Fabrication of SPES/Nano-TiO2 Composite Ultrafiltration Membrane and Its Anti-fouling Mechanism*
LUO Mingliang (罗明良), WEN Qingzhi (温庆志), LIU Jialin (刘佳林), LIU Hongjian (刘洪见) and JIA Zilong (贾自龙)
Fabrication of SPES/Nano-TiO2Composite Ultrafiltration Membrane and Its Anti-fouling Mechanism*
LUO Mingliang (罗明良)**, WEN Qingzhi (温庆志), LIU Jialin (刘佳林), LIU Hongjian (刘洪见) and JIA Zilong (贾自龙)
College of Petroleum Engineering, China University of Petroleum (East China), Qingdao 266555, China
Membrane fouling is one of the major obstacles for reaching a high flux over a prolonged period of ultrafiltration (UF) process. In this study, a sulfonated-polyethersulfone (SPES)/nano-TiO2composite UF membrane with good anti-fouling performance was fabricated by phase inversion and self-assembly methods. The TiO2nanoparticle self-assembly on the SPES membrane surface was confirmed by X-ray photoelectron spectroscopy (XPS) and FT-IR spectrometer. The morphology and hydrophilicity were characterized by scanning electron microscopy (SEM), atomic force microscopy (AFM) and contact angle goniometer, respectively. The anti-fouling mechanism of composite UF membrane was discussed through the analysis of the micro-structure and component of UF membrane surface. The results showed that the TiO2content and the micro-structure of the composite UF membrane surface had great influence on the separation and anti-fouling performance.
anti-fouling, ultrafiltration membrane, sulfonated-polyethersulfone, TiO2nanoparticle, phase inversion, self-assembly
1 INTRODUCTION
In recent years, more and more attention in ultrafiltration (UF) membrane has been attracted for a variety of applications in wastewater treatment, substance separation, solute concentration, and so on. The major drawback in the extensive use of membranes includes membrane fouling, which results in flux decline during operation [1]. Polyethersulfone (PES) is a special engineering plastics. It possesses many good characteristics such as high mechanical property and heat distortion temperature, good heat-aging resistance, environmental endurance as well as easy processing. It has become an important membrane material, but its hydrophobicity controlled by PES molecular structure leads to low membrane flux and poor anti-fouling performance [2]. Recently, modification methods of UF membrane involve ultraviolet irradiation [3], graft polymerization [4, 5], glow discharge [6], ozone [7, 8], and so on in order to improve PES hydrophilicity. Among these methods, blending with inorganic materials, especially nanoparticles, has attracted much interest due to their convenient operation and mild conditions.
Nanoparticles used to modify organic membranes include SiO2, TiO2, Al2O3, and so on [9], among which TiO2receives most attention because of its stability, availability, and hydrophilicity [10]. Molinari. prepared TiO2/polymer composite membranes in order to develop photocatalytic membrane reactors for wastewater treatment [11-13]. Kwak and coworkers studied the ability of a TiO2/polymer thin film composite (TFC) reverse osmosis membrane under ultraviolet (UV) radiation to mitigate biofouling by a photobactericidal effect [14, 15], focusing on the photocatalytic or photobactericidal effects of TiO2nanoparticles under UV radiation. Recently, Luo. [16] and Yang. [17] prepared PES/nano-TiO2and polysulfone(PSF)/nano- TiO2composite UF membrane, respectively. The hydrophilicity, mechanistic performance and thermal stability of the composite UF membrane were improved greatly, but in the UF operation, the low washing resistance of nanoparticles on the membrane surface affects the anti-fouling period and service performance due to the weak interaction between PES (or PSF) and nano-TiO2resulted from the less active groups.
In this study, sulfonated-polyethersulfone (SPES) is used as the membrane material due to its good performance as well as PES, the active site and functional group in SPES. The SPES/nano-TiO2composite UF membrane is prepared by phase inversion and self-assembly methods, which is expected to present good anti-fouling and washing resistance performance. The morphology and hydrophilicity of UF membrane are characterized by scanning electron microscopy (SEM), atomic force microscopy (AFM) and contact angle goniometer, respectively. X-ray photoelectron spectroscopy (XPS) and FT-IR spectrometer are employed to analyze the mechanism of nano-TiO2self-assembly on the SPES membrane surface. To examine the fouling mitigation ability of membranes, a filtration experiment is carried out and the anti-fouling mechanism is discussed through the analysis of the micro-structure and composition of UF membrane surface.
2 EXPERIMENTAL
2.1 Materials
The SPES powder was obtained from Jilin University, China. The inherent viscosity (inh) of the polymer in-methyl pyrrolidone solvent and the equilibrium moisture uptake under ambient condition (relative humidity of 82% and 26°C) were 1.27 dl·g-1and 2.07%, respectively. The solvent,-dimethyl acetamide (DMAc) was in AR grade, and TiO2colloidal suspension was prepared by the sol-gel method in our laboratory [2].
2.2 Fabrication of SPES/TiO2 composite ultrafiltration membranes
The SPES ultrafiltration membrane was fabricated by phase inversion method.,-dimethyl acetamide (DMAc) solvent was used to dissolve the SPES for several hours under continuous agitation for complete dissolution and elimination of bubbles. The uniform transparent solution was then cast on a smooth glass plate with a knife edge. The thickness of the membrane was controlled by varying the thickness of adhesive tapes on the sides of the glass plate. The glassplate was kept in an environment with controlled temperature and humidity during membrane casting. After casting, the glass plate was immediately immersed in a gelling bath, which is generally demineralized water maintained at certain temperature, and the phase inversion started. After a few minutes, the thin polymeric film was taken from the glass. The film was washed repeatedly with demineralized water and stored wetted.
The wet UF membrane was rinsed in a sodium carbonate solution [0.2% (by mass)] and then washed with demineralized water. The neat SPES membrane with an area of 38.5 cm2was dipped in the transparent TiO2colloidal solution, stirred for 1 min by ultrasonic method and immersed for 1 h, and then washed with demineralized water.
2.3 Characterization
2.3.1Characterization of the SPES/TiO composite UF membrane
The size of TiO2nanoparticles was determined by a JEOL transmission electron microscope (TEM, JEOL JEM-200CX) at 120 kV. The surface morphology, roughness and mean pore size of the neat SPES membrane and the composite membrane were observed with a JSM-5800 scanning electron microscope (SEM) and a DualSopeTMatomic force microscope (AFM). The pore sizes were measured by inspecting line profiles of different low valleys (.. pores) and high peaks (.. nodules) on the AFM images at different locations of a membrane surface. Then the mean pore size of membrane was calculated.

Figure 1 TEM micrograph of TiO2nanoparticles
The contact angle of membrane surface was the average value of 10 measurements by Eromag-1 contact angle goniometer and the measurement error was±3°.
A FT-IR spectrum of the composite was recorded on a Perkin-Elmer RXI over the range of 400-4000 cm-1. The spectral resolution was 4 cm-1. X-ray photoelectron spectroscopy (XPS) was employed to analyze the component of the composite membrane surface with a PHI-5400 spectrometer. The spectra were taken at the takeoff angle (defined as the angle between the detected photoelectron beam and the membrane surfaces) of 45°to give a sampling depth of ca. 2.3 nm.
2.3.2Separation performance of the SPES/TiO composite UF membrane
The mass transfer characteristics of UF membrane for 0.02% (by mass) polyethylene glycol (PEG-5000) aqueous solution were determined in an apparatus with a continuous flow at 0.2 MPa and 25°C for 30 min. The water flux was calculated by direct measurement of the mass of the permeate flow:
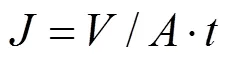
whereis the membrane flux (L·m-2·h-1),is the permeate volume (L),is the membrane area (m2), andis ultrafiltration time (h).
The solute rejection was defined as

whereis the solute rejection,fis the feed concentration, andpis the permeate concentration.
3 RESULTS AND DISCUSSION
3.1 Morphology, roughness and pore size of the composite membrane surface
The TiO2nanoparticle size assembled on the SPES membrane is determined by TEM, as shown in Fig. 1. The particle size is about 5-42 nm. The SEM graphs of the surface morphology before and after treated by TiO2colloidal solution are shown in Fig. 2. The neat SPES membrane has the typical surface morphology of a ridge-and-valley structure [Fig. 2 (a)], but the structure is not apparent. Fig. 2 (b) displays the surface morphology of the TiO2self-assembled composite membrane, where TiO2nanoparticles appear as nodular shapes on the ridges so that the membrane surface has clear ridge-and-valley structure. Fig. 3 demonstrates the 3D AFM image of membrane surface for the neat SPES membrane and the SPES/TiO2composite membrane at a scan size of 5 µm×5 µm. The brightest area presents the highest point of the membrane surface and the dark regions indicate valley or membrane pores. The surface morphology is greatly changed due to TiO2nanoparticles assembled on SPES membrane and the ridge-and-valley structure on the composite membrane is distinct.
The average pore size and roughness of membrane surface were obtained from AFM images using Danish Micro Engineering Scanning Probe Microscopes (DME SPM) software. The size of 30 pores in 1 µm×1 µm area of membrane surface was measured from height profile of two-dimensional AFM images using SPM software and the average value was reported. The results are given in Table 1. The mean pore size of the composite membrane surface decreases slightly due to the self-assembly of TiO2nanoparticles on the SPES membrane surface. The surface roughness parameters of the membrane, expressed in terms of the mean roughness (a), the root mean square of thedata (q) and the mean difference between the highest peaks and lowest valleys (z), were calculated by DME SPM software in 10 µm×10 µm scan size and are presented in Table 1. The roughness parameters for the composite membranes increase remarkably. Since the roughness parameters depend on the-value, which is the vertical distance that the piezoelectric scanner moves, this relationship is expected. When the surface includes deep depressions (pores) and high peaks (nodules), the tip moves up and down over a wide range and the roughness parameter of surface is high.
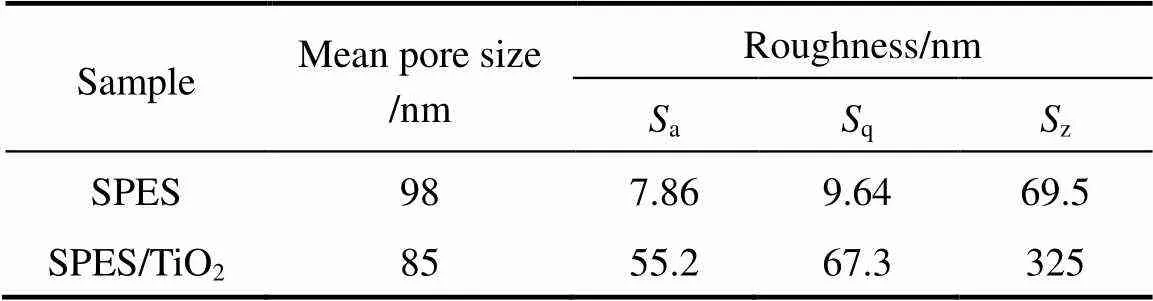
Table 1 Mean pore size and roughness of the neat SPESand SPES/TiO2 membrane surface

Figure 2 SEM micrograph of UF membrane surface
Figure 3 AFM micrographs of UF membrane
3.2 XPS and FT-IR analyses of the composite membrane surface
To confirm the self-assembly TiO2nanoparticles on the composite membrane surface and further to estimate the abrasive resistance of the membrane surface, FT-IR method was employed to analyze the interaction between nano-TiO2and SPES. X-ray photoelectron spectroscopic (XPS) was carried out for investigating the change of membrane surface elements under various UF conditions.

Figure 4 FT-IR spectra of pure SPES and SPES/TiO2composites
a—SPES; b—SPES/TiO2


Figure 5 Chemical bond structure models of SPES/TiO2composite
The constituent elements of the composite membrane surface are hydrogen, carbon, oxygen, sulfur, chlorine, and titanium. XPS analyses were performed on the elements of carbon, oxygen, sulfur, chlorine, and titanium, but not on hydrogen because its photoelectron cross-section is too small to be characterized by XPS. The core-electron binding energies of the constituent elements are typically 287eV (C1s), 537eV (O1s), 23eV (O2s), 229eV (S2s), 270eV (Cl2s), 199eV (Cl2p) and 458eV (Ti2p) [23]. Fig. 6 shows the resulting spectrum, in which all the photoelectron peaks appear at positions similar to the above values and Ti peaks appear. The results provide the evidence of TiO2self-assembly on the composite membrane surface.
On the basis of the observed photoelectron peaks and corresponding sensitivity factors, the relative atomic concentrations of the individual elements can be calculated:

Figure 6 XPS spectra of the elements on the composite membrane
whereAis the photoelectron peak area of element,Sis the sensitivity factor for element, andis the number of the elements in the sample. In Table 2, the elemental compositions determined by an angle-resolved XPS analysis are summarized for the composite membranes with different washing conditions and UF operation time. There is an initial drop in the relative atomic concentration of Ti element after washing the composite membrane, which attaches to the surface when dipping into the TiO2colloidal solution. An additional loss of TiO2nanoparticles is observed in the UF operation for 5 h. The UF process was operated in the cross-flow mode where the feed solution was pumped across the composite membrane parallel to its surface. Some TiO2particles are wiped out and the loosely bound TiO2nanoparticles cannot overcome the shear force. However, the TiO2loss does not continue as the UF operation time increases, and the amount of TiO2changes little after 15 h of UF operation as shown by the samples No. 4 and No. 5. This result indicates that a considerable amount of TiO2nanoparticles remains tightly bound on the membrane surface under actual UF operation conditions, which is expected to improve the hydrophilicity of SPES membrane and prevent the membrane from fouling. The results of samples No. 4 and No. 6 indicate that the washing resistance of the SPES/TiO2composite membrane increases remarkably compared to the PES/TiO2composite membrane [16]. Thus the increase of active site, functional group and electronegativity on the SPES membrane surface enhances the interaction between SPES and nano-TiO2based on the FT-IR and XPS analyses.

Table 2 Elements compositions of the SPES/TiO2composite membrane under various washingconditions and UF time
①Analyses for the TiO2self-assembled SPES UF membranes (1) just after preparation, (2) after washing with flowing water, (3) after UF operation for 5.0 h, (4) after UF operation for another 15.0 h, and (5) after UF operation for another 45.0 h; (6) analysis for the TiO2self-assembled PES UF membranes after the same UF operation time as No. 4 sample [16].

Table 3 Contact angle and UF separationperformance of membrane
① (7) neat SPES; (8) SPES/TiO2; (9) PES/TiO2UF membranes [16].
3.3 Anti-fouling mechanism and separation performance of the composite membrane
TiO2nanoparticles in the anatase form are very hydrophilic, photoactive and practical for wide environmental applications such as water purification, wastewater treatment, hazardous waste control, air purification, and water disinfection [24]. In this work, the composite UF membrane is devised by the self-assembly between TiO2nanoparticle and SPES with the ether bond, sulfuryl group and sulfonic group (as shown in Fig. 5) because of the strong electronegative oxygen in the ether bond, sulfuryl group and sulfonic group of the SPES. As the UF process was operated in the cross-flow mode under high pressure, simply adsorbed particles may be detached from the membrane surface. XPS results in Table 2 indicate that some TiO2particles in the composite membrane have sufficient binding strength for the actual operation, which agrees with other researches on the interaction behavior of TiO2nanoparticles [25]. It is concluded that a novel organic-inorganic membrane is successfully prepared by self-assembly process.
The hydrophilic and separation performance of the membrane surface untreated and treated by TiO2colloidal solution are presented in Table 3. The area of UF membrane is 38.5 cm2, the applied pressure is 0.2 MPa, operation temperature is 25°C, and the feed concentration is 0.02% (by mass) (PEG-5000) in this test. As shown in Table 3, the contact angle of the UF membrane treated by TiO2colloidal solution is smaller, but the flux and retention increase to some degree. Combined to the results of FT-IR and XPS analysis, it is shown that the hydrophilicity of the PES itself is improved through sulfonation and nano-TiO2self-assembly on the SPES membrane surface and the antifouling performance is better. On the other hand, the SEM and AFM photographs show that the microstructure of SPES membrane surface is changed due to nano-TiO2self-assembly. The ridge-and-valley structure with micro- or nano-scale is distinct on the SPES membrane surface (Fig. 3) and the surface roughness is changed (Table 1). Generally, the contact angle of hydrophilic surface decreases with the increases of roughness [26], so the contact angle of the composite UF membrane declines remarkably. Since the hydroxyl is rich on the nano-TiO2surface and TiO2nanoparticles have high hydrophilicity and large specific surface, the hydroxyl content on the composite membrane is increased greatly by the self-assembly of nanoparticles on the membrane surface and the membrane hydrophilicity is higher. The water molecules are easy to permeate through the membrane and the flux increases significantly. At the same time, the pore structure is changed with TiO2incorporated into membrane. The pore sizes become more uniform and the surface becomes more compact, so the retention is improved.
4 CONCLUSIONS
Membrane fouling by hydrophobic substances is the main cause to deteriorate the ultrafiltration (UF) performance of polyethersulfone (PES)-type membranes. A new type of composite membrane is developed as an approach to solve the fouling problem. TiO2nanoparticles are incorporated onto the sulfonated polyethersulfone membrane surface by self-assembly. The micro- or nano-scale ridge-and-valley structure of the composite UF membrane is examined with scanning electron microscopy (SEM) and atomic force microscopy (AFM) and the roughness of membrane surface is determined. The FT-IR and X-ray photoelectron spectroscopy (XPS) demonstrate that TiO2particles are tightly self-assembled with sufficient bonding strength for the actual UF process. The contact angle test of the composite membrane shows that the hydrophilicity of the membrane surface is improved remarkably. The separation experiment verifies the prevention of the composite membrane from the fouling of hydrophobic substances, suggesting a possible use as a new type of antifouling composite membrane.
1 Wang, Y., Kim, J.H., Choo, K.H., Lee, Y.S., Lee, C.H., “Hydrophilic modification of polypropylene microfiltration membranes by ozone-induced graft polymerization”,..., 169 (2), 269-276 (2000).
2 Luo, M.L., Tang, W., Zhao, J., Q, Pu, C.S., “Hydrophilic modification of polyethersulfone used TiO2nanoparticles by a sol-gel process”,..., 172 (3), 431-436 (2006).
3 Nystrom, M., Jarvinen, P., “Modification of polysulfone ultrafiltration membranes with UV irradiation and hydrophilicity increasing agents”,..., 60 (2-3), 275-296 (1991).
4 Wu, Y.T., Shi, Y.J., “Polysulfone ultrafiltration membranes modified by irradiation grafting with acrylic acid monomer”,, 21 (1), 21-25 (1995). (in Chinese)
5 Fujimoto, K., Takebayashi, Y., Inoue, H., Ikada, Y., “Polyurethane surface modification by graft polymerization of acrylamide for reduced protein adsorption and platelet adhesion”,, 14 (6), 442-448 (1993).
6 Suzuki, M., Kishida, A., Iwata, H., Ikada, Y., “Graft copolymerization of acrylamide onto a polyethylene surface pretreated with a glow discharge”,, 19 (7), 1804-1808 (1986).
7 Fujimoto, K., Takebayashi, Y., Inoue, H., Ikada, Y., “Ozone-induced graft polymerization onto polymer surface”,....., 31 (4), 1035-1043 (1993).
8 Yamauchi, J., Yamaoka, A., Ikemoto, K., Matsui, T., “Graft copolymerization of methyl methacrylate onto polypropylene oxidized with ozone”,...., 43 (6), 1197-1203(1991).
9 Kim, K.M., Park, N.G., Ryu, K.S., Chang, S.H., “Characteristics of PVdF-HFp/TiO2compositemembrane electrolytes prepared by phase inversion and conventional casting methods”,., 51 (26), 5636-5644 (2006).
10 Cao, X.C., Ma, J., Shi, X.H., Ren, Z.J., “Effect of TiO2nanoparticle size on the performance of PVDF membrane”,..., 253 (4), 2003-2010 (2006).
11 Molinari, R., Mungari, M., Drioli, E., Di Paola, A., Loddo, V., Palmisano, L., Schiavello, M., “Study on a photocatalytic membrane reactor for water purification”,., 55 (1-2), 71-78 (2000).
12 Molinari, R., Grande, C., Drioli, E., Palmisano, L., Schiavello, M., “Photocatalytic membrane reactors for degradation of organic pollutants in water”,., 67 (1-3), 273-279 (2001).
13 Molinari, R., Palmisano, L., Drioli, E., Schiavello, M., “Studies on various reactor configurations for coupling photocatalysis and membrane process in water purification”,..., 206 (1-2), 399-415 (2002).
14 Kwak, S.Y., Kim, S.H., Kim, S.S., “Hybrid organic/inorganic reverse osmosis (RO) membrane for bactericidal anti-fouling (I) Preparation and characterization of TiO2nanoparticle self-assembled aromatic polyamide thin film composite (TFC) membrane”,..., 35 (11), 2388-2394 (2001).
15 Kim, S.H., Kwak, S.Y., Sohn, B.H., Park, T.H., “Design of TiO2nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane as an approach to solve biofouling problem”,..., 211(1), 157-165 (2003).
16 Luo, M.L., Zhao, J.Q., Tang, W., Pu, C.S., “Hydrophilic modificationof polyethersulfone ultrafiltration membrane surface by self-assembly of TiO2nanoparticles”,..., 249 (1-4), 76-84 (2005).
17 Yang, Y.N., Wang, P., Zheng, Q.Z., “Polysulfone/TiO2hybrid ultrafiltration membrane prepared by the sol-gel process”,, 64 (6), 569-573 (2006).
18 Lu, Y.Q., Deng, Z.H., Applied Infrared Spectra Analysis, Electronic Industry Press, Beijing (1989). (in Chinese)
19 Lu, H.J., Shen, L.S., Wang, C.X., Jiang, D.Z., “Sulphonation and characterization of polyethersulfone(PES)”,..., 17 (5), 833-835 (1998).
20 Wang, X.L., Organic Chemistry, Higher Education Press, Beijing (1987). (in Chinese)
21 Sun, H.W., Zhong, S.H., “Preparation of IR spectra analysis of TiO2-polyethylene complex membrane”,..., 17 (5), 42-46 (1997). (in Chinese)
22 Tang, F.Q., Hou, L.P., Guo, G.S., “Preparation of TiO2nanometer powders”,..., 16 (4), 615-619 (2001).
23 Wang, J.Q., Wu, W.H., Feng, D.M., Electron Spectroscopy, National Defence Industry Press, Beijing (1992). (in Chinese)
24 Mills, A., Davies, R.H., Worsley, D., “Water purification by semiconductor photocatalysis”,..., 22 (6), 417-434 (1993).
25 Mills, A., Hunte, S.L., “An overview of semiconductor photocatalysis”,...:., 108 (1), 1-35 (1997).
26 Xu, J.H., Li, M., Zhao, Y., “Advance of wetting behavior research on the superhydrophobic surface with micro- and nano- structures”,, 18 (11), 1425-1433 (2006). (in Chinese)
** To whom correspondence should be addressed. E-mail: yfsailing_wxg@163.com
2009-12-08,
2010-09-20.
the Natural Science Foundation of Shandong Province (Q2007B01).
 Chinese Journal of Chemical Engineering2011年1期
Chinese Journal of Chemical Engineering2011年1期
- Chinese Journal of Chemical Engineering的其它文章
- Effect of Boundary Layers on Polycrystalline Silicon Chemical Vapor Deposition in a Trichlorosilane and Hydrogen System*
- Experimental and CFD Study on the Role of Fluid Flow Pattern onMembrane Permeate Flux
- Separation of Eu3+ Using a Novel Dispersion Combined LiquidMembrane with P507 in Kerosene as the Carrier*
- Adsorption and Ozonation Kinetic Model for PhenolicWastewater Treatment*
- Properties of Bio-oil from Fast Pyrolysis of Rice Husk*
- Induction of Recombinant Uridine Phosphorylase and Its Application in Biosynthesis of Pyrimidine Nucleosides*
