Induction of Apoptosis in HL60 Cells by Selenosulfate
——Cytotoxicity of Selenium Anion
HUANG Bo,ZHANG Jinsong,CHEN Chang
1.Institute of Biophysics,Chinese Academy of Sciences,Beijing 100101,China;
2.Graduate School of the Chinese Academy of Sciences,Beijing 100049,China;
3.University of Science and Technology of China,Hefei 230052,China
Induction of Apoptosis in HL60 Cells by Selenosulfate
——Cytotoxicity of Selenium Anion
HUANG Bo1,2,ZHANG Jinsong3,CHEN Chang1
1.Institute of Biophysics,Chinese Academy of Sciences,Beijing 100101,China;
2.Graduate School of the Chinese Academy of Sciences,Beijing 100049,China;
3.University of Science and Technology of China,Hefei 230052,China
The work is supported by grant from The National Natural Science Foundation of China(90606020)
The effect of Cd2+releasing in the cytotoxicity of nanoparticle cadmium selenide(CdSe)has been reported;however,the role of Se2-ion remains unclear.In this study,the authors explored the cellular effects of selenosulfate(SSeO3)2-on HL60 cells.They found that 10 μmol/L selenosulfate significantly suppressed the cell viability,and induced apoptosis characterized by condensation of chromatin,DNA ladder and the sub-G0/G1peak measured by flow cytometry.The mitochondrial membrane potential decreased and the immunofluorescence of pro-apoptosis protein Bax increased.The investigation reveals that the reductive Se2-ion may cause significant cytotoxicity,which suggests that the release of Se2-may be an alternative mechanism for the cytotoxic effects of Se2--containing nanoparticles such as quantum dots CdSe.
Selenosulfate;Nanoparticles;Selenium(Se);Apoptosis;Quantum dots
0 Introduction
Early studies have raised concerns that tiny man-made nanoparticles (those smaller than 100 nanometers)might pose threats to human health and the environment.Therefore,calls are rising for more research on toxicology of nanomaterials[1,2].Quantum dots(QDs)have been adopted as a new class of fluorescent labels.There remains a pressing need for further investigations into QD toxicity especially when they are used to study live cells and animals because they contain elements such as cadmium and selenium[3].The cytotoxicity of QD CdSe has been reported.It is regarded that the release of Cd2+(Thishappened when the QD surface coating was not stable,exposing the CdSe to oxidization by air or UV damage)and the stability toward aggregation play important roles[4,5].However,the role of Se2-anion can not be excluded and remains unclear.
Selenosulfate is wildly used nowadays in nanotechnology as a Se2-donor to produce nanoparticles or films such as CdSe,PbSe,HgSe[6,7].It can be synthesized by the reaction of elemental selenium and sodium sulfite as following[6,7]:
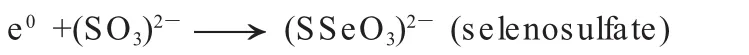
Therefore,the role of Se2-anion in the toxicity of Se2--containing nanoparticles can be elucidated by the cellular effects of selenosulfate.
In thisstudy,we synthesized selenosulfate with a modified method which used the red elemental selenium nanoparticles instead ofelementalselenium. Human promyelocytic leukemia cell line(HL60)was chosen as a model and the results showed that this reductive Se-compound induced cellapoptosis via mitochondria pathway.One potential mechanism for the cellular toxicity of selenium-containing nanoparticles was proposed.
1 Materials and Methods
1.1 Reagents
3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide(MTT),Hoechst 33342,propidium iodine(PI),rhodamine123(Rh123) were from Sigma. Mouse IgG2banti-human Bax mAb(SC-7480),and FITC-conjugated Goat IgG anti-mouse IgG mAb(ZF-0312)were from Santa Cruz.All other chemicals were of analytical grade.
1.2 Selenosulfate Solution Preparation
Sodium selenosulfate solution is usually prepared by elemental selenium and sodium sulfite[6,8].Due to low activities of the normal element selenium,we used red elemental selenium nanoparticles with huge surface area[9],to react with 2-fold sulfite(mol/mol)at 37℃for 10 min to obtain selenosulfate solution composing of selenosulfate and sulfide at 1∶1 ratio.Both separation from sulfide and long-term store may result in the decomposition of selenosulfate,so the selenosulfate solution used composed of the same concentration sulfide and must be freshly made.For nano-Se,we used the method with some modifications to eliminate BSA[9].First,we changedBSA/Se (w/w) from 10/1 to 1/1; second,we used two-cycle centrifugation and washed by distilled water to separateBSA and nano red elemental selenium. The BSA/Se in the final Nano-Se used forpreparation of selenosulfate is below 1/100,and the trace BSA in the selenosulfate solution did not cause any cytotoxicity.
1.3 Cell Culture
The human promyelocytic leukemia cell line(HL60),originated from the American Type Culture Collection,was kindly provided by Prof.Hui-tu Liu of Beijing Normal University.HL60 cells were cultured in RPMI1640(Gibco)medium supplemented with 10%(v/v)newborn calf serum(Hyclone),25 mmol/L HEPES,25 mmol/L sodium bicarbonate,100 U/mL penicillin and 100 μg/mL streptomycin at 37℃in 100%humidified atmosphere with 5%CO2.
1.4 Assessment of Cell Viability
MTT was dissolved in 0.9%NaCl with a concentration of 5.0 mg/mL.Lysing buffer was prepared as follows:20% SDS,25% DMF(N,N-dimethyl formamide),and 2.5% glacial acetic acid. Cells were seeded at5×104cells/mL in 100 μl medium in 96-well plates and were treated with different concentrations of selenosulfate for the indicated time.After the treatment,cells were incubated with 10 μl/well MTT for 4 hours and were lysed.The optical densities at 595 nm were measured using a microplatereader(Bio-Rad Lab,Richmond,CA,USA).The data were shown by means±SD(n=8).
1.5 Morphological Change in Nucleus
About 1×106cells were seeded in 3 ml medium in 35 mm cell dishes.After 24 hours of incubation,selenosulfate was added as treatment for 24 hours.Cells were then centrifuged and rinsed twice by PBS.For transmission electron microscopy(TEM)tests,cells were routinely processed and photographs(amplified 6,000×)were taken randomly.For fluorescence microscopy(FM)tests,cells were stained with 0.1 μg/mL Hoechst 33342 at 37℃for 30 min and were observed at 340 nm by fluorescence microscopy(Olympus).
1.6 DNA Integrity Test
About 1×106cells in 3 ml medium were planted in 35 mm cell dishes.After 3 hours of incubation,different concentrations of selenosulfate were added.Cellswere lysed in 6 hours and the DNA samples were extracted.The DNA samples were separated with electrophoresis in 1.2% agarose gel dyed with EB and scanned with UV Gel Analyzer.
1.7 Flow Cytometry Analysis
About 1×106cells in 3 ml medium were planted in 35 mm cell dishes.After 24 hours of incubation,different concentrations of selenosulfate were added. After 24 hours of treatments,the cells were centrifuged at 300×g at 4℃ for 5 min and rinsed twice by PBS.For the PI dyeing,the cells were fixed in 75%ethanol and kept at-20℃ overnight.Then cells were washed twice by PBS and incubated at 37℃ for 30 min with 5 μg/mL PI and 1 mg/mL RNase in PBS(Since PI can also bind to double-stranded RNA,it is necessary to treat the cells with RNase for optimal DNA resolution).Cell suspensions were analyzed by flow cytometry(CoulterEpics). For Rh123 dyeing,the cells were dyed with Rh123 at 37℃for 30 minutes,then washed by PBS and analyzed by flow cytometry(Coulter Epics).
For the immunofluorescence of Bax,the cells were fixed by 4%poly-formaldehyde at 4℃for overnight.Cells were washed twice with PBS,suspended in 1 ml PBS supplemented with 5%NCS and 0.2%Triton and incubated at 4℃for 20 min.Then the cells were washed twice with PBS and divided into two aliquots(one for the negative control,one for the Bax analysis).IgG antihuman Bax mAb wasfirstly added and incubated with cells at 4℃for 40 min.Then cells were washed twice with PBS and mouse anti-human IgG mAb was added to incubate at 4℃for 40 min.At last,cells were washed twice with PBS and analyzed by flow cytometry(Coulter Epics).
2 Results
2.1 Cell Viability Affected by Selenosulfate
As shown in Figure 1,the viability of HL60 cells significantly decreased with the increased concentration of selenosulfate.When the incubation time with selenosulfate increased,the cell viability was suppressed significantly compared to the control group.After the treatment of 10 or 15 μmol/L selenosulfate for 48 hours,there was significant difference compared to the control group.The results indicated that selenosulfate caused the decrease of cell viability probably by inhibition of cell growth or in-duction of cell death.The EC50(median effective concentration)is about 10 μmol/L under 24 hour treatment.

Fig.1 Cell viability determined by MTT assay treated with various concentrations of selenosulfate for different time as indicated OD meansthe absorbance of the product of MTT,which is proportional to the viability of cells.Date were presented asmean±SD,n=8,and "*"means significant different compared with the control group atP<0.05图1 MTT方法检测硒代硫酸钠处理细胞后的细胞活力 OD表示MTT紫色产物的吸光值,反映了细胞活力,数值越高,细胞活力越高。*P<0.05
2.2 Selenosulfate Induced Typical Apoptosis
The morphological changes of apoptotic cells were observed by transmission electron microscopy(TEM)and fluorescence microscopy(FM)with Hoechst 33342 staining.After the treatment of 10 μmol/L selenosulfatefor 24 hours,the cells showed typical condensed chromatin at the early apoptotic stage and apoptotic bodies at the later stage(Figure 2A-2,3)compared to the control cells(Figure 2A-1).The PI fluorescence treated with 20 μmol/L selenosulfate for 24 hours increased significantly as shown in Figure 2B-2 and the typical apoptotic body formed in Figure 2B-3.The condensed chromatins and the increased fluorescence indicated cell apoptosis.

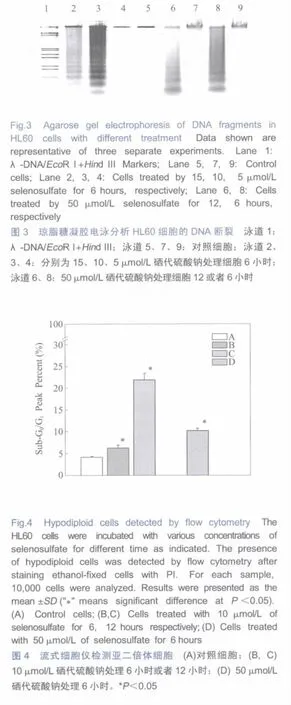
Asshown in Figure3,HL60 cellsshowed typical apoptotic DNA ladders with the treatments of selenosulfatefrom 10 μmol/L to 50 μmol/L.Theseresultsconfirmed the apoptotic death ofHL60 cells treated by selenosulfate.
In order to get the quantitative results of the apoptotic cells,theinduction of hypodiploid cellswasexamined with PI staining. The cells in sub-G0/G1peak increased with the selenosulfate treatmentdose-and time-dependently(Figure 4).Thepercentage of cellsin sub-G0/G1peak increased from 4.1%of control group to 6.3% and 23%after 6 h,12 h treatment of 10 μmol/L selenosulfate; and after treated with 50 μmol/L of selenosulfate for 6 hours,there were 10%cells presented in the sub-G0/G1peak.
2.3 Effects of Selenosulfate on Mitochondrial Membrane Potential(MMP)and Bax
The dropping of MMP is one early stage of apoptotic cells[10].Our resultsindicated that the dropping of the MMP was dose-and time-dependent upon treatment with selenosulfate(Figure 5).After treated with 10 μmol/L of selenosulfate for 6 h and for 12 h,the average MMP dropped from 13.9 of the control group to 8.8 and 6.2,respectively;and after treated with 50 μmol/L of selenosulfate for 6 hours,the MMP dropped to 3.6.
In order to evaluate the potential biochemical effects of the selenosulfate on HL60 cells,we monitored the change of immunofluorescence of Bax with flow cytometry(FCM).The FS-SS graphs were shown in Figure 6A(Forward Scatter is a rough indicator of a cell's size.Side Scatter is a rough indicator of cellular granularity,membrane complexity,number of organelles).Apart from the group 1 cells which is similar to the control cells,one new group separated to the right-down of group 1 as Group 2 in the selenosulfate-treated cells.With the help of the analyzing softwareof FCM,wefound that the Group 2 represented for the shrunk cells which had entered the progress of apoptosis;while the Group 1 represent for the normal cellswhich did not enter the apoptotic program.

Fig.5 Mitochondria membrane potential of cells indicated by the immunofluorecence of Rh123 staining measured with flow cytometry (A)Control cells;(B,C)Cells treated with 10 μmol/L of selenosulfate for 6,12 hoursrespectively;(D) Cells treated with 50 μmol/L ofselenosulfate for 6 hours.Date were presented as mean±SD,n=3."*"means significant different compared with the control group atP<0.05图5 罗丹明123染色分析线粒体膜电位 (A)对照细胞;(B,C)10 μmol/L硒代硫酸钠处理 6小时或者 12小时;(D)50 μmol/L硒代硫酸钠处理6小时。*P<0.05

Up regulation of Bax is one indicator for the induction ofapoptosis[11].Immunofluorescence ofBax was monitored as shown in Figure 6B.Cells treated with selenosulfate showed two immunofluorescencepeaks.It was found that the two peaks represented for their counterparts in Fiugre 6A.These results indicated that HL60 cells had two stages after the treatment of selenosulfate:at the first stage,the cells did not entered the apoptotic program(Group 1 cells)but the expression of the protein Bax in cytosol increased indicated by the higher immunofluorescence(Figure 6B);while atthe second stage,the cells executed the apoptotic program(Group 2 cells)and the protein Bax transported to the mitochondria which resulted in the reduced immunofluorescence(Figure 6B)in cytosol[12,13].
3 Discussion
The cytotoxicity of selenium has been studied extensively and the mechanism is mainly through oxidative stress induced by over-dosed selenium[14].Toxicological studies have shown thatselenium compound could induce cell death:in primary murine hepatocytes,EC50 was 20 μmol/L for selenite,270 μmol/L for selenate,and 30 μmol/L for Se-methionine.Similar results were found in murine Hepa1-6 cells[15].Sulfite has little effect on cell viability below 100 μmol/L[16,17]but10 μmol/L selenosulfate solution has significant effects of induction of apoptosis on HL60 cells in our study,which indicates that the effect of the selenosulfate solution comes mainly from selenosulfate or the Se2-anion.In our study,we showed that the EC50 for selenosulfate is about 10 μmol/L(Figure 1),lower than that of selenite and Se-methionine[15].According to our results,selenosulfate caused the decrease ofthe mitochondrialmembrane potential,implying selenosulfate induced the apoptosis via the mitochondria pathway.
The reaction of beef kidney rhodanese with selenosulfate wasstudied by Cannella[18].They identified that the productin this reaction was a cysteinyl-selenium derivative.These resultsindicated that the non-specific exchanges of S and Se in the functional proteins containing S may occur and disturb the cell viability.Thiol is important for the redox regulation of cell viability and the functions of many proteins,and thiol modification could regulate many pathway of signal transduction such as:S-nitrosation,S-thiolation,glutathionylation[19,20].The modification or replacement of S by Se might be one explanation for the regulation effects of selenium on cell functions.
Selenosulfate was found as a main composition in the rootexudates in a known selenium accumulator model plant[21],which indicated that selenosulfate could be produced byin vivo metabolism of selenium.And in the“zinc-specific autometallographic in vivo selenium methods”[22],sodium selenite or selenosulfate is proved to have the ability to generate ZnSe or ZnS nanocrystalsin vivo.Although the method is used to identify so-called zinc-enriched(ZEN)neurons,their study implies that the Se-containing nanoparticles may be generatedin vivoby selenium treatment.
In the study of the cytotoxicity of nanoparticles containing selenium such as quantum dots CdSe,the release of Cd2+is important for the cytotoxicity of CdSe nanoparticles.In the mean time,SeO2is proposed to be produced.Therefore,there is the possibility that Se2-ions could be released originally and cause cell apoptosis through inducing reactive oxygen species[14,23].In the present study,we found that thetoxicity may comefrom Se2-atom as well,which suggests that the release of Se2-be another main contributor to the cytotoxicity of Se-containing nanoparticles such as quantum dots CdSe.
Acknowledgment Thank Lin Lifor discussion and valuable advice.
1. Service RF.American Chemical Society meeting.Nanomaterials show signs of toxicity.Science,2003,300(5617):243
2. Service RF.Nanotechnology.Calls rise for more research on toxicology of nanomaterials.Science,2005,310(5754):1609
3. Alivisatos AP,Gu W,Larabell C.Quantum dots as cellular probes.Annu Rev Biomed Eng,2005,7:55~76
4.Derfus AM,Chan WCW,Bhatia SN.Probing the cytotoxicity of semiconductor quantum dots.Nano Lett,2004,4(1):11~18
5. Kirchner C,Liedl T,Kudera S,Pellegrino T,MunozJavier A,Gaub HE,Stolzle S,Fertig N,Parak WJ.Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles.Nano Lett,2005,5(2):331~338
6. Yochelis S,Hodes G.Nanocrystalline CdSe formation by direct reaction between Cd ions and selenosulfate solution.Chem Materials,2004,16(14):2740~2744
7. Pejova B,Grozdanov I.Chemical deposition and characterization of Cu3Se2 and CuSe thin films.J Solid State Chem,2001,158(1):49~54
8. HodesG,Grunbaum E,FeldmanY,BastideS,Levy-Clement C. Variable optical properties and effective porosity of CdSe nanocrystalline films electrodeposited from selenosulfate solutions.J Electrochem Soc,2005,152(12):G917~G923
9. Huang B,Zhang J,Hou J,Chen C.Free radical scavenging efficiency of Nano-Sein vitro.Free Radic Biol Med,2003,35(7):805~813
10.KuroseI,Higuchi H,WatanabeN,MiuraS,TomitaK,Yonei Y,Takaishi M,Zeki S,Nakamura T,Saito H,Kato S,Ishii H.CD18/ICAM-1-dependent nitric oxide production of Kupffer cells as a cause of mitochondrial dysfunction in hepatoma cells:influence of chronic alcohol feeding.Free Radic Biol Med,1997,22(1-2):229~239
11.Yin C,Knudson CM,Korsmeyer SJ,Van DT.Bax suppresses tumorigenesis and stimulates apoptosis in vivo.Nature,1997,385(6617):637~640
12.Jia L,Patwari Y,Srinivasula SM,Newland AC,Fernandes-Alnemri T,Alnemri ES,Kelsey SM.Bax translocation is crucial for the sensitivity of leukaemic cells to etoposide-induced apoptosis. Oncogene,2001,20(35):4817~4826
13.Murphy KM,Ranganathan V,Farnsworth ML,Kavallaris M,Lock RB.Bcl-2 inhibits Bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells.Cell Death Differ,2000,7(1):102~111
14.Stewart MS,Spallholz JE,Neldner KH,Pence BC.Selenium compounds have disparate abilities to impose oxidative stress and induce apoptosis.Free Radic Biol Med,1999,26(1-2):42~48
15.WeillerM,Latta M,Kresse M,LucasR,Wendel A.Toxicity of nutritionally available selenium compounds in primary and transformed hepatocytes.Toxicology,2004,201(1-3):21~30
16.Marshall KA,Reist M,Jenner P,Halliwell B.The neuronal toxicity of sulfite plus peroxynitrite is enhanced by glutathione depletion:implicationsfor Parkinson'sdisease.Free Radic Biol Med,1999,27(5-6):515~520
17.Labbe P,Pelletier M,Omara FO,Girard D.Functional responses of human neutrophils to sodium sulfite(Na2SO3)in vitro.Hum Exp Toxicol,1998,17(11):600~605
18.CannellaC,Pecci L,Finazzi AA,Federici G,PensaB,Cavallini D.Selenium binding to beef-kidney rhodanese.Eur J Biochem,1975,55(1):285~289
19.Moran LK,GutteridgeJM,Quinlan GJ.Thiolsin cellular redox signalling and control.Curr Med Chem,2001,8(7):763~772
20.Georgiou G.How to flip the (redox)switch.Cell,2002,111(5):607~610
21.Vonderheide AP,Mounicou S,Meija J,Henry HF,Caruso JA,Shann JR.Investigation ofselenium-containing root exudates ofBrassica juncea using HPLC-ICP-MS and ESI-qTOF-MS.Analyst,2006,131(1):33~40
22.Danscher G,Stoltenberg M.Zinc-specific autometallographic in vivo selenium methods:tracing of zinc-enriched (ZEN)terminals,ZEN pathways,and pools of zinc ions in a multitude of other ZEN cells.J Histochem Cytochem,2005,53(2):141~153
23.Lovric J,Cho SJ,Winnik FM,Maysinger D.Unmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell death.Chem Biol,2005,12(11):1227~1234
硒代硫酸钠诱导HL60细胞凋亡——还原态硒的细胞毒性
黄 波1,2,张劲松3,陈 畅1
1.中国科学院生物物理研究所,北京 100101;
2.中国科学院研究生院,北京 100049;
3.中国科学技术大学,合肥 230052
2010-03-12;接受日期:2010-03-31
国家自然科学基金委重大研究计划面上项目(90606020)
陈畅,电话/传真:(010)64888406,E-mail:changchen@moon.ibp.ac.cn
细胞毒性研究认为Cd2+的释放是硒化镉(CdSe)纳米粒子的细胞毒性机制之一,而Se2-阴离子在纳米粒子中的毒性机制未知。作者研究了硒代硫酸钠(selenosulfate(SSeO3)2-)对HL60细胞的细胞毒性作用,发现10 μmol/L的硒代硫酸钠可以显著抑制细胞活力,诱导细胞凋亡,出现了染色质凝聚、DNA ladder和G0/G1凋亡亚峰。线粒体膜电位显著降低的同时,促凋亡蛋白Bax的免疫荧光增加。结果表明还原态的Se2-阴离子有显著的细胞毒性作用,可以诱导HL60细胞凋亡。同时也暗示Se2-阴离子的释放可能是含Se2-纳米粒子(比如硒化镉的量子点)细胞毒性的机制之一。
硒代硫酸钠;纳米粒子;硒;凋亡;量子点
Q26
Mar 12,2010 Accepted:Mar 31,2010
CHEN Chang,+86(10)64888406,E-mail:changchen@moon.ibp.ac.cn

