Preparation and Dyeing Performance of a Novel Crosslinking Polymeric Dye Containing Flavone Moiety*
TANG Lijun (汤立军)**, TANG Bingtao (唐炳涛) and ZHANG Shufen (张淑芬),**
1 College of Chemistry and Chemical Engineering, Bohai University, Jinzhou 121013, China
2 State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024, China
1 INTRODUCTION
In the past decade, polymeric dyes have attracted extensive interest by virtue of their application in various aspects, particularly in fiber dyeing [1-4].Crosslinking dyes such as basazol [5], indosol [6] and aminoalkyl crosslinking dyes [7] present excellent characteristics including less color effluent and excellent fixation. A common feature of these crosslinking dyes is that they have suitable nucleophilic groups,which react with crosslinker to form covalent bonds.Thus, the dyes link to the fiber through covalent bonding with the aid of a crosslinking agent, giving high fixation and good wet fastness.
Although water-soluble polymeric dyes are of considerable biological and technological interest due to their various properties, investigations on the dyes containing natural dye moiety are rare. Flavones are one of the most common classes of natural dyes that present significant biocidal [8-10], pharmaceutical[11-14], and antioxidant [15-17] activities. Thus, incorporating flavone dye moiety into a polymer skeleton may lead to an interesting and potential biological useful polymeric dye.
Herein, we report the preparation and dyeing performance of a novel flavone moiety containing crosslinking polymeric dye, in which polyvinylamine acts as backbone and a flavone containing azo dye acts as chromophore. The plenty amino groups in the polymeric dye crosslink with fiber in the presence of crosslinking agent to improve the dye fixation and washing and rubbing fastness of fibers.
2 EXPERIMENTAL
The synthesis of the polymeric dye is shown in Fig. 1.
2.1 Reagents and instruments
Polyvinylamine hydrochloride was prepared according to the method in Ref. [18], 7-hydroxyflavone (1)was prepared following the method in Ref. [19], and the crosslinking agent, 2-chloro-4,6-di(aminobenzene-4′-βsulphatoethylsulphone)-1,3,5-s-triazine (DAST) was prepared according to the method in literature [20].Other reagents were commercial analytical grade and used as received.
Melting points were measured on an X-6 micromelting point apparatus and uncorrected.1H NMR spectra were recorded with a Varian INOVA 400 NMR spectrometer at 400 MHz and chemical shifts were expressed in ppm relative to tetramethylsilane (TMS)used as an internal standard. Infrared (IR) spectra were measured as KBr pellets with an FT/IR-430 spectrophotometer. Mass spectra (MS) were performed on a HP1100 LC/MSD spectrometer.
2.2 Synthesis of 7-hydroxy-8-(p-nitrophenylazo)flavone (2)
In our experiments, coupling reaction ofp-benzenesulfanilamide diazonium salt with 7-hydroxyflavone was first tried under alkaline condition and was unsuccessful, due to the relatively lower reactivity of the diazonium salt as well as the coupling component. Whenp-nitroanilinediazonium salt was used, the coupling reaction proceeded smoothly to give azo compound 2. The preparation is as follows.
A mixture of 7-hydroxyflavone (1) (0.50 g, 2.1 mmol), 1% aqueous sodium hydroxide (10 ml) and aqueous sodium acetate (AcONa) (4.0 g in 10 ml H2O)was stirred until the flavone was completely dissolved,then the temperature was reduced to 0-5 °C. A ready-madep-nitroanilinediazonium salt (2.2 mmol)solution was added dropwise to the coupling component while maintaining the pH value of the reactant at 8.5-9. The coupling process was examined by spotting on a filter paper a drop of the reaction mixture close to a spot of an alkaline aqueous solution of H-acid, and then checking any color appearance at the interface of the two boundaries. If no color appeared at the interface, the mixture was acidified and the formed precipitate was filtered and washed with water.After being dried, the product was recrystallized from acetic acid to afford 0.79 g of dark orange needles with 78% yield, melting point 298-300 °C (λmax=371 nm, in acetone). IR (KBr): 3445, 1645, 1595, 1521,1450, 1373, 1344 cm-1.1H NMR (CDCl3):δ=14.32(s, 1H), 8.48 (d, 2H,J=8.8 Hz), 8.32 (d, 1H,J=9.2 Hz), 8.11 (d, 2H,J=8.8 Hz), 8.01-8.03 (m, 2H), 7.61(m, 3H), 7.11 (d, 1H,J=9.2 Hz), 6.99 (s, 1H). MS(APCI, negative mode)m/z(%) 386 ([M-H]-, 48), 387(M-, 100).
2.3 Preparation of polymeric dye 4
The flavone containing sulfonyl chloride 3 was prepared by a modified procedure based on the method in Refs. [21, 22] and was used directly for the next step without further purification. Chlorosulfonic acid (30 ml) was added to a 100 ml three-necked flask equipped with a mechanical stirrer and then it was cooled with ice-water bath. Compound 2 (1.05 g,mmol) was added in portion-wise, and the resulted mixture was heated slowly to 80 °C and kept for 9 h.After cooling to room temperature, the mixture was poured carefully onto 200 g of crushed ice in a beaker with vigorously stirring. The precipitate thus formed was collected by filtration and washed with ice cooled water until the filtrate was neutral. The filter cake was suspended in 100 ml tetrahydrofuran (THF) for use.MS (APCI, negative mode)m/z(%) 484 ([M-H]-,100), 486 ([M-H+2]-, 42).
Poly(vinylamine)chloride (2.0 g) was added to a stirred mixture of THF/H2O (100 ml, 1∶1 volume ratio), the pH of the resulted solution was adjusted to 9.0 with 10% aqueous sodium hydroxide. At room temperature, the suspension of 3 in THF was added dropwise to the mixture with vigorously stirring, and the pH of the reactant was kept at approximately 9.0.The completion of the reaction was determined by thin layer chromatography (TLC) [Silica GF254; eluent:n-butanol∶acetic acid∶water=4∶1∶5 (volume ratio)]. After reaction for 2 h, the spot of 3 on TLC was disappeared (Rfof the polymeric dye=0;Rfof 3=0.86). Then the pH of the mixture was adjusted to 3 with concentrated HCl. The resultant was poured into 400 ml methanol. The precipitate was filtered and washed thoroughly with methanol. It was dried in vacuum to constant mass to give 2.70 g of the polymeric dye 4, yield 86.7% (λmax=382 nm, in water).
2.4 Dyeing and fixing processes
The dyeing performance of polymeric dye 4 on silk and cotton were investigated by the similar methods described in our previous studies [23, 24], in which the effects of dyeing conditions, such as pH value of fixing bath, fixation temperature and concentration of crosslinking agent on fixation had been investigated.It can be concluded that when polyamine type crosslinking dye and DAST are used in the crosslinking dyeing process, the pH of the crosslinker bath is preferred to be 8 based on the fixation mechanism.Thus in the dyeing process of cotton and silk with polymeric dye 4, the pH value of DAST solution was adjusted to 8 before use. Under the weak alkaline condition, many nucleophilic amino groups in the polymeric dye can be released due to the neutralization of HCl salt. The reactions of DAST with polymeric dye and fiber polymer are all nucleophilic substitution reactions in the crosslinking-fixing processes,so this weak alkaline condition is favorable for fixation of the polymeric dye on fabrics.
Dyeing of silk and cotton with the crosslinking polymeric dye was performed by use of a “3-dip 3-nip”padding operation at room temperature. The dwell time of the dyed fiber in the padding bath containing 4%(by mass) polymeric dye was 3 min, and the pressure on the mangle was adjusted to give 70% pick-up. After the dyed fiber became dry at room temperature, it was dipped into fixing bath containing various amount(0-5%, by mass) of crosslinking agent (DAST) at pH 8 for 3 min and nipped once. The pressure on the mangle was adjusted to give approximately 80% wet pick-up,then the fiber was heated to 50 °C and kept for 10 min in an oven. The dyed fiber was soaped using 2 g·L-1anionic detergent (sodium dodecyl benzene sulphonate; 10 min, 100 °C) and was then washed and dried.Finally, the soaped fibers were treated with DMF/water(1∶1) to evaluate whether the polymeric dye was linked to the fiber by covalent bonding.
2.5 Measurement of dye fixation
The fixation of the yellow polymeric dye is calculated as follows. The reflectance (R) of the dyed fiber at the wavelength of maximum absorption on a Pye-Unicam SP8400 spectrophotometer (Pye-Unicam,Cambridge, UK) is determined. Then the color yield(K/S) value is calculated according to the Kubelka-Munk equation,
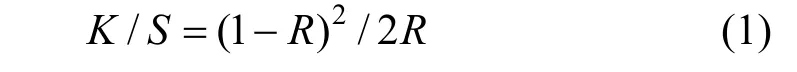
in whichK/Svalue is measured twice, before soaping(K/S)band after soaping (K/S)a. The dye fixation (%)is calculated as follows [25],
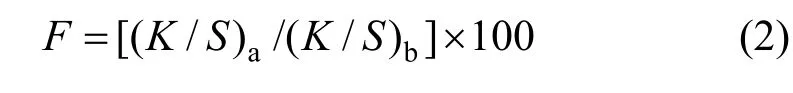
2.6 Fastness testing
The color fastness of the dyed fabrics was tested according to Chinese National standard methods including fastness to washing (GB/T 3921-97) and rubbing (GB/T 3920-97).
3 RESULTS AND DISCUSSION
3.1 Compound 2
The infrared spectrum (Fig. 2) of 2 shows a strong band of hydroxyl group, demonstrating that compound 2 exists mainly in azo form.1H NMR spectrum (Fig. 3) of 2 shows double peaks at 8.32 and 7.11, each corresponds to one proton and they have the identical coupling constant of 9.2 Hz. Thus they are assigned to flavone C5H and C6H respectively,which also demonstrates that the azo group only attaches to position 8 of 7-hydroxyflavone. The Uv-vis spectrum (Fig. 4) of 2 in DMF shows two strong absorption bands at 368 nm and 520 nm.
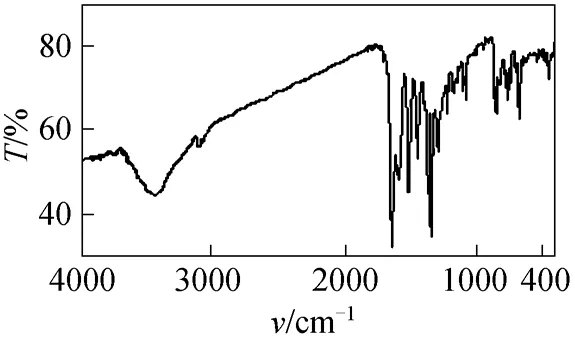
Figure 2 IR spectrum of compound 2
3.2 Polymeric dye 4
The polymeric dye was detected by TLC analysis(a mixture ofn-butanol∶acetic acid∶H2O=4∶1∶5 was used as eluent), which was proved by the appearance of typical stretching bands of sulfonamide group at 1344 cm-1and 1155 cm-1in its infrared spectrum(Fig. 5). The Uv-vis spectrum (Fig. 6) shows that the maximum absorption wavelength of this polymeric dye in water is 382 nm.

Figure 3 1H NMR spectrum of compound 2
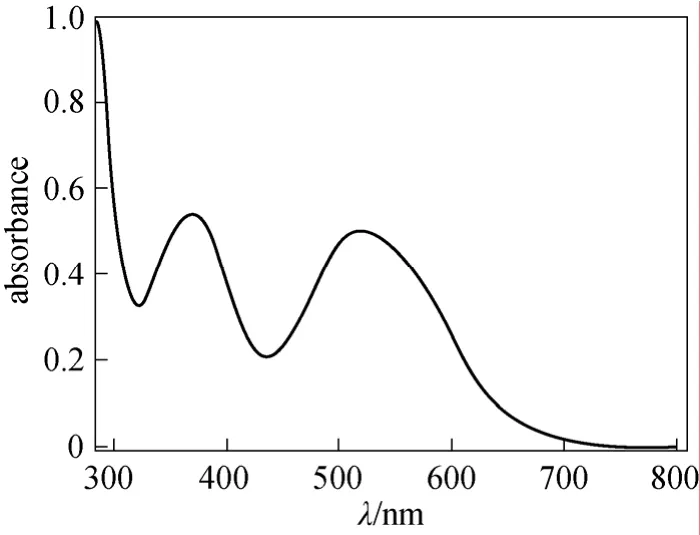
Figure 4 Uv-vis spectrum of 2 in DMF
Figure 5 IR spectrum of polymeric dye containing flavone moiety
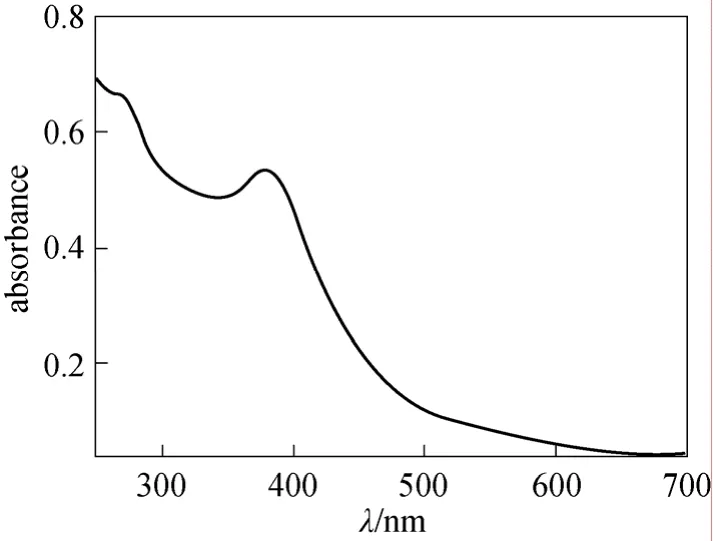
Figure 6 Uv-vis spectrum of dye 4 in water
3.3 Dyeing characteristics
The effect of DAST concentration on fixation was investigated due to its critical roles on the fixation of the dye. As shown in Fig. 7, the fixation on silk increases from 83% to 99% and that on cotton is from 72% to 99% when the concentration of DAST increases from 1% to 2% (by mass). When the concentration of DAST is less than 2%, a portion of the polymeric dye is not linked to the fiber, leading to a relatively low fixation. When the concentration increases to 2% and higher, the fixation maintains at 99%. At concentration of 2%, DAST molecules are enough to fully complete the crosslinking reaction so that the fixation level is high.
The dyed samples were evaluated for their fastness to rubbing and washing. The fastness can reach grades 4-5 and 5, respectively (Table 1). The dyed fibers soaped with mild detergent and subsequently treated with DMF do not show bleeding, which demonstrate that the yellow crosslinking polymeric dye is fixed on the fabric through covalent bonding during the crosslinking fixation process with crosslinking agent (DAST).
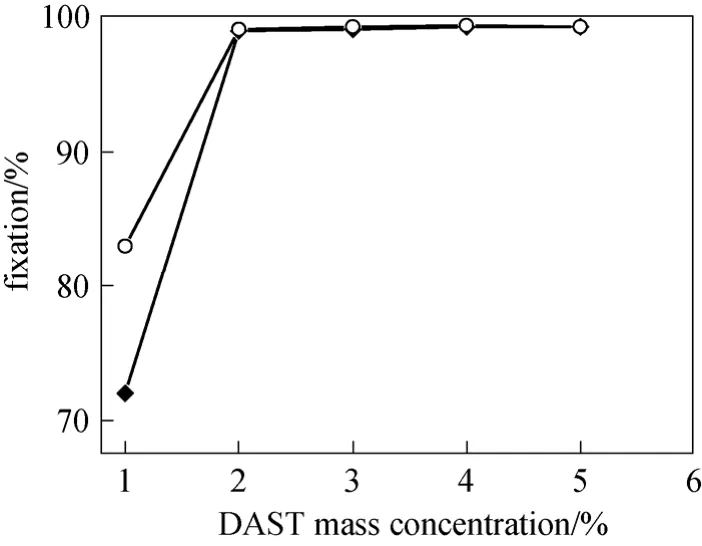
Figure 7 Effect of DAST concentration on fixation◆ cotton; ○ silk

Table 1 Fixation and fastness of the crosslinking polymeric dye
4 CONCLUSIONS
A yellow water-soluble crosslinking polymeric dye containing flavone moiety was synthesized and its dyeing performance was investigated. Silk and cotton were dyed with this polymeric dye and fixed by means of crosslinking agent (DAST). The dye fixation on silk and cotton reached 99% through covalent bonding with the aid of crosslinking agent molecule, which acts as a bridge between the fiber polymer and the polymeric dye. The rubbing and washing fastness of the dyed samples are excellent, reaching grades 4-5 and 5, respectively.
1 Maradiya, H.R., Patel, V.S., “Polymeric dyes based on thiadiazole derivatives”,Fiber Polym., 2, 212-220 (2001).
2 Maradiya, H.R., Patel, V.S., “Studies of novel monomeric and polymeric azo disperse dyes”,J.Appl.Poly.Sci., 84, 1380-1389 (2002).
3 Maradiya, H.R., Patel, V.S., “Synthesis, characterization and application of monomeric and polymeric dyes based onN-arylmaleimides”,High Perform.Polym., 12, 335348 (2000).
4 Sokol, P.E., Tsai, H.C., “Method for dyeing human hair with cationic polymeric dyes”, U.S. Pat., 4182612 (1980).
5 Lewis, D.M., “New possibilities to improve cellulosic fibre dyeing processes with fibre-reactive systems”,J.Soc.Dyers Colour., 109,357-364 (1993).
6 Lewis, D.M., Wang, Y.N., Lei, X.P., “Level fast dyeing of wool with nucleophilic aminoalkyl dyes and crosslinking agents. part 1-using a trifunctional crosslinking agent”,J.Soc.Dyers Colour., 111, 12-18(1995).
7 Lei, X.P, Lewis, D.M., Shen, X.M., “Crosslinking nucleophilic dyes on wool”,Dyes Pigm., 30, 271-281 (1996).
8 Rao, K.V., Chattopadhyay, S.K., Reddy, G.C., “Flavonoids with mosquito larval toxicity”,J.Agric.Food Chem., 38, 1427-1430(1990).
9 Weidenborner, M., Jha, H.C., “Antifungal activity of flavonoids and their mixtures against different fungi occurring on grain”,Pestic.Sci., 38, 347-351 (1993).
10 Silva, A.M., Weidenbörner, M., Cavaleiro, J.A.S., “Growth control of different fusarium species by selected flavones and flavonoid mixtures”,Mycol.Res., 102, 638-640 (1998).
11 Wu, E.S.C., Loch, J.T., Toder, B.H., Borrelli, A.R., Gawlak, D., Radov, L.A., Gensmantel, N.P., “Flavones. 3. synthesis, biological activities, and conformational analysis of isoflavone derivatives and related compounds”,J.Med.Chem., 35, 3519-3525 (1992).
12 Wolfman, C., Viola, H., Marder, M., Wasowski, C., Ardenghi, P.,Izquierdo, I., Paladini, A.C., Medina, J.H., “Anxioselective properties of 6,3′-dinitroflavone, a high-affinity benzodiazepine receptor ligand”,Eur.J.Pharmacol., 318, 23-30 (1996).
13 Akama, T., Ishida, H., Kimura, U., Gomi, K., Saito, H., Fuse, E.,Kobayashi, S., Yoda, N., Kasai, M., “Design and synthesis of potent antitumor 5,4′-diaminoflavone derivatives based on metabolic considerations”,J.Med.Chem., 40, 1894-1900 (1997).
14 Gee, J.M., Johnson, I.T., “Polyphenolic compounds: interactions with the gut and implications for human health”,Curr.Med.Chem.,8, 1245-1255 (2001).
15 Rice-Evans, C.A., Miller, N.J., Paganga, G., “Structure antioxidant activity relationships of flavonoids and phenolic acids”,Free Rad.Biol.Med., 20, 933-956 (1996).
16 Rice-Evans, C., “Flavonoid antioxidants”,Curr.Med.Chem., 8,797-807 (2001).
17 Pietta, P.G., “Flavonoids as antioxidant”,J.Nat.Prod., 63,1035-1042 (2000).
18 Hu, Z.Y., Zhang, S.F., Yang, J.Z., Chen, Y., “Some properties of aqueous-solutions of poly(vinylamine chloride)”,J.Appl.Polyl.Sci.,89 (14), 3889-3893 (2003).
19 Tang, L.J., Zhang, S.F., Yang, J.Z., Gao, W.T., Cui, J., “Synthesis of ring-A hydroxylated flavones by sodium hydroxide-catalyzed cyclization of 1,3-diketone in water”,Org.Prep.Proced.Int., 36 (5),453-459 (2004).
20 Lewis, D.M., “Textile treatment”, E.P. Pat., 0174794 (1985).
21 Bozdag, O., Verspohl, E. J., Ertan, R., “Synthesis and hypoglycemic activity of some new flavone derivatives. Part 1: Flavonylsulphonylurea derivatives”,Arch.Pharm.Pharm.Med.Chem. 332, 435-438(1999).
22 Silva, A.M.G., Tomé, A.C., Silva, A.M.S., Cavaleiro, J.A.S., “Chlorosulfonation of flavones”,Phosphorus,Sulfur Silicon Relat.Elem.,140, 113-124 (1998).
23 Tang, B.T., Zhang, S.F., Yang, J.Z., “Synthesis and dyeing performance of a novel yellow crosslinking polymeric dye”,Color.Technol.,120, 180-183 (2004).
24 Li, Y.L., Zhang, S.F., Yang, J.Z., Jiang,S., Li, Q., “Synthesis and application of novel crosslinking polyamine dyes with good dyeing performance”,Dyes Pigm., 76, 508-514 (2008).
25 Tang, Y.F., Li, Y.L., Zhang, S.F., Yang, J.Z., Dang, N.Y., Liang, X.F.,“Fixation of a tetraethylene pentamine dye on cotton and silk by bifunctional crosslinkers”,Color.Technol., 122, 82-85 (2006).
 Chinese Journal of Chemical Engineering2011年4期
Chinese Journal of Chemical Engineering2011年4期
- Chinese Journal of Chemical Engineering的其它文章
- Synthesis of Methyl Isopropyl Ketone and Diethyl Ketone over Ni-Na/ZrO2-MnO2-ZnO Catalyst*
- Isotherm Equation Study of F Adsorbed from Water Solution by Fe2(SO4)3-modified Granular Activated Alumina*
- Solubility of Paclitaxel in Mixtures of Dichloromethane and Supercritical Carbon Dioxide*
- The Kinetics for Electrochemical Removal of Ammonia in Coking Wastewater*
- Solvent Recovery from Soybean Oil/Hexane Miscella by PDMS Composite Membrane*
- Direct Optical Resolution of Chiral Pesticides by High Performance Liquid Chromatography*
