Phase Separation Behavior of Cocamidopropyl Betaine/Water/Polyethylene Glycol System*
YOU Xiaoyan (游晓艳), QIN Wei (秦炜)** and DAI Youyuan (戴猷元)
Phase Separation Behavior of Cocamidopropyl Betaine/Water/Polyethylene Glycol System*
YOU Xiaoyan (游晓艳), QIN Wei (秦炜)** and DAI Youyuan (戴猷元)
State Key Laboratory of Chemical Engineering, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
Phase separation behavior of cocamidopropyl betaine/water/polyethylene glycol (PEG) system was studied. The effects of concentration and molecular weight of PEG on the phase separation behavior were investigated. Clouding occurred when the concentration of PEG was large enough in the betaine aqueous solution, and the concentration of PEG at cloud point decreased with the increase of PEG molecular weight for a constant betaine concentration. The bottom phase was the PEG-rich phase, and the upper phase was the betaine-rich phase. The volumetric ratio of PEG-rich phase to betaine-rich phase, at the same difference between the PEG concentration and the one at the cloud point,Dcp(0.1 g·ml-1), decreased as the PEG molecular weight increased and approached 1 for higher PEG molecular weight (about 20000), which was similar to the typical aqueous two-phase system. This volumetric ratio depended on the initial PEG concentration, but independent of PEG molecular weight. The concentration ratio of betaine to PEG in both phases depended on theDcp, independent of PEG molecular weight.
phase behavior, cocamidopropyl betaine, polyethylene glycol
1 INTRODUCTION
Because of their better surface properties, surfactant mixtures are widely applied in domestic chemicals as one of the main ingredients of detergents and cosmetics [1]. Upon heating, aqueous solution of many nonionic surfactants becomes turbid at a temperature that is called cloud point (CP), above which the solution separates into two phases: a surfactant-depleted phase and a surfactant-rich aggregate phase [2]. For the zwitterionic surfactants, or the mixtures of ionic surfactants and an electrolyte, clouding also occurs under certain condition [2, 3]. Nowadays, this phenomenon is widely used as the separation method, especially, to keep activities of solutes in the separation process [4],.., the isolation and purification of biomaterials, proteins, viruses, antibiotics, and enzymes by the mixed surfactant solution [5-8].
The studies usually focus on anionic, cationic, nonionic and gemini surfactants [9-17], and that on amphoteric surfactants is seldom reported. Recently, these biodegradable compounds arouse more interest, since they are environmentally friendly. Amphoteric surfactants, such as betaine, have specific properties and structure, two ionic centers with different signs in one molecule, so that their behavior is between that of nonionic and ionic compounds [1]. Materna. [18] investigated the recovery of crystal violet with aqueous solutions containing oxyethylated methyl dodecanoate and cocamidopropyl betaine, and the dynamics of the surfactant-rich phase separation. It was found that the addition of betaine improved the dye transfer to the surfactant-rich phase and about 90% of the dye was recovered from the aqueous solution.
Inorganic salts/water/polyethylene glycol (PEG) is a typical two aqueous phase widely used in extracting biomolecules such as proteins, enzymes, and cells. However, these systems lead to high concentrations of sulfate and phosphate salts in the effluent streams and affect the activity of biomass. In this study, the phase separation behavior of cocamidopropyl betaine/PEG/ water mixture is studied, and the effect of molecular weight and concentration of PEG on the phase separation behavior is investigated.
2 EXPERIMENTAL
2.1 Materials
The cocamidopropyl betaine solution, from Rhodia Specialty Chemicals Wuxi Co., is a clear liquid with 30% (by mass) betaine and 5.2% (by mass) NaCl. The structure of cocamidopropyl betaine is shown in Fig. 1. The PEGs (PEG 400, 1000, 2000, 4000, 6000, 8000, 10000, 20000 and 35000) were supplied by Fluka. They are all chemically pure reagents. PEG 400 is a liquid, and the others are white powder. Double distilled water was used in all experiments.

Figure 1 Structure of cocamidopropyl betaine
2.2 Experiments
The samples for determination of the clouding condition and phase distribution behavior were prepared by mixing the stock solutions with a series of PEG concentrations and betaine solution in 10 ml centrifuge tubes, and the volume of PEG solution was the same as that of betaine. The tubes were shaken for 2 min by hands, then placed in a centrifuge (Eppendorf 5702RH) with temperature control within ±0.1°C for 30 min at 3000 r·min-1and 25°C. The equilibrium state was verified by Turbiscan,.., the clear interface was stable and volumes of both phases changed little when the mixture stood for 20 min.
The cloud point of the solutions was determined by direct observation for disappearance of the turbidity. The PEG concentration for a molecular weight of PEG at cloud point of 25°C was the average of two measurements and reproducible within 0.001 g·ml-1. In the experiments of phase distribution, the samples of two phases were obtained using a syringe with long needle, and the volumes of the two phases and the concentrations of PEG and betaine in the two phases were measured. The uncertainty in the volume measurement was ±0.02 ml, and the density measurements were reproducible within 0.002 g·ml-1.
2.3 Analysis
The concentrations of main compositions in two phases were obtained by the measurement of the elements, total carbon, oxygen and nitrogen, with Element Analyses System (Vario EL), and the calculation based on the material balance for total carbon, oxygen and nitrogen elements. The precision of these three elements was ≤0.1% in mass fraction.
3 RESULTS AND DISCUSSION
3.1 Effect of PEG molecular weight on the cloud point condition
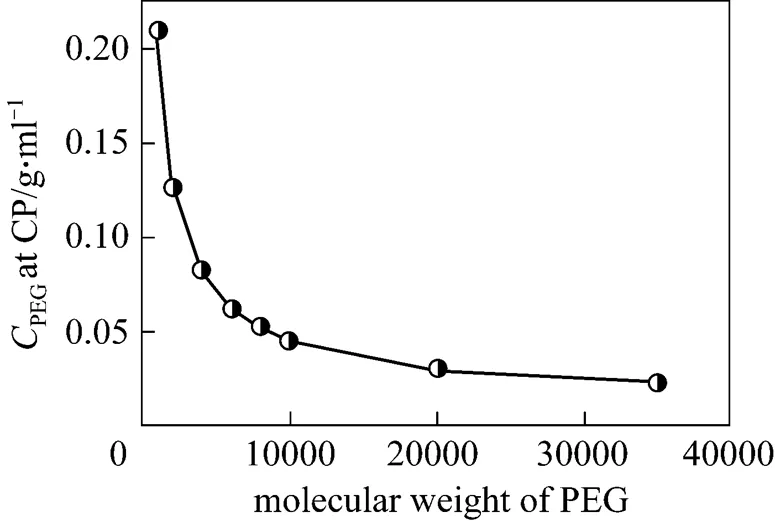
Figure 2 Effect of PEG molecular weight on the clouding condition of PEG/water/betaine system at 25°C
It had been suggested that additives with high polarity reduce the CP of nonionic surfactant, while those with less polarity have opposite effect. Qiao and Easteal [19] proposed two models to describe the polymer-surfactant interactions based on the cloud point dependence of Triton X surfactants on the ethylene oxide chain length and PEG molecular weight. In one model, intra-chain micelles of polysoap are formed among the surfactant monomers and long polymer chains. The bridging attraction between the two intra-chain micelles in such structure enhances the collisions among the micelles. In another model, flocculation depletion for the polymer chains exists between two regular micelles, providing the driving force for the neighboring micelles to approach each other and destabilizing the colloidal system. The flocculation effect is more significant for the polymer with a long chain. According to the two models above, higher PEG molecular weight is good for aggregating the micelle and achieving the CP more easily.
3.2 Effect of PEG molecular weight on phase distribution
The cloud point extraction is usually conducted at 15°C above the CP to make the phase separation easy and stable. For the PEG/water/betaine system in this study, the PEG concentration is higher than the value of cloud point. Fig. 3 shows the effect of PEG molecular weight on the phase distribution behavior at the PEG concentrationPEG(0.1)0.1 g·ml-1. In Fig. 3, theis defined as the phase volume, and theis defined as the mass fraction. The phase separation occurs when the PEG concentration is higher than the value at cloud point, and betaine is concentrated in the upper phase [>90% (by mass)], which is the betaine-rich phase, while PEG is mainly in the bottom phase [>90% (by mass)], the PEG-rich phase. In the upper phase, the betaine concentrations are higher than the initial concentration of betaine solution. The PEG concentrations in the bottom phase depend on the PEG molecular weight. As the PEG molecular weight increases, the difference between PEG concentration in bottom phase and its initial concentration is decreased.
As the PEG molecular weight increases, the volumetric ratio of PEG-rich phase to betaine-rich phase atPEG (0.1)decreases and approaches 1 for higher PEG molecular weight. The distribution of PEG/water/betaine system is similar to that of a typical aqueous two-phase system (Dextran/water/PEG), and may be used for extracting bioactive substances.
3.3 Effect of PEG concentration on phase distribution
PEG molecular weights of 4000, 10000 and 20000 were selected to investigate the effect of PEG concentration on phase distribution behavior at a constant betaine concentration (≈15%). As shown in Fig. 4, the volumetric ratio of the bottom phase to the upper phase increases with the initial PEG concentration, independent of PEG molecular weight. The length of the tie line increases with the total PEG concentration. Betaine and PEG are dominant in the upper and bottom phase, respectively. The concentration of betaine in the upper phase and that of PEG in the bottom phase increase slightly withDcp, the PEG concentration difference between the initial concentration and the value at CP, but independent of PEG molecular weight. Therefore, higher total PEG concentration and larger PEG molecule weight are beneficial to obtaining a higher phase ratio and separation factor.
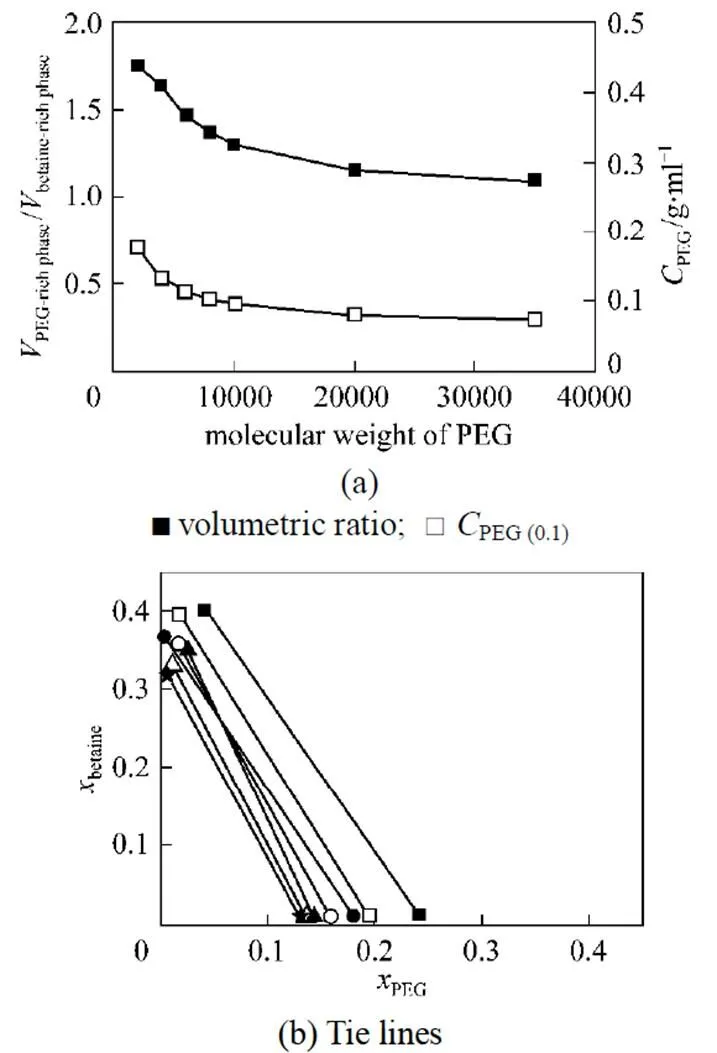
Figure 3 Influence of PEG molecular weight on the phase distribution behavior of PEG/water/betaine system at 25°C [PEG(0.1)0.1 g·ml-1]
Some relevant physical properties were also measured for better understanding the system. The density difference of the two phases is small and close to that of water,.., the density of the upper phase is 1.030 g·ml-1and that of the bottom phase is 1.037 g·ml-1for the lowest point in Fig. 4 (a), and the density difference is 0.054 g·ml-1for the highest point in Fig. 4 (a).
4 CONCLUSIONS
Clouding occurred when PEG was introduced into betaine aqueous solutions at a certain concentration. The bottom phase was the PEG-rich phase, and the upper phase was the betaine-rich phase. For a constant betaine concentration, the concentration of PEG at CP decreased as PEG molecular weight increased. At the same PEG concentration difference [PEG(0.1)], the volumetric ratio of PEG-rich phase to betaine-rich phase decreased and approached 1 as PEG molecular weight increased. The volumetric ratio of the bottom phase to the upper phase increased with the initial PEG concentration, independent of PEG molecular weight. The concentration ratio of betaine to PEG in the upper phase and that of PEG to betaine in the bottom phase depended on the PEG concentration difference between the initial concentration and that at CP, independent of PEG molecular weight. Both concentration ratios increased with the PEG concentration difference slightly. The distribution behavior of the PEG/water/betaine system was similar to the typical aqueous two-phase system, and may be used for extracting bioactive substances.
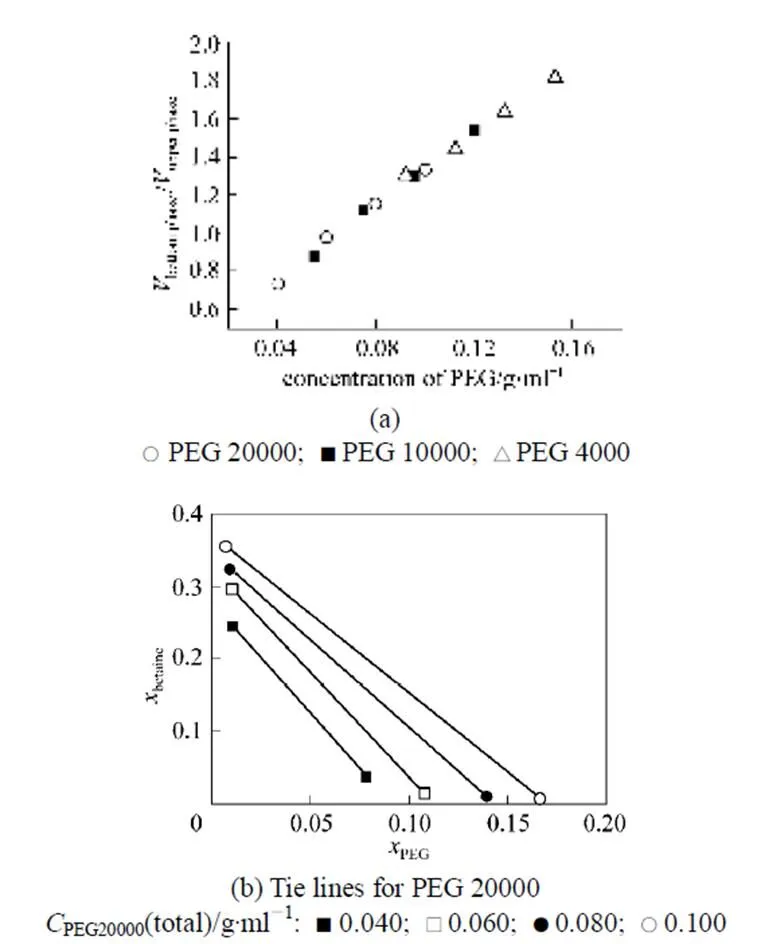
Figure 4 Influence of PEG concentration on the phase distribution behavior at 25°C
1 Wydro, P., “The influence of the size of the hydrophilic group on the miscibility of zwitterionic and nonionic surfactants in mixed monolayers and micelles”,.., 316 (1), 107-113 (2007).
2 Quina, F.H., Hinze, W.L., “Surfactant-mediated cloud point extractions: an environmentally benign alternative separation approach”,...., 38 (11), 4150-4168 (1999).
3 Nilsson, P.G., Pacynko, W.F., Tiddy, G.J.T., “‘Clouding’ in zwitterionic surfactant/water systems—The influence of additives on the upper consolute loop of the decyldimethylammonioethane sulfate/water system”,..., 9 (1/2), 117-123 (2004).
4 Trakultamupatan, P., Scamehorn, J.F., Osuwan, S., “Removal of volatile aromatic contaminants from wastewater by cloud point extraction”,..., 37 (6), 1291-1305 (2002).
5 Mazzola, P.G., Lopes, A.M., Hasmann, F.A., Jozala, A.F., Vessoni Penna, T.C., Magalhaes, P.O., Rangel-Yagui, C.O., Pessoa Jr, A., “Liquid-liquid extraction of biomolecules: An overview and update of the main techniques”,..., 83 (2), 143-157 (2008).
6 Lee, C.K., Su, W.D., “Nonionic surfactant-mediated affinity cloud-point extraction of vancomycin”,..., 34 (16), 3267-3277 (1999).
7 Wang, Z.L., Xu, J.H., Chen, D.J., “Whole cell microbial transformation in cloud point system”,...., 35 (7), 645-656 (2008).
8 Wang, Z.L., “The potential of cloud point system as a novel two-phase partitioning system for biotransformtion”,.., 75 (1), 1-10 (2007).
9 Yin, H.Q., Mao, M., Huang, J.B., Fu, H.L., “Two-phase region in the DTAB/SL mixed surfactant system”,, 18 (24), 9198-9203 (2002).
10 Shang, Y.Z., Liu, H.L., Hu, Y., “Phase separation and microstructure of mixed surfactants solution containing cationic geminis and traditional anionic surfactant”,...., 12 (4), 486-492 (2004).
11 Shang, Y.Z., Liu, H.L., Hu, Y., Prausnitz, J.M., “Phase behavior and microstructures of the gemini (12-3-12, 2 Br-)-SDS-H2O ternary”,.:.., 294 (1-3), 203-211 (2007).
12 Lin, Y.Y., Han, X., Cheng, X.H., Huang, J.B., Liang, D.H., Yu, C.L., “pH-regulated molecular self-assemblies in a cationic-anionic surfactant system: from a ‘1-2’ surfactant pair to a ‘1-1’ surfactant pair”,., 24 (24), 13918-13924 (2008).
13 Jiang, R., Huang, Y.X., Zhao, J.X., Huang, C.C., “Transformation of vesicles in aqueous two-phase system of an anionic Gemini surfactant and a cationic conventional surfactant mixture”,..., 26 (4), 635-639 (2008).
14 Lu, T., Li, Z.H., Huang, J.B., Fu, H.L., “Aqueous surfactant two-phase systems in a mixture of cationic gemini and anionic surfactants”,, 24 (19), 10723-10728 (2008).
15 Yao, B.J., Yang, L., Hu, Q., Shigendo, A., “Cloud point extraction of polycyclic aromatic hydrocarbons in aqueous solution with silicone surfactants”,...., 15 (4), 468-473 (2007).
16 Qin, W., Huang, Y., Ding, Y.W., Dai, Y.Y., “Surfactant distribution in the clouding of TX114”,...., 16 (5), 722-725 (2008).
17 Jozala, A.F., Lopes, A.M., Mazzola, P.G., Magalhães, P.O., Vessoni Penna, T.C., Pessoa Jr, A., “Liquid-liquid extraction of commercial and biosynthesized nisin by aqueous two-phase micellar systems”,.., 42 (2), 107-112 (2008).
18 Materna, K., Schaadt, A., Bart, H.J., Szymanowski, J., “Dynamics of surfactant-rich phase separation from solutions containing non-ionic and zwitterionic surfactants”, Colloids., 254 (1-3), 223-229 (2005).
19 Qiao, L., Easteal, A.J., “The interaction between triton X series surfactants and poly (ethylene glycol) in aqueous solutions”,.., 276 (4), 313-320 (1998).
2008-12-10,
2009-05-25.
the National Natural Science Foundation of China (20676069).
** To whom correspondence should be addressed. E-mail: qinwei@tsinghua.edu.cn
 Chinese Journal of Chemical Engineering2009年5期
Chinese Journal of Chemical Engineering2009年5期
- Chinese Journal of Chemical Engineering的其它文章
- Molecular Simulation of CO2/H2 Mixture Separation in Metal-organic Frameworks: Effect of Catenation and Electrostatic Interactions*
- Deactivation Kinetics of Nitrile Hydratase in Free Resting Cells*
- Corrosion Behavior of TP316L of Superheater in Biomass Boiler with Simulated Atmosphere and Deposit
- Influence of A-type Zeolite on Methane Hydrate Formation*
- Effects of Sintering Atmosphere on the Microstructure and Surface Properties of Symmetric TiO2Membranes*
- Improvement of Isomerization Process of Crude Isoamylene with Tertiary-amyl-alcohol Addition
