热分解法制备Y2O2S∶Eu3+纳米粒子
谭宁会 王园媛 廖佩坤 刘艺成 孟建新 刘应亮
(广州市暨南大学化学系暨南大学纳米化学研究所,广州 510632)
热分解法制备Y2O2S∶Eu3+纳米粒子
谭宁会 王园媛 廖佩坤 刘艺成 孟建新 刘应亮*
(广州市暨南大学化学系暨南大学纳米化学研究所,广州 510632)
采用热分解法和硫熔法分别合成了纳米Y2O2S∶Eu3+和体相Y2O2S∶Eu3+。其中硫氧化钇纳米粒子的制备是以水热法合成的Y(OH)3为前驱体,随后在激活剂和硫的共同作用下焙烧得到的。结果表明,所得Y2O2S∶Eu3+为单一纯相纳米粒子,粒径分布集中,大小约80 nm,而前驱体Y(OH)3为纳米棒状,形貌上的这一巨大变化是由激活剂和硫粉在高温煅烧过程所形成的熔融物的腐蚀作用造成的。荧光光谱分析表明,Eu3+能有效地掺入硫氧化钇基质中,并具有良好的发光性能。此外,还探讨了纳米粒子的形成机理。
硫氧化钇;纳米粒子;发光性能;机理
0 Introduction
Rare earth oxysulfide materials often exhibit high luminescence efficiency.These materials are widely used as luminescent host materials such as red emitting phosphors for color television picture tube and long afterglow materials[1].Coarser particles of rare earth oxysulfide phosphors obtained from conventional hightemperature solid state reaction can not meet the requirements for higher resolution image rendering devices.The fabrication of Y2O2S∶Eu3+nano-crystals has attracted much attention recently.Jagannathan et alsynthesized nano-crystal Y2O2S∶Eu3+by two step sol-gel polymerthermolysismethod[2-6]and hydrothermal method[7].Pires et al[8]prepared Y2O2S ∶Yb(4%),Er(0.1%)and Y2O2S∶Yb(4%),Tm(0.1%)from polymeric and basic carbonate precursors.Besides,an ethanol assisted combustion synthesis method was adopted to prepare Y2O2S∶Eu3+nano-crystals by Luo et al[1].But the products are either seriously aggregated or still big in particle size.Although Fu et al[9]obtained welldispersed Y2O2S∶Eu3+nanocrystals with a combustion method by adding polyvinyl alcohol(PVA)to prevent the particles from aggolomeration.The raw material CS(NH2)2they used is toxic.The fabrication of nanocrystals Y2O2S∶Eu3+with small size remains a challenge.To the best of our knowledge,there has been no detail information on the growth mechanism of Y2O2S∶Eu3+nano-crystals.
In our previous work,long-lasting phosphorescent material Y2O2S∶Eu3+,Ti4+[10]and Y2O2S∶Eu3+,Mg2+,Ti4+[11]were prepared by co-precipitation method and hydrothermal-microwave method,respectively.In this work,Y2O2S∶Eu3+nano-crystals were synthesized through hydrothermal method followed by subsequent thermal decomposition in presence of sulfur.The experimental results confirm that the Y2O2S∶Eu3+nanocrystals are narrow distributed small particles with an average size of 80 nm.The mechanism is discussed too.
1 Experimental
1.1 Synthesis of Eu3+do ped yttrium oxysulfide nano-crystals
The main starting materials were Y2O3(99.99%),Eu2O3(99.99%)and sulfur(A.R.grade).In a typical synthesis,Y2O3was dissolved in nitric acid(65%)to get a clear solution and the pH value was adjusted to 12~13 by adding NaOH solution(5 mol·L-1)under vigorous stirring.The mixture was then transferred to Teflon lined stainless autoclave and heated at 180℃ for 12 h.A white precipitate was collected,washed with distilled water and ethanol for several times,and dried in air at 60℃.Then the as prepared Y(OH)3nano-rod was mixed with certain amount of Eu2O3,Na2CO3and sulfur,in which Y2O3,S and Na2CO3with a mass ratio of 10∶3∶3 while Y3+and Eu3+with a molar ratio of 1 ∶0.03.The mixture was thoroughly mixture and ground then heated in muffle furnace at 850℃for 5 h under carbon monoxide atmosphere.Then the products were dipped in dilute HCl(0.1 mol·L-1)for about 1 h and washed with distilled water and ethanol for several times.For comparison,bulk Y2O2S∶Eu3+was synthesized by conventional sulfur flux method[9].
1.1 Characterization
X-ray power diffraction(XRD)data of the products were collected at room temperature using MSAL-XRD2 automatic X-ray Diffractometer with Cu Kα(40 kV,20 mA, λ =0.154 06 nm)and a scanning rate of 8°·min-1.Transmission electron microscopy(TEM)was used to detect the shape of the products,using an accelerating voltage of 100 kV.Samples for TEM were prepared by dropping a diluted suspension of the sample powders onto a standard carbon-coated film on a copper grid and air-dried.Photoluminescence measurements were performed on a Hitachi F-4500 Fluorescence spectrophotometerequippedwitha150Wxenonlampas theexcitationsourceatroomtemperature.
2 Results and discussion
2.1 Crystal structure and morphology of Y(OH)3 precursor

Fig.1 XRD patterns of Y(OH)3
The XRD pattern of the precursor after hydrothermal route is shown in Fig.1.The well developed sharp diffraction peaks and flat baseline indicate good crystallinity of the powder.All the peaks can be indexed into hexagonal structure,and the lattice parameters are calculated from the pattern with a=6.026 1 nm and c=3.544 nm,in good agreement with the standard powder diffraction file,PDF#24-1422.No peaks attributable to other products are identified,suggesting that a pure phase of hexagonal Y(OH)3powder is prepared by hydrothermal route.
As can be seen from Fig.2a,Y(OH)3powder with uniform shape and size is obtained by the hydrothermal method,showing the advantage of this method in preparing particles with uniform size.All the particles show rod-like shape,and the diameter is 70~80 nm.

Fig.2 TEM images of Y(OH)3(a),Y2O2S:Eu3+nano-crystals(b)and bulk Y2O2S∶Eu3+(c)
2.2 Phase identification of Y2O2S∶Eu3+
Fig.3 shows the XRD patterns of bulk and nanocrystalline Y2O2S∶Eu3+.Both of the patterns for bulk and nanocrystalline Y2O2S∶Eu3+are different from the Y(Oh)3precursor shown in Fig.1,suggesting a new phase.Another hexagonalstructure with lattice parameter a of 0.385 2 nm and c of 0.666 7 nm could be indexed from the pattern,which is in good consistence with the standard XRD data of Y2O2S(PDF#24-1424).No diffraction peaks attributable to other products are observed,indicating high purity of the products.Na2CO3servesasco-activaterand the byproducts are washed away completely.The XRD pattern shows sharp diffraction features in bulk Y2O2S∶Eu3+but much broader and less intense peaks in Y2O2S∶Eu3+nano-crystals because of their much smaller grain size and lower degree of crystallinity.
2.3 Morphology and growth mechanism of Y2O2S∶Eu3+nano-crystals
Transmission electron microscope images of nanocrystalline and bulk Y2O2S∶Eu3+are shown in Fig.2(b,c).As estimated from the TEM micrograph,the diameter of the bulk Y2O2S∶Eu3+prerared by conventional method are several microns while the diameter of Y2O2S∶Eu3+nanocrystals prerared by hydrothermal method are 70~80 nm,correspounding to the size of the precursor Y(OH)3.However,in this case,quite different from our expectance,the rod-like structure of the precursor Y(OH)3is broken,and uniform particles are obtained.This indicates a tremendous shape change from the precursor to the product.This may result from the unstablility of the precursor Y(OH)3or the etching effect of the flux formed by the alkali carbonates and sulfur powder during the calcination.Perhaps both factors are functioning synergistically.
Further exprements reveal that the shape change from the precursor to the product is attributed to the etching effect of the flux.If the precursor is heated alone without the fiux at 850℃or higher for 5 h,the Y2O3obtained is nano-rod too(Fig.4b),indicating that the shape change is not caused by the decomposition of the precursor.If the mixture is heated at lower tempreture,rod-like morphology of the precursor is partly preserved.The morphology of the final product in this case is neither rod-like nor nano-particles,but rod-like with some crevasses(Fig.4c).Therefore we suggest that the flux of Na2CO3and sulfurcorruptthe precursor[9].The thermolysis reaction proceeds as follows:

Fig.3 XRD patterns of Y2O2S∶Eu3+

Fig.4 TEM images of Y(OH)3(a),Y2O3(b),800 ℃ 2 h for intermediate(c)and Y2O2S∶Eu3+(d)
Y(OH)3+Eu2O3+flux(Na2CO3+S)→Y2O2S∶Eu3++flux residue(Na2Sx+Na2SO4)+byproducts(H2S+H2O+SO2+···)
The flux corrupts the precursor Y(OH)3and then crevassesare formed.Astime passses,rod-like structure is broken,nano-particles are formed.When the products are dipped in dilute HCl,flux residue is dissoved.All the byproducts are washed away by distilled water and anhydrous ethanol,only Y2O2S∶Eu3+nano-particles are left.This result is in accrodance with the XRD and TEM analysis.No impurity phases are detected,and the particles are well-dispersed(Fig.4d).According to the literature[12],different flux will produce oxysulfide with different shapes.A scheme for the formation of spherical particles is illustrated in Fig.5.

Fig.5 A scheme for a nanorod to nano-crystals conversion by the etching of alkali melt during the calcination
2.4 Luminescent spectra analysis
The excitation spectrum was performed by monitoring the emission of Eu3+5D0→7F2transition at 626 nm.There are two charge transfer(CT)bands in the excitation spectra for Y2O2S∶Eu3+from Fig.6a,the one around 280 nm attributes for S2-→Eu3+while the other around 346 nm attributes for O2-→Eu3+[6].The emission intensities caused by O2-→Eu3+are much stronger than S2-→Eu3+.Upon excitation at 314 nm,the emission spectra of the products are obtained(Fig.6b).The strong red-emission lines at 626 nm and 614 nm are due to the transition from5D0→7F2level of Eu3+and its strongest peak is at 626 nm.Other transition from the5DJ(J=0,1)excited levels to7FJ(J=0,1,2,3,4,5,6)ground states are very weak.Both the excitation peaks and the emission peaks are amost the same in position for bulk and nanocrystalline Y2O2S:Eu3+but all the peaks of nanocrystalline Y2O2S∶Eu3+are much less intense than the uncoated Y2O2S∶Eu3+,which may result from the great demission of the particle size.
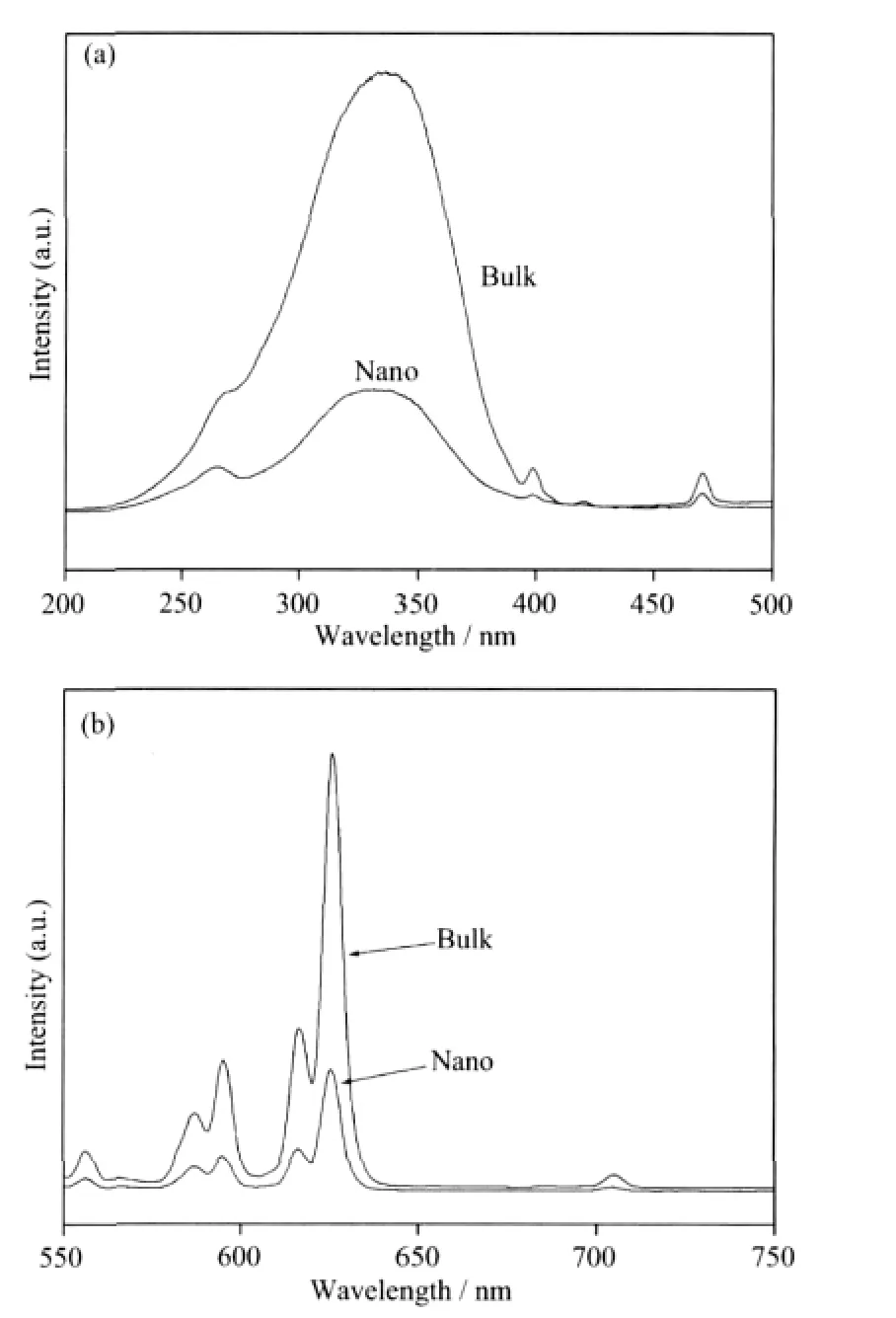
Fig.6 (a)Excitation and(b)emission spectra of Y2O2S∶Eu3+
3 Conclusions
In summary,well-dispersed Y2O2S∶Eu3+nanocrystals were synthesized through hydrothermal method followed by thermal decomposition in presence of sulfur.The results indicate that both bulk and nanocrystals Y2O2S∶Eu3+are in pure phase,hexagonal crystal structure.The hydrothermally prepared Y(OH)3reveals a rod-like morphology,while the calcined Y2O2S∶Eu3+are narrow distributed nano-crystals about 80 nm.The morphological change can be explained by the etching effect of the melt formed by the carbonate and S powder during the high temperature calcination.It is therefore concluded that Eu3+could be effectively incorporated into yttrium oxysulfides,resulting in good luminescent properties from the excitation and emission spectra.Besides,the mechanism is discussed too.Hydrothermal method offers a very simple way of fabricating wellcrystallized nano-crystals.
[1]Luo X X,Cao W H,Xing M M.J.Rare Earths,2006,24:20-24
[2]Dhanaraj J,Jagannathan R,Trivedi D C.J.Mater.Chem.,2003,13:1778-1782
[3]Dhanaraj J,Geethalakshmi M,Jagannathan R,et al.Chem.Phys.Lett.,2004,387:23-28
[4]Thirumalai J,Jagannathan R,Trivedi D C.J.Lumin.,2007,126:353-358
[5]Nakkiran A,Thirumalai J,Jagannathan R.Chem.Phys.Lett.,2007,436:155-161
[6]Thirumalai J,Chandramohan R,Sekar M,et al.J.Nanopart.Res.,2008,10:455-463
[7]Thirumalai J,Chandramohan R,Auluck S,et al.J.Colloid Interface Sci.,2009,336:889-897
[8]Pires A M,Serra O A,Davolos M R.J.Alloys Compd.,2004,374(1/2):181-184
[9]Fu Z L,Geng Y,Chen H W,et al.Opt.Mater.,2008,31:58-62
[10]HE Gan-Wu(贺干武),LIU Ying-Liang(刘应亮),ZHANG Jun-Wen(张俊文).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2007,23(2):315-318
[11]LI Wen-Yu(李文宇),LIU Ying-Liang(刘应亮),Al Peng-Fei(艾鹏飞).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24(5):772-776
[12]Hang T,Liu Q,Mao D L,et al.Mater.Chem.Phys.,2008,107:142-147
Nanocrystals Y2O2S∶Eu3+Prepared by Thermolysis
TAN Ning-HuiWANG Yuan-Yuan LIAO Pei-Kun LIU Yi-Cheng MENG Jian-Xin LIU Ying-Liang*
(Department of Chemistry,Jinan University,Guangzhou 510632)
This paper reports the preparation process of Y2O2S∶Eu3+nano-crystals by thermolysis method and the corresponding bulk Y2O2S∶Eu3+by conventional sulfur flux method.Nano-crystals Y2O2S∶Eu3+were synthesised by calcining the rod-like Y(OH)3precursor,obtained from hydrothermal method,with co-activator and S powder.The results show that both bulk and nano-crystals Y2O2S:Eu3+are with pure phase,hexagonal crystal structure.The hydrothermally prepared Y(OH)3is in a rod-like morphology,while the calcined Y2O2S∶Eu3+are narrow distributed nano-crystals,with an average size of 80 nm.The morphological change can be explained by the etching effect of the flux formed by the carbonate and S powder during high temperature calcination.It is concluded that Eu3+could be effectively incorporated into yttrium oxysulfides and therefore shows good luminescent properties from the excitation and emission spectra.Besides,the mechanism is discussed too.
yttrium oxysulfide;nano-crystals;luminescent properties;mechanism
O614.32
A
1001-486(2010)08-1421-05
2010-03-29。收修改稿日期:2010-05-10。
国家自然科学基金(No.20671042),广东省自然科学基金(No.0520055)资助项目。
*通讯联系人。 E-mail:tliuyl@jnu.edu.cn,Fax:+86-20-85220597;会员登记号:S060017521P。
谭宁会,女,25岁,硕士研究生;研究方向:纳米材料与环境材料。

