一维链状锰配位聚合物[Mn(1,5-nds)(Him)4]n的合成、晶体结构及热稳定性研究
钱保华 马卫兴 陆路德 杨绪杰 汪 信
(1淮海工学院化学工程学院,连云港 222005)(2南京理工大学材料化学实验室,南京 210094)
研究简报
一维链状锰配位聚合物[Mn(1,5-nds)(Him)4]n的合成、晶体结构及热稳定性研究
钱保华1马卫兴1陆路德*,2杨绪杰2汪 信2
(1淮海工学院化学工程学院,连云港 222005)(2南京理工大学材料化学实验室,南京 210094)
锰配合物;配位聚合物;1,5-萘二磺酸根;晶体结构;热稳定性
0 Introduction
Coordination polymers,consisting of metal-ligand coordination compounds,have drawn enormous interests in recent years due to their diverse structural topologies and potential applications in areas of catalysis and materials science[1-4].The connection of metal-organic coordination polymers based on transition metals andmultifunctional bridging ligands has proven to be a promising field[5].Hence,proper selection of metal ions and ligands is crucial to the construction of coordination polymers[6-9].Arenesulfonate anions are widely used industrially as surfactants and dyes[10].There are a few researches of transition metal with arenesulfonate have achieved in the last years[11].Therefore,its coordination chemistry remains largely unexplored compared with the well studied metal phosphates[12-13].Generally,sulfonate group shows weak coordination ability with transition metals,whereas extensive studies have demonstrated that the coordination behavior of arenesulfonates with transition metals can be tailered by decorating the metal center with other ligands[14-16].For example 1,3-bis(benzimidazol-l-yl-methyl)-2,4,6-trimethylbenzene(mbbimb)used to form[Mn(mbbimb)2(1,5-nds)]npolymer[17].In our efforts to investigate the coordination behavior of arenesulfonates with transition metals,we have chosen imidazole as auxiliary ligand to react with cadmium naphthalene-1,5-disulfonate,resulting in a novel Cd polymer,{[Cd(1,5-nds)(Him)2(H2O)]·2H2O}n[18].Herein we report the synthesis,crystal structure and thermal stability of a novel one-dimensional manganesecomplex[Mn(1,5-nds)(Him)4]n(1,5-nds is naphthalene-1,5-disulfonate and Him is imidazole).
1 Experimental
1.1 Materials and instruments
All reagents and solvents were used as commercial source without further purification.Crystal structure determination was carried out on a Bruker SMART 1000 CCD X-ray diffractometer.Elemental analyses for C,H and N were performed on a Perkin-Elmer 240C analyzer.IR spectra were obtained for KBr pellets on a Shimadzu IRPrestige-21 spectrophotometerin the 4000~400 cm-1region.Thermal analysis was performed on a Q50 thermal analyzer(TA)in flowing nitrogen gas at a heating rate of 10 ℃·min-1.
1.2 Synthesis of the title compound
To a stirred solution of 1,5-naphthalenedisulfonic acid tetrahydrate(C10H8O6S2·4H2O,0.72 g,2.0 mmol)in water(20 mL),0.23 g MnCO3(2.0 mmol)was added with heating in 60 ℃,until all of the solid dissolved.To this solution was added imidazole (C3H4N2,0.27 g,4.0 mmol)in methanol(10 mL)with heating and stirring for another 2 h.The solution was left to crystallize at room temperature,colorless prismatic crystals was separated after two weeks (yield 62%).Anal.Calcd.(%)for C22H22MnN8O6S2:C 43.07,H 3.61,N 18.26;found(%):C 43.11,H 3.66,N 18.27.IR:3 460(vs),3 278(vs),3 140(vs),1628(s),1536(s),1499(s),1433(m),1327(s),1206(vs),1 101(m),1 063(s),1 040(vs),937(m),764(s),646(s),604(vs),529(s)cm-1.
1.3 Structure determination and refinement
A suitable single crystal with dimensions of 0.47 mm×0.43 mm×0.40 mm was put on a Bruker SMART 1000 CCD diffractometer equipped with a graphitemonochromatized Mo Kα radiation(λ=0.071073 nm)by using a φ/ω scan mode at 298(2)K.Out of the total 3 495 reflections collected in the range of 2.39°≤θ≤25.00°,2 332 were independent with Rint=0.042 8,of which 1 772 were considered to be observed (I>2σ(I))and used in the succeeding refinement.An empirical absorption correction was applied.The crystal structure was solved with direct methods and refined using the program SHELXTL[19].All the non-hydrogen atoms were located from the direct method and then refined anisotropically with SHELXTL using full-matrix leastsquares techniques on F2.The hydrogen atom positions were fixed geometrically at calculated distances and allowed to ride on the parent atoms.A full-matrix leastsquares refinement gave the final R=0.050 2,wR=0.125 7(w=1/[σ2(Fo2)+(0.059 7P)2+0.401 3P],where P=(Fo2+2Fc2)/3),(Δ/σ)max=0.000,S=1.047,(Δρ)max=497 e·nm-3and (Δρ)min=-391 e·nm-3.Crystallographic data and selected bond lengths and angles are summarized in Table 1 and 2.
CCDC:773063.
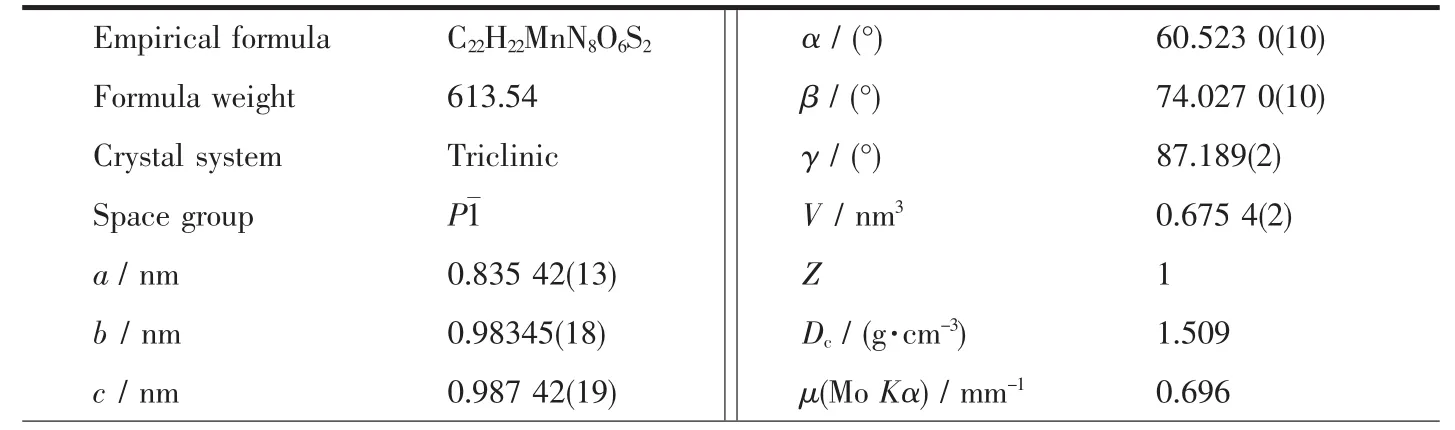
Table 1 Crystal data and structure refinement parameters for title complex
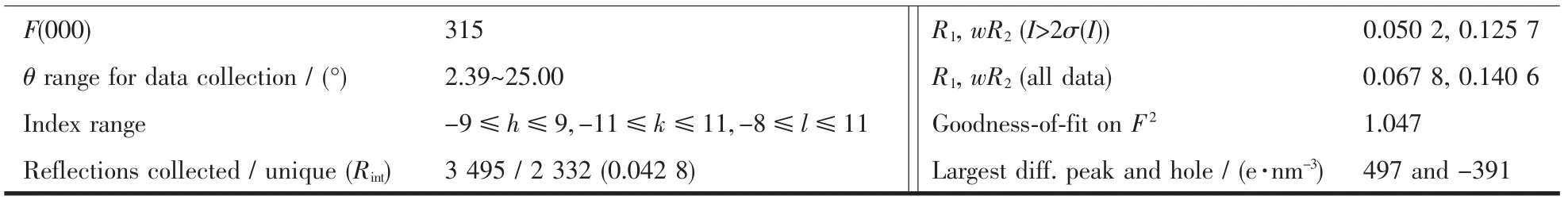
Continued Table 1
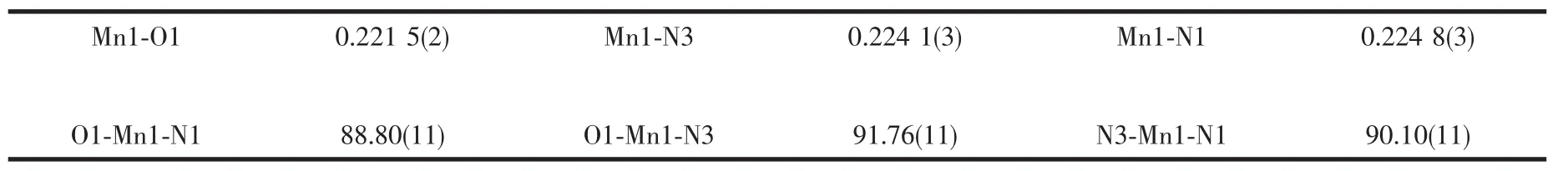
Table 2 Selected bond lengths(nm)and angles(°)for title complex
2 Results and discussion
2.1 IR spectrum
The strong peak at 3226 cm-1is obviously from the N-H stretching vibration of Him.Peaks at 1 198 and 608 cm-1could be assigned to characteristic peaks of the-SO3-groups[20],and the characteristic peaks at 3 140,1 536 and 765 cm-1may be assigned to Him ligand.Compared to free Him (3 126,1 544 and 758 cm-1),these peaks shift to some extent,suggesting that Him has participated in coordination[21],which is consistent with the structural analysis.
2.2 TG analysis
The thermal behavior of the title complex was performed in the temperature range 20~700 ℃ under nitrogen stream.The TG curve showed that the complex is thermally stable up to 250℃and then undergoes a three stage decomposition process.In the first stage,the complex starts to lose the two imidazole ligands between 250 and 300℃,with an experimental mass loss of 24.00%(Calcd.22.19%).The second stage in the temperature range 300~500℃ is related to the release of the remaining two imidazole ligands,giving loss 21.50%(Calcd.22.19%).In the last stage,a quick decomposition was finished at ca.530 ℃ with a total weight loss of 87.94%(Calcd.88.44),suggesting that MnO is the end product.
2.3 Structure description
Structural analysis revealed that the title complex consists of 1D coordination chains with 1,5-nds bridges.The structure of a portion of polymer is shown in Fig.1.The central Mnion assumes the octahedral geometry completed by four nitrogen atoms from four imidazole ligands in the equatorial positions and two oxygenatoms from half of the two 1,5-nds ligands in the axial positions.The Mn-O bond distance is 0.221 5(2)nm and Mn-N bond distances are 0.224 1(3)and 0.2248(3)nm,which are typical of those found in sixcoordinate manganesecomplexes with O-and N-ligation[22-23].The square planar[Mn(Him)4]2+units are linked by 1,5-nds which act as bidentate bridging ligands,leading to the forming of a neutral one-dimensional linear chain polymer with Mn…Mn distance of 1.14499(12)nm along the[111]direction.
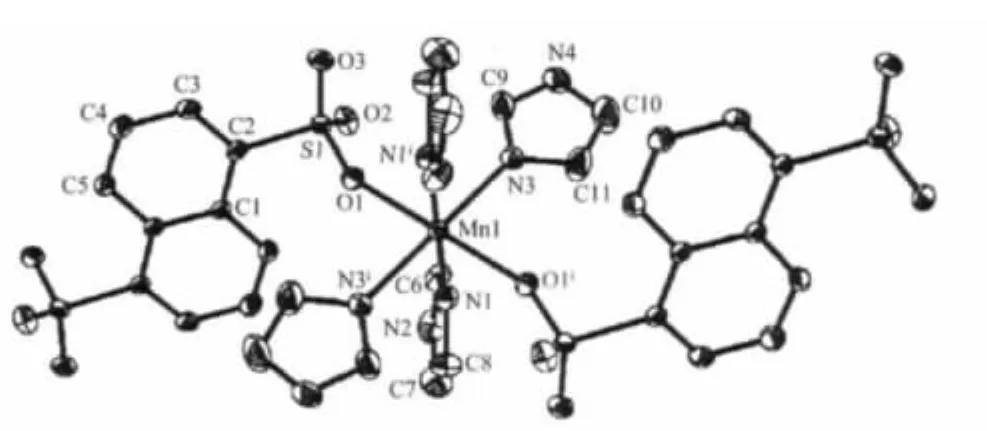
Fig.1 Coordination environment of the metal centers in the complex,displacement ellipsoids are drawn at the 30%probability level,hydrogen atoms are omitted for clarity
There are significant interchain hydrogen-bonding and C-H…π interactions involving the-SO3-groups and imidazole ligands(Table 3).The hydrogen bonds between O2 and N2[O2…N2 0.279 9(5)nm]associate the chains into two-dimensional layers extending in the(0 1 1)planes (Fig.2a).Furthermore,the layers are linked to form overall a three-dimensional framework structurethrough H-bonds[O3…N4 0.287 6(4)nm]between adjacent 2D layers (Fig.2b).The interchain C-H…π interactions involving mainly the imidazole ligands may be important in stabilizing the structure(Fig.3).
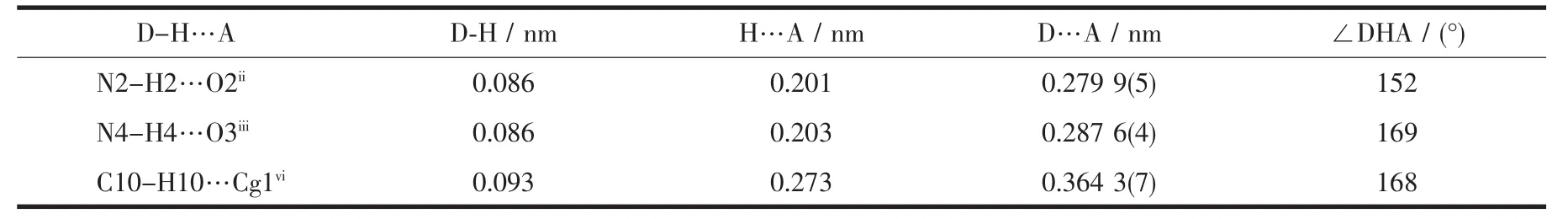
Table 3 Hydrogen bond lengths and bond angles

Fig.2(a)Hydrogen bonded network of title complex,(b)View of a section of the 1D chain of title complex

Fig.3 View of C-H…π weak interaction showing as dashed lines
3 Conclusion
In this paper,we have prepared a novel onedimensional manganesecoordination polymer bridged by 1,5-nds,which is assembled into a three-dimensional supramolecular architecture via hydrogen bonds and C-H…π interactions.The successful synthesis demonstrates that arenesulfonate ligands may provide multiple binding forces such as coordinate covalent,hydrogen-bonding and C-H…π weak interactions which may endow enormous potential for assembling multidimensional supramolecular architectures.
[1]Jacob W,Mukherjee R.Inorg.Chim.Acta,2008,361:1231-1238
[2]Hill R J,Long D L,Champness N R,et al.Acc.Chem.Res.,2005,38:337-350
[3]Champness N R.Dalton Trans.,2006:877-880
[4]Biradha K,Sarkar M,Rajput L.Chem.Commun.,2006:4169-4179
[5]Steel P J.Acc.Chem.Res.,2005,38:243-250
[6]Baten S R,Hoskins B F,Robson R.J.Am.Chem.Soc.,1995,117(19):5385-5386
[7]Gardner G B,Venkataraman D,Moore J S,et al.Science,1995,374:792-795
[8]Hoskins B F,Robson R.J.Am.Chem.Soc.,1990,112(4):1546-1554
[9]Xu H T,Zheng N W,Xu H H,et al.J.Mol.Struc.,2002,610:47-52
[10]Gunderman B J,Kabell I D,Squattrito P J,et al.Inorg.Chim.Acta,1997,258:237-246
[11]Natarajan S,Mahata P.Current Opinion in Solid State and Materials Science,2009,13:46-53
[12]Shubnell A J,Kosnic E J,Squattrito P J.Inorg.Chim.Acta,1994,216:101-112
[13]Cai J W,Chen C H,Liao C Z,et al.J.Chem.Soc.,Dalton Trans.,2001:1137-1142
[14]Chen C H,Cai J W,Liao C Z,et al.Inorg.Chem.,2002,41:4967-4974
[15]Wen L L,Li Y Z,Lu Z D,et al.Cryst.Growth Des.,2006,6:530-537
[16]CAI Ji-Wen(蔡继文),CHEN Cai-Hong(陈彩虹),ZHOU Jin-Sen(周金森).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2003,19(1):81-85
[17]Zhou H,Du S W,Li Z H.Chinese J.Struct.Chem.,2007,26:929-932
[18]Qian B H,Ma W X,Xu X Y,et al.Chinese J.Struct.Chem.,2008,27:1533-1537
[19]Sheldrick G M.SHELXTL 6.12 for Windows NT:Structure Determination Software Programs,Bruker AXS Inc.
[20]Smith G,Cloutt B A,Lynch D E,et al.Inorg.Chem.,1998,37(13):3236-3242
[21]Carolina A P,Elena B A,Duane C L,et al.Chem.Commun.,2006,9(9):903-906
[22]Papaefstathiou G S,Escuer A,Mautner F A,et al.Eur.J.Inorg.Chem.,2005:879-893
[23]Stoumpos C C,Gass I A,Milios C J,et al.Inorg.Chem.Commun.,2008,11:196-202
Synthesis,Crystal Structure and Thermal Stability of 1D Manganese Coordination Polymer[Mn(1,5-nds)(Him)4]n
QIAN Bao-Hua1MA Wei-Xing1LU Lu-De*,2YANG Xu-Jie2WANG Xin2
(1School of Chemical Technology,Huaihai Institute of Technology,Lianyungang,Jiangsu 222005)(2Materials Chemistry Laboratory,Nanjing University of Science&Technology,Nanjing 210094)
The synthesis,structural characterization and thermal behavior of a novel one-dimensional manganesecomplex of formula [Mn(1,5-nds)(Him)4]n[1,5-nds=naphthalene-1,5-disulfonate,Him=imidazole]is reported.The complex crystallizes in the triclinic system,space group P1,with a=0.83542(13)nm,b=0.98345(18)nm,c=0.987 42(19)nm,α=60.523 0(10)°, β=97.748(2)°,γ=87.189(2)°and Z=2.The 1,5-nds ligand assumes the μ2coordination mode and interlinks Mnions into infinite one-dimensional chain structure along [111]direction,with the adjacent Mn…Mn distance being 1.14499(12)nm.The chains are assembled into a three-dimensional supramolecular architecture via hydrogen bonds and C-H…π interactions.IR spectra and thermal analysis data are in agreement with the crystal structure.CCDC:773063.
manganesecomplex;coordination polymer;1,5-naphthalenedisulfonate;crystal structure;thermal stability
O614.7+11
A
1001-4861(2010)07-1289-05
2009-10-13。收修改稿日期:2010-03-03。
江苏省高校科研成果产业化推进项目(No.JHZD07-038)资助。
*通讯联系人。 E-mail:Lldnju@yahoo.com.cn
钱保华,男,49岁,博士,教授;研究方向:功能配合物。

