Pyrolysis Mechanisms of Quinoline and Isoquinoline with Density Functional Theory*
LING Lixia (凌丽霞), ZHANG Riguang (章日光), WANG Baojun (王宝俊)** and XIE Kechang(谢克昌)
Pyrolysis Mechanisms of Quinoline and Isoquinoline with Density Functional Theory*
LING Lixia (凌丽霞), ZHANG Riguang (章日光), WANG Baojun (王宝俊)** and XIE Kechang(谢克昌)
Key Laboratory of Coal Science and Technology (Taiyuan University of Technology), Ministry of Education and Shanxi Province, Taiyuan 030024, China

quinoline, isoquinoline, coal, pyrolysis mechanism, density functional theory
1 INTRODUCTION
Emission of nitrogen oxides (NO, NO2and N2O) in the utilization process of coal is an important environmental problem [1]. It is well known that the presence of nitrogen in coal and coal-derived products is mainly in the form of heterocycles such as pyrrole and pyridine ring systems [2, 3]. Pyrrole, pyridine, indole and quinoline are commonly selected as N-containing model compounds in coal. Many detailed experimental and theoretical studies on the pyrolysis of pyrrole [4-9], pyridine [10-13] and indole [14, 15] have been carried out. Quinoline and isoquinoline, both the molecules containing a pyridine ring fused to benzene, are more authentic than pyridine for describing the coal pyrolysis, considering the actual structures of N-containing components in the aromatic matrix of coal.

The presence of 1-indene imine has not been identified experimentally although it is widely believed to be an important intermediate during pyrolysis of quinoline and isoquinoline. In order to better understand the fact that the total disappearance rates of quinoline and isoquinoline are the same and the pyrolysis products are also the same for both reactants, and in order to verify the importance of 1-indene imine intermediate, we carry out a study on the kinetic mechanism of pyrolysis of quinoline and isoquinoline using quantum chemistry calculation.
2 COMPUTATION
Density functional theory (DFT) method of quantum chemistry calculation was adopted and the calculations were performed using the Dmol3program [21] mounted on Materials Studio Modeling 4.0 package. Generalized gradient approximation (GGA) with PW91 function [22] was adopted. The DND basis set, equivalent in accuracy to the 6-31G* Gaussian orbital basis set, was also used. Spin unrestricted was chosen. Total self-consistent field (SCF) tolerance criteria, integration accuracy criteria and orbital cutoff quality criteria were set at medium. Multipolar expansion was set at octupole.

Table 1 The calculated and experimental bond lengths and bond angles of N-containing compounds
All the reactants, products and possible intermediates in reaction paths were optimized, and their single point energies (SPE) were determined at the same time. All transition state searches were carried out using the Linear Synchronous Transit/Quadratic Synchronous Transit (LST/QST) method. The vibration analyses about the molecular structure of the species involved in the pyrolysis mechanism were carried out at the same theoretical level to verify whether a stationary point corresponding to the structure was a local minimum (equilibrium structure) or a first-order saddle point (transition state) according to the vibration modes, meanwhile, the corresponding zero-point energies (ZPE) were determined. Transition state confirmation calculation was carried out using the nudged elastic band (NEB) method to confirm that the transition states lead to the desired reactants and products. All calculations were performed in HP Proliant DL 380 G5 server system.
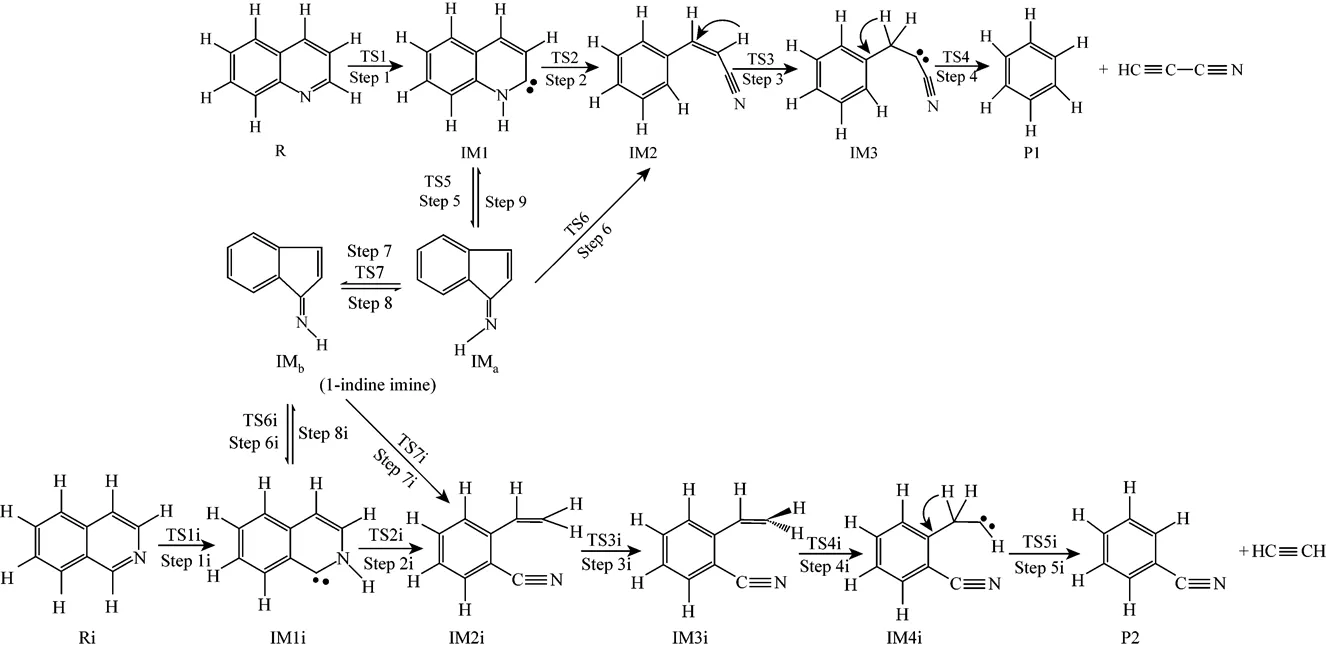
Figure 1 The pyrolysis mechanisms of quinoline (R) and isoquinoline (Ri)
In order to evaluate the reliability of the selected calculation method and parameters, some structural parameters of several N-containing heterocyclic compounds were calculated, and the calculated and experimentalresults [23] are shown in Table 1. The calculated results are in agreement with the experimental structural parameters, the largest deviation of bond angles is 0.617°, and the deviation of bond lengths is smaller than 0.002 nm. In addition, some results about the reaction mechanism have been obtained using the above method and parameters in our previous studies [24, 25].
3 RESULTS AND DISCUSSION
3.1 The pyrolysis mechanisms of quinoline and isoquinoline


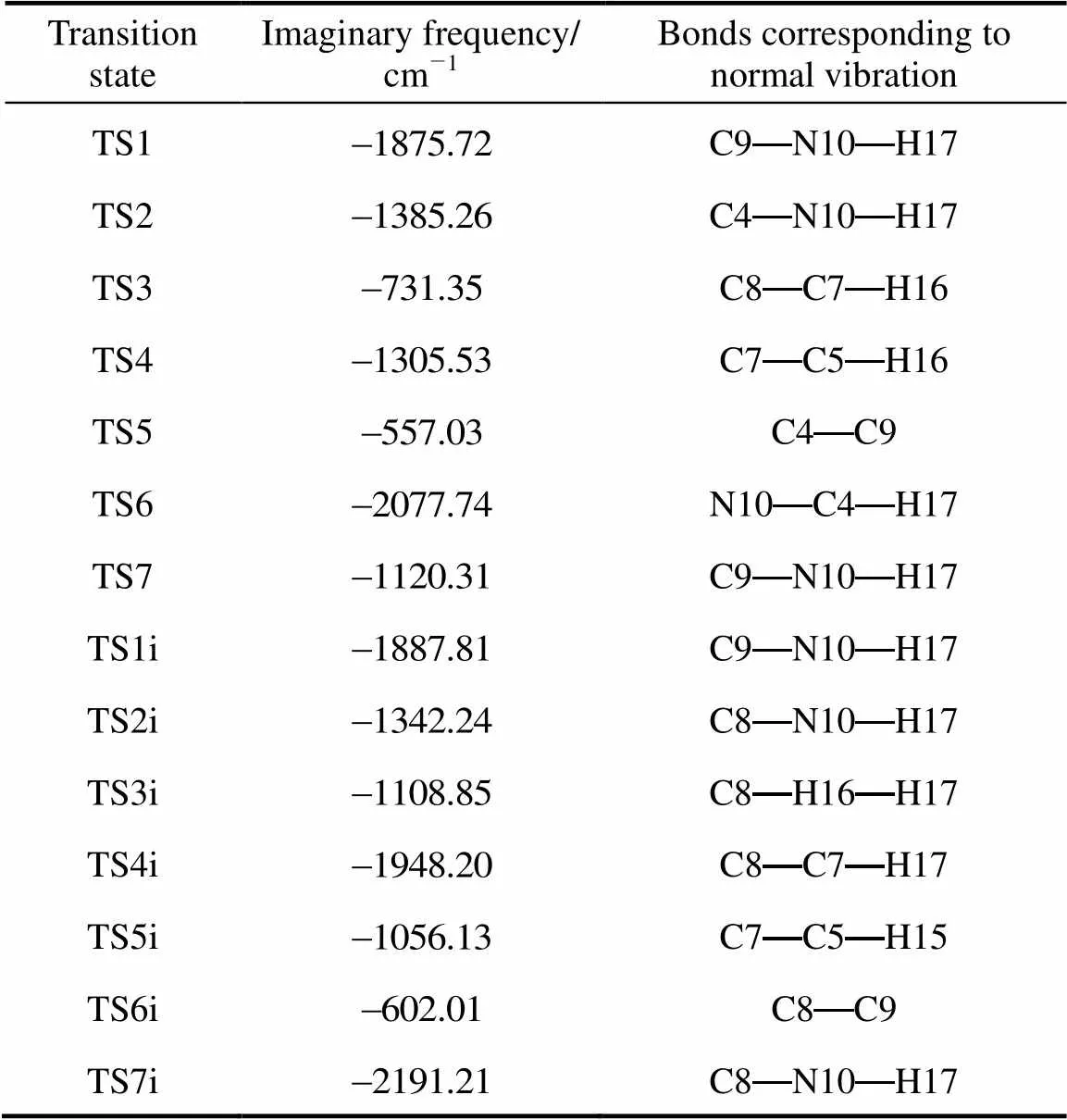
Table 2 Imaginary frequencies of the transition states and the bonds corresponding to relative normal vibrations
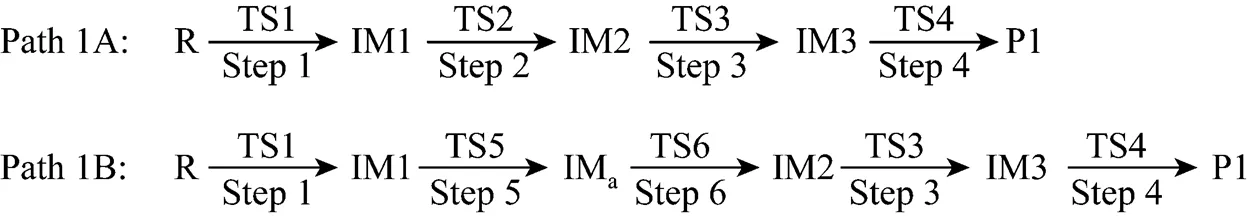

Figure 2 The optimized structures of all species relating to Path 1A and Path 1B

Path 1B has the same initial step to IM1 as Path 1A. The next step is that the C4 in IM1 links to C9 to yield an intermediate 1-indene imine (IMa)TS5, then the migration of H17 in IMafrom N10 to C4 also results in the formation of IM2 with an activation energy of 361.93 kJ·mol-1. From IM2 to P1, Path 1B and Path 1A have the same steps.
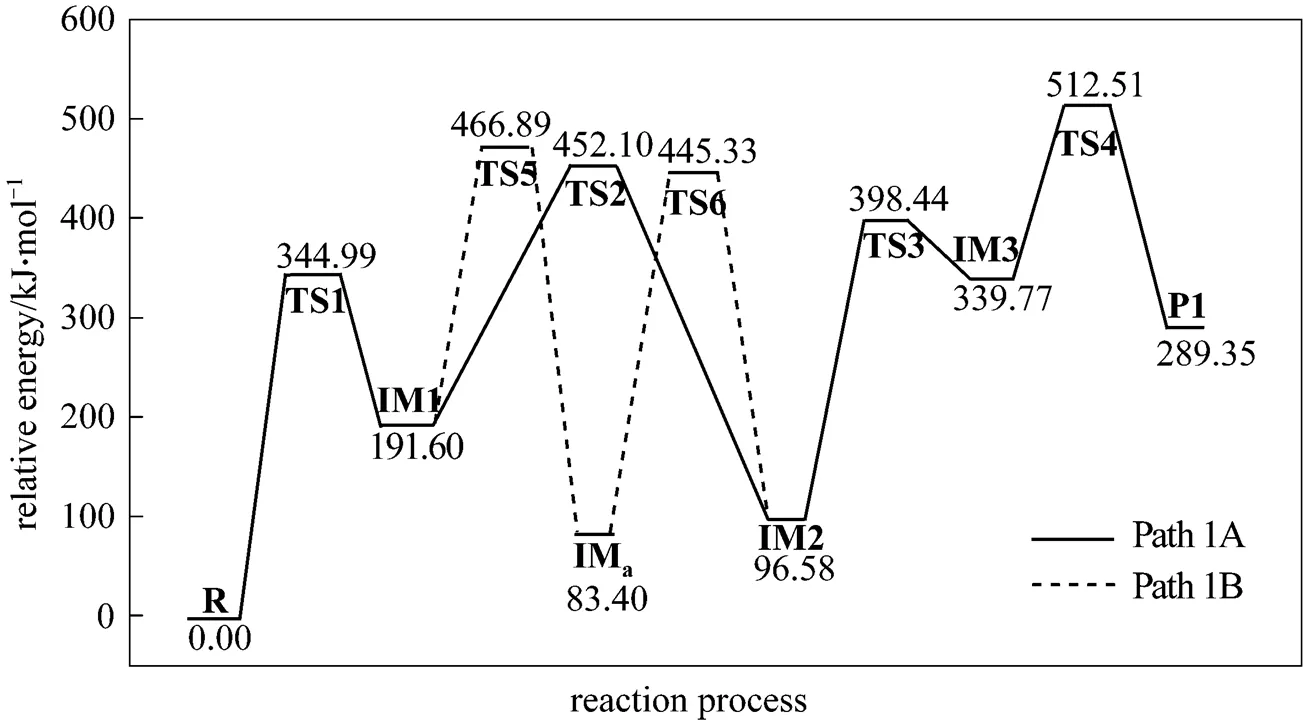
Figure 3 Energetic sketch of the stationary points for Path 1A and Path 1B along the reaction process


Figure 3 shows that the rate determining steps of Path 1A and Path 1B are Step 1 and Step 6, with activation energies of 344.99 kJ·mol-1and 361.93 kJ·mol-1, respectively. There is only a difference of 16.93 kJ·mol-1in the activation energy, so Path 1A and Path 1B may be considered to be the coexistent competitive reaction paths.


2018年11月,第二十六届世界计量组织大会将在巴黎召开,届时将对我们所熟知的基本计量单位做出重大调整。千克作为国际单位制中质量的基本单位,将基于普朗克常数进行重新定义,而沿用了129年的国际千克原器也将退出历史舞台。



Figure 5 shows that the rate determining steps of Path 2A and Path 2B are Step 1i and Step 7i, with activation energies of 338.94 kJ·mol-1and 370.76 kJ·mol-1, respectively. There is only a difference of 31.82 kJ·mol-1in activation energy, so Path 2A and Path 2B may be considered to be the coexistent competitive reaction paths like the case of Path 1A and Path 1B.
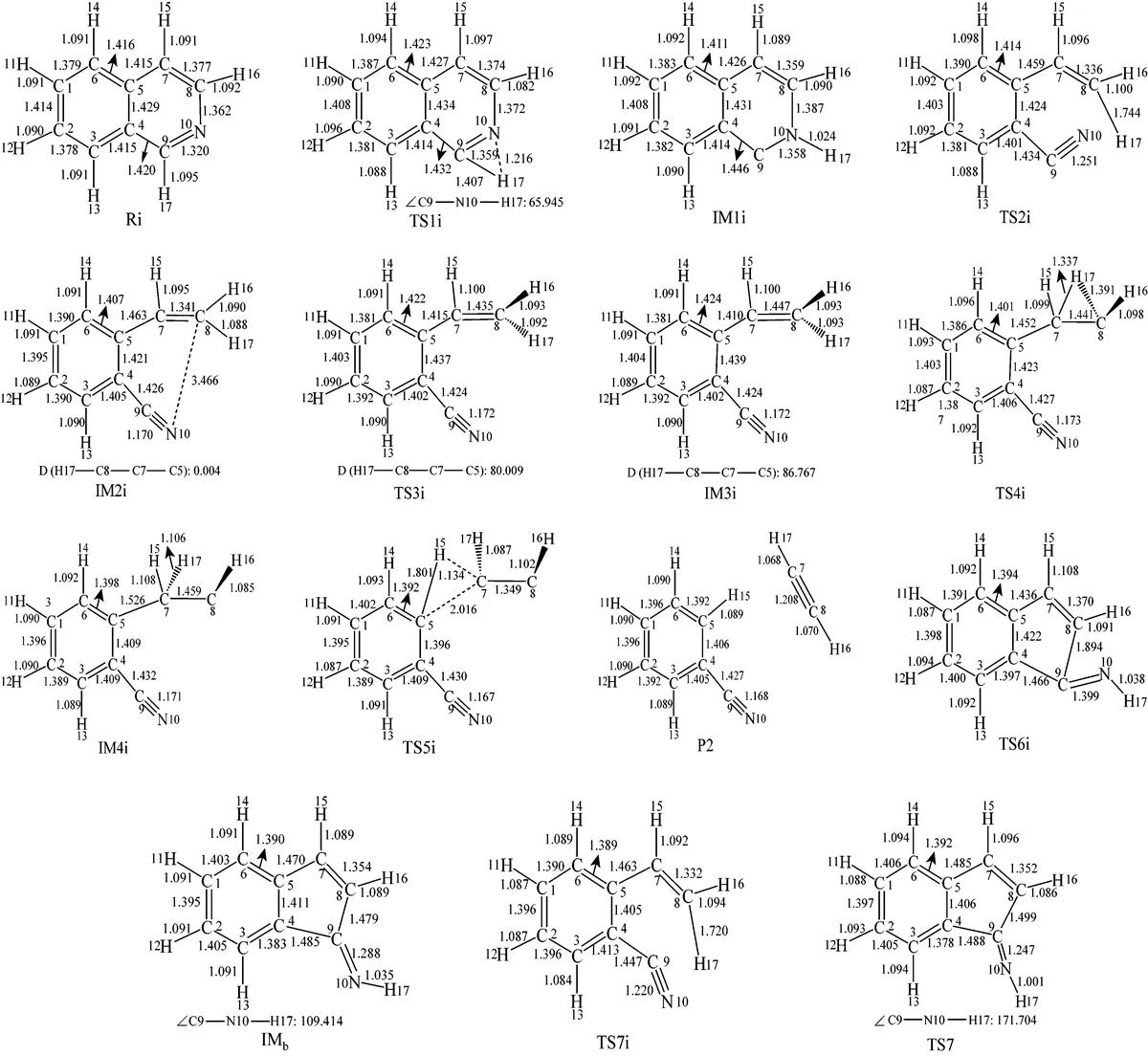
Figure 4 The optimized structures of all species relating to Path 2A and Path 2B
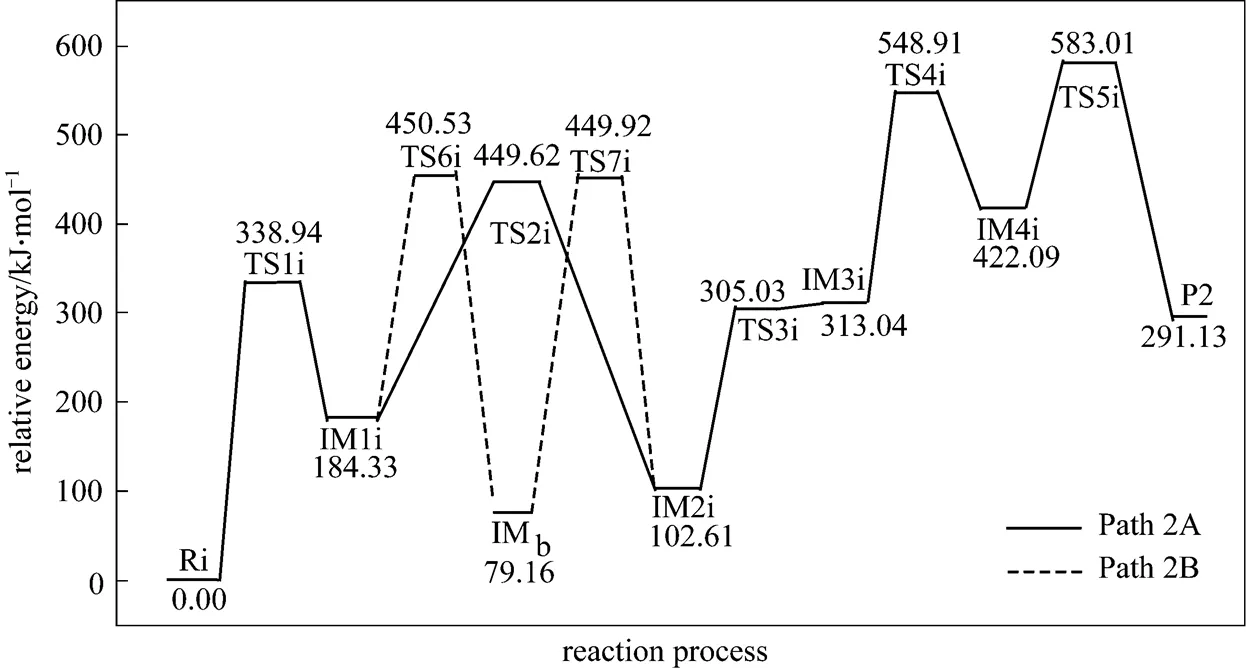
Figure 5 Energetic sketch of the stationary points for Path 2A and Path 2B along the reaction process
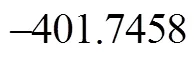




It can be seen from Fig. 6 that the activation energies of the rate determining steps are 370.76 kJ·mol-1in Path 3A and 371.37 kJ·mol-1in Path 3B, so Path 3A and 3B are considered to be the coexistent competitive paths.




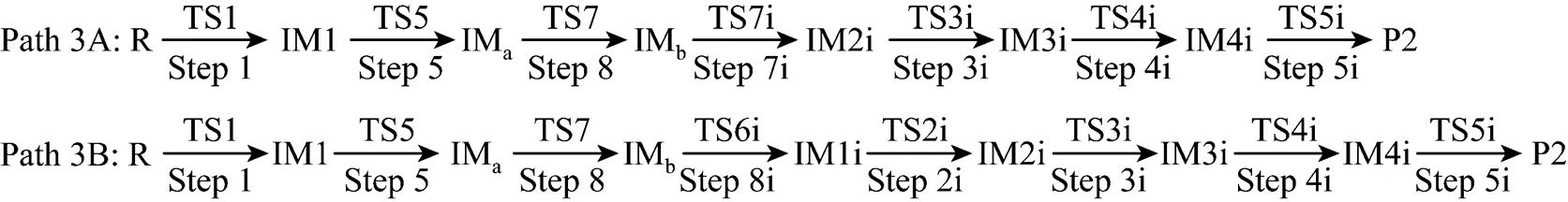

Figure 6 Energetic sketch of the stationary points for Path 3A and Path 3B along the reaction process

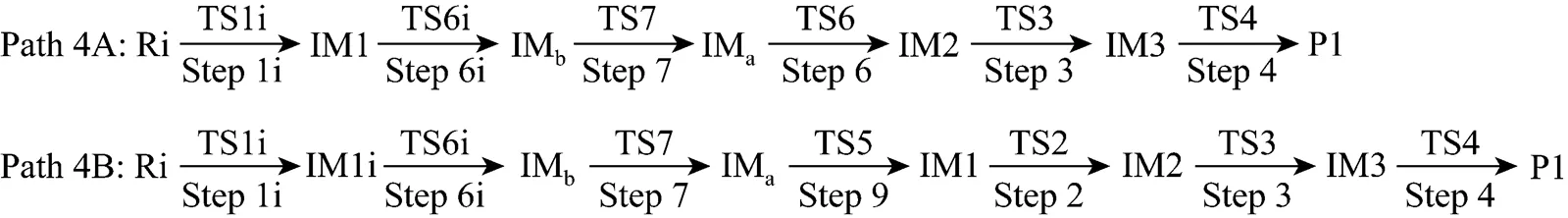

Figure 7 Energetic sketch of the stationary points for Path 4A and Path 4B along the reaction process
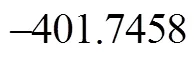
It can be seen from Fig. 7 that the activation energies of rate determining steps are 361.93 kJ·mol-1in Path 4A and 383.48 kJ·mol-1in Path 4B, so Path 4A and 4B may be considered to be the coexistent competitive paths.
3.1.5

Quinoline goes through three paths (Path 1B, Path 3A and Path 3B) towards IMaand isoquinoline goes through other three paths (Path 2B, Path 4A and Path 4B) towards IMbby the kinetic mechanism involving the intramolecular hydrogen migration, in which the highest energy barriers of 344.99 kJ·mol-1in Step 1 and 338.94 kJ·mol-1in Step 1i have to be overcome, which are close to the experimental activation energies 326.00 kJ·mol-1[17] and 316.47 kJ·mol-1[19]. Because of the similar activation energies, the production rates of 1-indene imine are almost the same, whether the original reactant is quinoline or isoquinoline. Then there is a quick conformational tautomeric equilibrium between IMaand IMbwith activation energies of 113.98 kJ·mol-1and 113.96 kJ·mol-1.
The common intermediate 1-indene imine goes through three paths (Path 1B, Path 4A and Path 4B) towards P1, and other three paths (Path 2B, Path 3A and Path 3B) towards P2. The calculated results show that the highest energy barriers yielding P1 and P2 are 361.93 kJ·mol-1in Step 6 and 370.76 kJ·mol-1in Step 7i. The highest energy barriers are almost the same, slightly higher than the experimental results [17, 19]. Thus it is concluded that the total disappearance rates of quinoline and isoquinoline are the same, and the composition of the pyrolysis products is also the same, no matter the original reactant is quinoline or isoquinoline, which is in agreement with the experimental results [19, 20].
3.2 The role of 1-indine imine during quinoline and isoquinoline pyrolysis

4 CONCLUSIONS
The kinetic mechanisms of pyrolysis of quinoline and isoquinoline are investigated in detail using the density functional theory of quantum chemistry. The results are summarized as follows:
(1) A rational reaction mechanism involving eight reaction paths and a common tautomeric intermediate is proposed, and the activation energies of the rate determining steps are obtained. The calculated results are in good agreement with the experimental results.
(2) The conformational tautomerism of 1-indene imine intermediate plays an important role in the mechanism, which decides the composition of the pyrolysis products to be the same, and also decides the total disappearance rates of the reactants to be the same, whether the original reactant is quinoline or isoquinoline.
(3) The intramolecular hydrogen migration is an important reaction step, which appears widely in the paths of the pyrolysis mechanism.
1 Tan, L.L., Li, C.Z., “Formation of NOand SOprecursors during the pyrolysis of coal and biomass. Part І. Effects of reactor configuration on the determined yields of HCN and NH3during pyrolysis”,, 79, 1883-1889 (2000).
2 Kelemen, S.R., Gorbaty, M.L., Kwiatek, P.J., Fletcher, T.H., Watt, M., Solum, M.S., Pugmire, R.J, “Nitrogen transformations in coal during pyrolysis”,., 12, 159-173 (1998).
3 Solomon, P.R., Colket, M.B., “Evolution of fuel nitrogen in coal devolatilization”,, 57, 749-755 (1978).
4 Mackie, J.C., Colket, M.B., Nelson, P.F., Esler, M., “Shock tube pyrolysis of pyrrole and kinetic modeling”,...., 23, 733-760 (1991).
5 Lifshitz, A., Tamburu, C., Suslensky, A., “Isomerization and decomposition of pyrrole at elevated temperatures. Studies with a single-pulse shock tube”,..., 93, 5802-5808 (1989).
6 Dubnikova, F., lifshitz, A., “Isomerization of pyrrole. Quantum chemical calculations and kinetic modeling”,..., 102, 10880-10888 (1998).
7 Zhai, L., Zhou, X.F., Liu, R.F., “A theoretical study of pyrolysis mechanisms of pyrrole”,..., 103, 3917-3922 (1999).
8 Martoprawiro, M., Bacskay, G.B., Mackie, J.C., “Ab initio quantum chemical and kinetic modeling study of the pyrolysis kinetics of pyrrole”,..., 103, 3923-3934 (1999).
9 Bacskay, G.B., Martoprawiro, M., Mackie, J.C., “The thermal decomposition of pyrrole: An ab initio quantum chemical study of the potential energy surface associated with the hydrogen cyanide plus propyne channel”,..., 300, 321-330 (1999).
10 Mackie, J.C., Colket, M.B, Nelson, P.F., “Shock tube pyrolysis of pyridine”,..., 94, 4099-4106 (1990).
11 Memon, H.U.R., Bartle, K.D., Taylor, J.M., Williams, A., “The shock tube pyrolysis of pyridine”,...., 24, 1141-1159 (2000).
12 Liu, R.F., Huang, T.T.S., Tittle, J., Xia, D.H., “A theoretical investigation of the decomposition mechanism of pyridyl radicals”,..., 104, 8368-8374 (2000).
13 Ninomiya, Y., Dong, Z.B., Suzuki, Y., Koketsu, J., “Theoretical study on the thermal decomposition of pyridine”,, 79, 449-457 (2000).
14 Laskin, A., Lifshitz, A., “Isomerization and decomposition of indole. Experimental results and kinetic modeling”,..., 101, 7787-7801 (1997).
15 Zhou, X.F., Liu, R.F., “A density functional theory study of the pyrolysis mechanisms of indole”,...., 461/462, 569-579 (1999).
16 Patterson, J.M., Issidorides, C.H., Papadopoulos, E.P., Smith, Jr. W.T., “The thermal interconversion of quinoline and isoquinoline”,.., 15, 1247-1250 (1970).
17 Bruinsma, O.S.L., Tromp, P.J.J., De Sauvage Nolting, H.J.J., Moulijn, J.A., “Gas phase pyrolysis of coal-related aromatic compounds in a coiled tube flow reactor 2. Heterocyclic compounds, their benzo and dienzo derivatives”,, 67, 334-340 (1988).
18 Axworthy, A.E., Dayan, V.H., Martin, G.B., “Reactions of fuel-nitrogen compounds under conditions of inert pyrolysis”,, 57, 29-35 (1978).
19 Laskin, A., Lifshitz, A., “Thermal decomposition of quinoline and isoquinoline. The role of 1-indene imine radical”,..., 102, 928-946 (1998).
20 Winkler, J.K., Karow, W., Rademacher, P., “Gas phase pyrolysis of heterocyclic compounds (3) Flow pyrolysis and annulation reaction of some nitrogen heterocycles. A product oriented study”,, 1 (4), 576-602 (2000).
21 Delley, B., “From molecules to solids with the Dmol3approach”,..., 113 (18), 7756-7764 (2000).
22 Perdew, J.P., Chevary, J.A., Vosko, S.H., Fiolhais, C., “Atoms, molecules, solids and surfaces: Application of the generalized gradient approximation for exchange and correlation”,.., 46 (11), 6671-6687 (1992).
23 Lide, D.R., Handbook of Chemistry and Physics, 82nd edition, CRC Press, New York, 9-40 (2001-2002).
24 Zhao, L.J., Ling, L.X., Zhang, R.G., Liu, X.F., Wang, B.J., “Theoretical study on pyrolysis mechanism of O-containing model compound anisole in coal”,.... (), 59 (8), 2095-2102 (2008). (in Chinese)
25 Zhang, R.G., Huang, W., Wang, B.J., “Theoretical calculation for interaction of CO2with ·H and ·CH3in synthesis of acetic acid from CH4and CO2”,... (), 28 (7), 641-645 (2007). (in Chinese)
2008-10-23,
2009-07-07.
the National Basic Research Program of China (2005CB221203), the National Natural Science Foundation of China (20576087, 20776093) and the Foundation of Shanxi Province (2006011022, 2009021015).
** To whom correspondence should be addressed. E-mail: wangbaojun@tyut.edu.cn
 Chinese Journal of Chemical Engineering2009年5期
Chinese Journal of Chemical Engineering2009年5期
- Chinese Journal of Chemical Engineering的其它文章
- Molecular Simulation of CO2/H2 Mixture Separation in Metal-organic Frameworks: Effect of Catenation and Electrostatic Interactions*
- Deactivation Kinetics of Nitrile Hydratase in Free Resting Cells*
- Corrosion Behavior of TP316L of Superheater in Biomass Boiler with Simulated Atmosphere and Deposit
- Influence of A-type Zeolite on Methane Hydrate Formation*
- Effects of Sintering Atmosphere on the Microstructure and Surface Properties of Symmetric TiO2Membranes*
- Improvement of Isomerization Process of Crude Isoamylene with Tertiary-amyl-alcohol Addition
