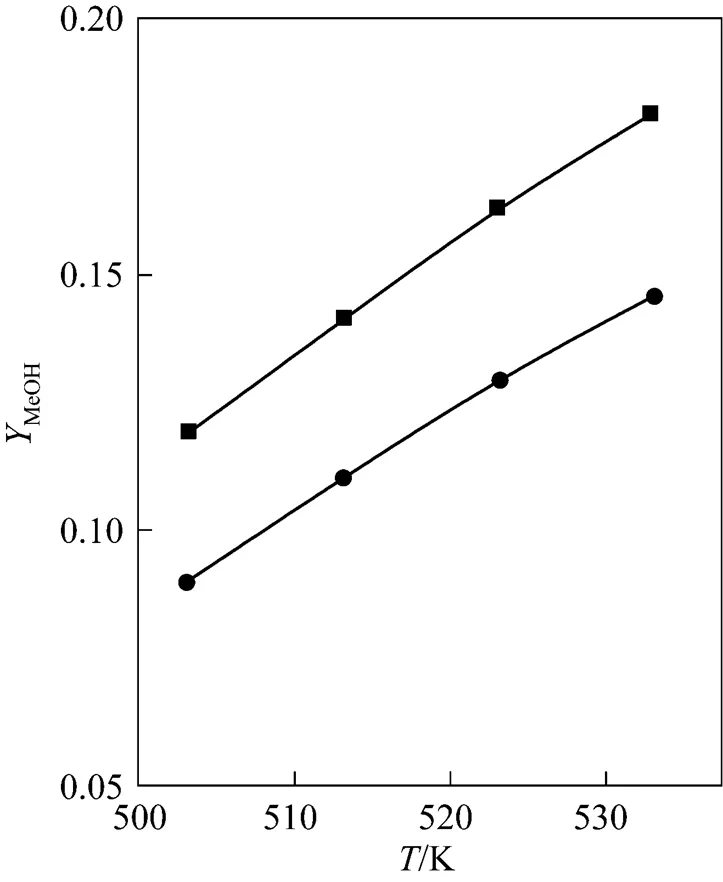
Figure 6 Effect of CO in the methanol synthesis process

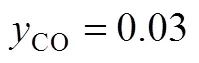
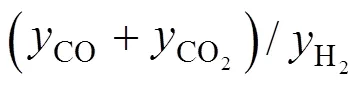
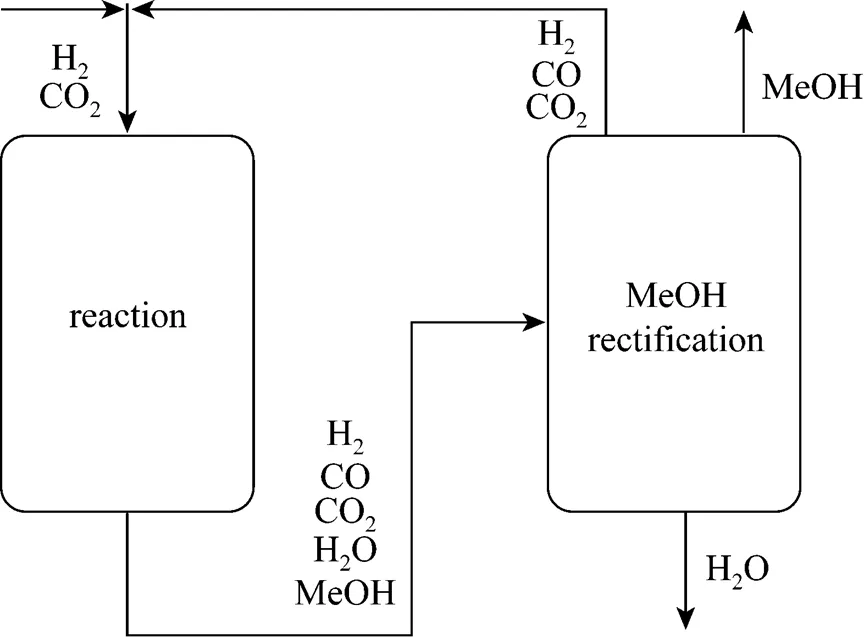
Figure 7 Proposed CO2hydrogenation process with CO recycled in the system
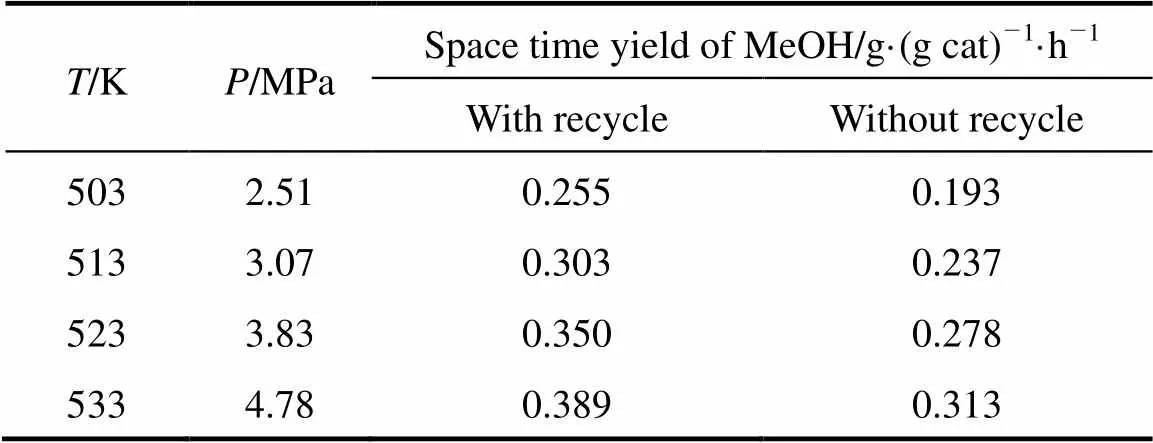
Table 2 Methanol yield from CO2 hydrogenation with/without CO recycle and pressures for a stable process with CO recycle at various temperatures
5 Conclusions
A fibrous Cu/Zn/Al/Zr catalyst was used to catalyze CO2hydrogenation. High CO2conversion and methanol yield were obtained at the optimum reaction temperature, high pressure and low space velocity. The kinetics parameters used were obtained by regression from experimental data. Simulation results showed good agreement with the experimental results. A process for CO2hydrogenation with CO recycle is proposed. Base on the simulation, the space time yield of methanol increased and there is no CO in the product stream, which shows good potential application for industry.
NOMENCLATURE
ffugacity of component, Pa
fiequilibrium constant for reaction
Kequilibrium constant for adsorption of component
molecular weight, g·mol-1
pressure, MPa
reaction speed, mol·(g cat)-1·s-1
space time yield, g·(g cat)-1·h-1
temperature, K
space time velocity, ml·(g cat)-1·h-1
conversion
yield
1 Jean-Paul, L., “Methanol synthesis: a short review of technology improvements”,., 64, 3-8 (2001).
2 Hironori, A., “Research and development on new synthetic routes for basic chemicals by catalytic hydrogenation of CO2studies”,.., 114, 19-30 (1998).
3 Roman-Martinez, M.C., Cazorla-Amoros, D., Linares-Solano, A., Salinas Martinez, C., “CO2hydrogenation under pressure on catalysts Pt-Ca/C”,.., 134, 159-167 (1996).
4 Takeshi, K., Itaru, H., Hirotaka, M., Kozo, M., Kenji, U., Taiki, W., Masahiro, S., “Kinetic study of methanol synthesis from carbon dioxide and hydrogen”,..., 15, 121-126 (2001).
5 Kenji, U., Kozo, M., Takeshi, K., Taiki, W., Masahiro, S., “Methanol synthesis from CO2and H2in a bench-scale test plant”,..., 14, 819-825 (2000).
6 Ortelli, E.E., Wambaca, J., Wokaun A., “Methanol synthesis reactions over a CuZr based catalyst investigated using periodic variations of reactant concentrations”,.., 216, 227-241 (2001).
7 Melian-Cabrera, I., Granados, M.L., Terreros, P., Fierro, J.L.G., “CO2hydrogenation over Pd-modified methanol synthesis catalysts”,., 45, 251-256 (1998).
8 Melian-Cabrera, I., Granados, M.L., Fierro, J.L.G., “Effect of Pd on Cu-Zn catalysts for the hydrogenation of CO2to methanol: stabilization of Cu metal against CO2oxidation”,.., 79, 165-170 (2002).
9 Melian-Cabrera, I., Granados, M.L., Fierro, J.L.G., “Reverse topotactic transformation of a Cu-Zn-Al catalyst during wet Pd impregnation: Relevance for the performance in methanol synthesis from CO2/H2mixtures”,.., 210, 273-284 (2002).
10 Melian-Cabrera, I., Granados, M.L., Fierro, J.L.G., “Structural reversibility of a ternary CuO-ZnO-Al2O3ex hydrotalcite-containing material during wet Pd impregnation”,.., 84, 153-161 (2002).
11 Cao, Y., Chen, L.F., Dai, W.L., Fan, K.N., Wu, D., Sun, Y.H., “Preparation of high performance Cu/ZnO/Al2O3catalyst for methanol synthesis from CO2hydrogenation by coprecipitation-reduction”,...., 24, 1296-1298 (2003).
12 An, X., Ren, F., Li, J.L., Wang, J.F., “A highly active Cu/ZnO/Al2O3nanofiber catalyst for methanol synthesis through CO2and CO hydrogenation”,..., 26, 729-730 (2005).
13 Yang, R.Q., Zhang, Y., Noritatsu, T., “Spectroscopic and kinetic analysis of a new low-temperature methanol synthesis reaction”,.., 106, 153-159 (2006).
14 Schuyten, S., Wolf, E.E., “Selective combinatorial studies on Ce and Zr promoted Cu/Zn/Pd catalysts for hydrogen productionmethanol oxidative reforming”,.., 106, 7-14 (2006).
15 Zhang, X.R., Wang, L.C., Yao, C.Z., Cao, Y., Dai, W.L., He, H.Y., Fan, K.N., “A highly efficient Cu/ZnO/Al2O3catalystgel-coprecipitation of oxalate precursors for low-temperature steam reforming of methanol”,.., 102, 183-190 (2005).
16 An, X., Li, J.L., Zuo, Y.Z., Zhang, Q., Wang, D.Z., Wang, J.F., “A Cu/Zn/Al/Zr fibrous catalyst that is an improved CO2hydrogenation to methanol catalyst”,.., 118, 264-269 (2007).
17 Sahibzada, M., Metcalfe, I.S., Chadwick, D., “Methanol synthesis from CO/CO2/H2over Cu/ZnO/Al2O3at differential and finite conversions”,.., 174, 111-118 (1998).
18 Li, F.X., Wang, Y., Wang, D.Z., Wei, F., “Characterization of single-wall carbon nanotubes by N2adsorption”,, 42, 2375-2383 (2004).
19 Zhang, Q., Qian, W.Z., Wen, Q., Liu, Y., Wang, D.Z., Wei, F., “The effect of phase separation in Fe/Mg/Al/O catalysts on the synthesis of DWCNTs from methane”,, 45, 1645-1650 (2007).
20 Wang, J.Y., Wang, X.H., Zeng, C.Y., Wu, C.Z., “Intrinsic kinetics of dimethyl ether synthesis directly from CO2hydrogeneration”,, 23, 62-68 (2007). (in Chinese)
21 Chen, L.J., Guo, S.H., Zhao, D.S., “Oxidative desulfurization of simulated gasoline over metal oxide-loaded molecular sieve”,...., 15, 520-523 (2007).
22 An, X., Zuo, Y.Z., Zhang, Q., Wang, D.Z., Wang, J.F., “Dimethyl ether synthesis from CO2hydrogenation on a CuO-ZnO-Al2O3-ZrO2/ HZSM-5 bifunctional catalyst”,...., 47, 6547-6554 (2008).
23 Ma, L., Zhang, Y., Yang, J.C., “Purification of lactic acid by heterogeneous catalytic distillation using ion-exchange resins”,...., 13, 24-31 (2005).
24 An, X., Li, J.L., Wang, J.F., “Thermodynamic analysis for direct synthesis of dimethyl ether from CO and/or CO2hydrogenation”,..., 32, 5-8 (2007). (in Chinese)
25 Natta, G., In Catalysis, Emmett, P.H., Eds, Reinhold, New York, USA, 3, 349 (1955).
26 Bakemeier, H., Laurer, P.R., Schroder, W., “Development and application of a mathematical model of the methanol synthesis”,....., 66, 1-10 (1970).
27 Leonov, V.E., Karavaev, M.M., Tsybina, E.N., Petrishcheva, G.S., “Kinetics of methanol synthesis on a low-temperature catalyst”,., 14, 970-975 (1973).
28 Klier, K., Chatikavanij, V., Herman, R.G., Simmons, G.W., “Catalytic synthesis of methanol from CO/H2(IV) the effects of carbon dioxide”,.., 74, 343-360 (1982).
29 Villa, P., Forzatti, P., Buzzi-Ferraris, G., Garone, G., Pasquon, I., “Synthesis of alcohols from carbon oxides and hydrogen (1) Kinetics of the low-pressure methanol synthesis”,......, 24, 12-19 (1985).
30 Graaf, G.H., Stamhuis, E.J., Beenackers, A.A.C.M., “Kinetics of low-pressure methanol synthesis”,..., 43, 3185-3195 (1988).
31 Graaf, G.H., Scholtens, H., Stamhuis, E.J., Beenackers, A.A.C.M., “Intra-particle diffusion limitations in low-pressure methanol synthesis”,..., 45, 773-783 (1990).
32 Herman, R.G., Klier, K., Simmons, G.W., Finn, B.P., Bulko, J.B., Kobylinski, T.P., “Catalytic synthesis of methanol from carbon monoxide/hydrogen (I) Phase composition, electronic properties, and activities of the copper/zinc oxide/M2O3catalysts”,.., 56, 407-429 (1979).
33 Wang, C.W., Ding, B.Q., Zhu, B.C., Luo, Z.C., “Study on phase equilibrium for binary system of gas components, methanol and water in three-phase methanol synthesis process with liquid paraffin”,....., 14, 588-592 (2000).
2008-05-19,
2008-11-11.
the National Natural Science Foundation of China (20576060, 20606021), and the Specialized Research Fund for the Doctoral Program of Higher Education (20050003030).
** To whom correspondence should be addressed. E-mail: wangjfu@mail.tsinghua.edu.cn
 Chinese Journal of Chemical Engineering2009年1期
Chinese Journal of Chemical Engineering2009年1期
- Chinese Journal of Chemical Engineering的其它文章
- Modeling and Optimization for Scheduling of Chemical Batch Processes*
- Simulation of Droplet-gas Flow in the Effervescent Atomization Spray with an Impinging Plate*
- Numerical Investigation of Constructal Distributors with Different Configurations*
- The Kinetics of the Esterification of Free Fatty Acids in Waste Cooking Oil Using Fe2(SO4)3/C Catalyst
- Multiple Model Soft Sensor Based on Affinity Propagation, Gaussian Process and Bayesian Committee Machine*
- Measurement and Correlation of Solid-Liquid Equilibria of Phenyl Salicylate with C4 Alcohols
