γ-Secretase Inhibitor, DAPT Inhibits Self-renewal and Stemness Maintenance of Ovarian Cancer Stem-like Cells In Vitro
Li-yu Jiang, Xiao-lei Zhang, Ping Du, Jian-hua Zheng
Department of Obstetrics and Gynecology, the First Affiliated Hospital, Harbin Medical University, Harbin 150001, China
γ-Secretase Inhibitor, DAPT Inhibits Self-renewal and Stemness Maintenance of Ovarian Cancer Stem-like Cells In Vitro
Li-yu Jiang, Xiao-lei Zhang, Ping Du, Jian-hua Zheng*
Department of Obstetrics and Gynecology, the First Affiliated Hospital, Harbin Medical University, Harbin 150001, China
Objective:The Notch signaling pathway plays an important role in the stem cell signaling network and contributes to tumorigenesis. However, the functions of Notch signaling in ovarian cancer stem cells (OCSCs) are not well understood. We aimed to investigate the effects of Notch blockade on self-renewal and stemness maintenance of OCSCs.
Methods:Ovarian cancer stem-like cells were enriched from ovarian cancer cell lines in serum-free medium. A γ-secretase inhibitor, (DAPT), was used to block Notch signaling. MTT assays were performed to assess self-renewal and proliferation inhibition, flow cytometry was performed to analyze cell surface marker and immunofluorescence, Western Blot and Real-time RT-PCR assays were performed to detect Oct4 and Sox2 protein and mRNA expression of the Ovarian cancer stem-like cells treated with DAPT.
Results:Notch blockade markedly inhibits self-renewal and proliferation of ovarian cancer stem-like cells, significantly downregulates the expression of OCSCs-specific surface markers, and reduces protein and mRNA expression of Oct4 and Sox2 in OCSC-like cells.
Conclusion:Our results suggest that Notch signaling is not only critical for the self-renewal and proliferation of OCSCs, but also for the stemness maintenance of OCSCs. The γ-secretase inhibitor is a promising treatment targeting OCSCs.
Ovarian cancer stem cells (OCSCs);Notch signaling pathway; γ-secretase inhibitor
INTRODUCTION
Ovarian cancer is the leading cause of death due to gynecological cancer[1]. Recurrence and chemoresistance are the major burdens in the treatment of ovarian cancer. Increasing evidence have shown that a small subset of cells, termed cancer stem cells (CSCs), are the only cells capable of initiating and sustaining tumor growth. CSCs are also responsible for tumor relapse, metastasis, and chemoresistance[2]. Present chemotherapy modalities eliminate the bulk of tumor cells, but cannot eliminate the core of these cancer stem cells, which have a high capacity for repair and renewal[3,4]. Therefore, focusing therapies on cancer stem cells is more effective for the treatment of ovarian cancer.
Researchers have identified the existence of CSCs in an increasingly longer list of solid tumors[5], and have proven the existence of ovarian cancer stem cells (OCSCs) in epithelial ovarian cancer[4,6–9]. OCSCs are characterized by 11111111111their capacity to survive and organize suspended spheroid structures in serum-free medium, overexpression of several stem cell markers (ABCG2, Nanog, Nestin, and Oct4)[4,10], CD44+, CD117+, CD133+, as well as resistance to conventional chemotherapies, resistance to apoptosis, and the ability to recapitulate the original tumorin vivo[4,6–8,11].
The signaling pathways that regulate the self-renewal and differentiation of normal stem cells are deregulated in CSCs, resulting in the continuous expansion of self-renewing cancer cells and tumor formation[12,13]. The Notch signaling pathway is one of the key pathways that constitute the stem cell signaling network[14,15], and plays multiple key functions in tumor initiation and progression[12,16]. In mammals, there are four Notch receptors (Notch 1–4). Ligand protein binding to Notch receptors leads to their cleavage by γ-secretase to release the Notch intracellular domain (NICD) following the nuclear translocation of NICD to induce transcriptional activation of Notch target genes[14]. Notch is both a transmembrane receptor and a transcription factor.
γ-secretase inhibitors prevent the proteolysis of Notch receptors and suppress the Notch activity. Treatment with γ-secretase inhibitor, DAPT (N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester) resulted in a marked reduction in medulloblastoma growth and induced G0-G1cell cycle arrest and apoptosis in a T-ALL mouse model[17]. And DAPT have been shown to inhibit renal cell carcinoma growthin vitroandin vivoand inhibit the growth of pancreatic cancer cells in vitro by inhibition of Notch signaling[18,19].
In this study, OCSC-like cells from ovarian cancer cell lines SKOV3 and HO8910 were enriched in serum-free medium. DAPT, which inhibits all four Notch receptors, was used to investigate the effects of Notch blockade on the self-renewal and stemness maintenance of OCSC-like cells.
MATERIALS AND METHODS
Cell Lines and Cell Culture
In this study, two human ovarian epithelial cancer cell lines, SKOV3 and HO8910, were used. The cells were cultured in Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 (DMEM/F12) medium supplemented with 10% fetal bovine serum (FBS) and in serum-free DMEM/F12 medium supplemented with 20 ng/mL human recombinant epidermal growth factor (EGF; Invitrogen), 10 ng/mL basic fibroblast growth factor (bFGF; Invitrogen), and 2% B27 supplement (Invitrogen). In medium containing serum, the cells adhered onto the wall to form cell monolayers, whereas in serum-free medium, both SKOV3 and HO8910 cells formed suspended spheroid structures. Primary spheres were dissociated with trypsin to generate single cells. They were then serially diluted and plated, at one cell per well, into 96-well plates. Mini-wells containing one single cell were marked after microscopic confirmation and assessed for secondary sphere generation. Secondary spheres were dissociated and replated at a density of 50 cells/cm2 in serum-free medium. Sphere-forming cells were passaged up to P5 and the subsequent experiments were begun.
Growth Inhibition Assays
3-4,5-Dimethylthiazol-2,5 diphenyl tetrazolium bromide (MTT) assays were used to assess self-renewal and proliferation inhibition. DAPT, dissolved in dimethyl sulfoxide (DMSO), was used to test the effect of Notch signaling blockade. DMSO alone was used as the vehicle control. Enriched OCSC-like SKOV3 and HO8910 cells were harvested and plated in 96-well plates at 5000 cells per well in 200 μL medium. Twenty-four hours after plating, each set of 10 wells of cells was treated with 0, 1, 2, 5, and 20 μg/mL DAPT (Sigma) or medium alone. The cells were incubated for 1, 2, or 3 days, then 20 μL of MTT solution (5 mg/mL) was added to each well. The cells were then incubated for 4 h at 37 °C. After incubation, the medium containing MTT was removed and replaced with 150 μL DMSO. The absorbance was measured at 490 nm using an ELISA microplate reader. The experiment was repeated three times.
Immunofluorescence
Parental SKOV3 and HO8910 cells were seeded onto glass coverslips in six-well plates before staining and spheroids were deposited by cytospin onto glass slides. Cell slides were fixed, permeabilized, and blocked. Coverslips were subsequently incubated overnight at 4°C with rabbit monoclonal antibodies against Oct4 (Abcam) or Sox2 (Millipore) (1:200 dilution each). After washing, the slides were incubated in the dark at room temperature for 30 min with FITC-labeled goat anti-rabbit IgG secondary antibodies (Santa Cruz; dilution 1:200). The nuclei were counterstained with DAPI (Santa Cruz). Reactions omitting the primary antibodies were used as controls. Microscopy was performed using a Nikon E800 fluorescence microscope and images were acquired digitally using MagnaFire Software (Optronics).
Flow Cytometry for Analyzing Cell Surface Marker
Sphere-forming, stem-like cells were treated with 5 μg/ml DAPT or DMSO alone for 24 h. Then, the cells were dissociated into single cells, washed, resuspended in PBS containing 5% bovine serum albumin and labeled with FITC-conjugated anti-human CD44 antibodies (eBioscience), APC-conjugated anti-human CD117 antibodies (eBioscience), and PE-conjugated anti-human CD133 antibodies (eBioscience) in the dark at room temperature for 30 min. Nonviable (i.e., membrane-permeable) cells were excluded by DAPI staining. The cells were analyzed on FACSAria (Becton Dickinson) using FACSDiva software (Becton Dickinson).
Western Blot
The cells were lysed in ice-cold RIPA lysis buffer containing protease inhibitors. After centrifugation, the supernates were analyzed for protein concentration using a kit (Pierce Biochemicals). Equal amounts of proteins from cell lysates (50 μg/lane) were subjected to 12% SDS-PAGE gel electrophoresis and transferred onto nitrocellulose sheets. Non-specific binding sites were blocked with non-fat dry milk. After overnight incubation at 4°C with anti-Notch intracellular domain (NICD) polyclonal antibodies (Millipore), anti-hairy and enhancer of split homologue-1 (HES1) polyclonal antibodies (Millipore), anti-Oct4 monoclonal antibodies, and anti-Sox2 monoclonal antibodies, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for two hours at room temperature, and revealed by chemiluminescence (ECL) reagent (Promega).
Real-time Reverse Transcription-Polymerase Chain Reaction (Real-time RT-PCR)
Total RNA from HO8910 and SKOV3 anchorageindependent cells were extracted with Trizol reagent (Invitrogen) and treated with DNAse I. Reverse transcription was performed with oligo dT and M-MuLV reverse transcriptase (Invitrogen) according to the manufacturer's instructions. The primers were as follows: forward of HES1 (5'-AAC GCA GTG TCA CCT TCC AG-3') and reverse (5'-TCT TCT CTC CCA GTA TTC AAG TTC C-3'); forward of Oct4 (5'-CGA AAG AGA AAG CGA ACC AGT ATC-3') and reverse ( 5'-GAA CCA CAC TCG GAC CAC ATC-3'); forward of Sox2 (5'-GCA AAA GAG GAG AGT AAG AAA CAG C-3') and reverse (5'-CGT GAG TGT GGA TGG GAT TGG-3'). cDNA was quantified on Opticon2 real-time PCR instrument (Bio-Rad). The PCR reaction contained the SYBR Green mix (TaKaRa) and 200 nM each of the primers. The expression levels of the transcripts were normalized usingβ-actin as the transcript internal control.
Statistical Analysis
Data were expressed as ¯x ± s. Statistical differences of MTT assays and Real-time quantitative PCR assays were analyzed by ANOVA. Frequency data of surface marker expression determined by flow cytometry were analyzed by Chi-square test. P < 0.05 was considered significant.
RESULTS
Anchorage-Independent, Self-renewing Cell Sphere Formation by A Subpopulation of Human Ovarian Cancer Cell Lines
Adult stem cells and cancer stem cells have been found in numerous tissues of the body. The ability to form anchorage-independent spherical structures is one of the most important characteristic of all these stem cells[4,6,20–23]. In this study, a self-renewing, stem-like cells subpopulation from the ovarian cancer cell lines SKOV3 and HO8910 were isolated and enriched using a method of non-adherent suspension culture (i.e., stem cell-selective culture). In serum-free medium, both SKOV3 and HO8910 cells are able to form anchorage-independent, three-dimensional ovarian cancer cell spheres (OCCS), but the efficiency of OCCS formation varied. After the dissociated cells were cultured in serum-free medium at one cell per well for 7 days, the HO8910 cells formed suspended clusters, whereas SKOV3 cells were still single. After culturing for 14 days, the HO8910 cells organized into spheroid structures while the SKOV3 cells began to form suspended clusters (Figure 1). In adherent culture containing 10% FBS, some semi-suspended cells, which were round, outnumbered the anchoragedependent cells. The more semi-suspended cells present, the more rapid the cells proliferated, and the shorter was the tumor cell culture doubling time. There were more semi-suspended cells in the HO8910 cell line than in the SKOV3 cell line. These semi-suspended cells are associated with proliferation and sphere formation.
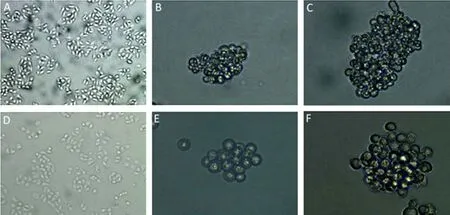
Figure 1.Ovarian cancer cell sphere (OCCS) formation. (A) Parental HO8910 cells. Magnification ×200 (B,C) in serum-free medium, cultured for 14 days, the HO8910 cells organized in spheroid structures, and after 21 days, the spheres grew larger. Magnification ×200. (D) Parental SKOV3 cells. Magnification ×200 (E,F) in serum-free medium, cultured for 14 days, the SKOV3 cells formed suspended clusters, and after 21 days, formed typical cell spheres. Magnification ×400
DAPT Prevented OCCS Formation
As Notch activity contributes to self-renewal of OCSCs, the effects of Notch inhibition on OCCS formation was determined using DAPT. DAPT was added to dissociated cells derived from HO8910 and SKOV3 spheres. OCCS formation was observed under an inverted microscope. DAPT was shown to reduce significantly the number and the size of the cell spheres. After culturing for 14 days, the ovarian cancer cells treated with DMSO generated larger, more numerous cell spheres (Figure 2A, 2C). The DAPT-treated cells only generated several cell clusters or smaller spheres (Figure 2B, 2D). The inhibitory effect of DAPT on OCCS formation was dose-dependent.
DAPT Inhibited Self-renewal and Proliferation of OCSCs
To confirm whether DAPT and the Notch inhibitor could prevent self-renewal and proliferation of OCSCs, the relative cell numbers of HO8910 and SKOV3 stem cell-like cells, treated or untreated with DAPT, were determined by MTT assays. After the cells were incubated with DAPT, a significant reduction in cell self-renewal and proliferation was observed. DAPT induced concentration-dependent anti-proliferative effects on the HO8910 and SKOV3 OCSC-like cells. (Figure 2E, 2F).
DAPT Downregulated OCSC-specific Surface Marker Expression
The expression of OCSC surface markers were examined in HO8910 and SKOV3 stem-like cells by flow cytometry. Of the HO8910 stem-like cells treated with DMSO, 50.4%, 60.1%, and 55.2% expressed CD44+CD133+, CD44+CD117+, or CD117+CD133+, respectively (Figure 3A) whereas the HO8910 stem cell-like cells treated with DAPT,the percentage of double positive cells was 28.0%, 29.7%, and 30.8%, respectively (Figure 3B). The difference in the constituent ratio was significant (P< 0.05). In the SKOV3 stem cell-like cells, the results were similar; 46.9%, 48.6%, and 37.1% of the cells expressed CD44+CD133+, CD44+CD117+, or CD117+CD133+, respectively (Figure 3C), whereas in the SKOV3 cells treated with DAPT, the percentage of double positive cells decreased to 16.3%, 13.6%, and 7.1% (Figure 3D) (P< 0.05). These results indicate DAPT inhibits the expression of these specific surface markers associated with OCSC identification

Figure 2.DAPT prevents OCCS formation and inhibits self-renewal and profiferation of OCSCs. (A,C) In serum-free medium without DAPT, HO8910 and SKOV3 cells formed larger spheres. (B<D) After DAPT treatment, HO8910 and SKOV3 cells formed smaller spheres or cell clusters. Scale bar 100 μm. (E,F) Cells were treated with different concentrations of DAPT, ranging from 0 to 20 μg/ml for 1,2, or 3 days, then cell growth was determined by MTT assay. After the cells were incubated with DAPT, a significant reduction in cell self-renewal and proliferation was observed. DAPT induced concentration-dependent anti-proliferative effects on the HO8910 and SKOV3 OCSC-like cells.
DAPT Treatment Decreased Oct4 and Sox2 Protein and mRNA Expression in OCSC-like Cells
The immunofluorescence assays showed that the stem cell marker proteins, Oct4 and Sox2, were overexpressed in HO8910 and SKOV3 OCCS (Figure 4A). We further investigated whether DAPT could regulate Oct4 and Sox2 protein and mRNA expression by blocking the Notch signaling pathway. HO8910 and SKOV3 OCSC-like cells were incubated with 2 μg/ml, 5μg/ml DAPT, or DMSO alone for 24 h. Western blot assays were performed to detect NICD, HES1, Oct4, and Sox2 protein expressions. Real-time quantitative PCR assays were performed to investigate the transcription of HES1, Oct4, and Sox2. Treatment with DAPT, which could inhibit γ-secretase and then inhibit the proteolytic process of Notch receptors, directly reduced the generation of NICD protein (Figure 4B), consequently reducing HES1 transcription and HES1 protein expression (Figure 4B and 4C), which showed that DAPT blocked the Notch signaling pathway. DAPT treatment also induced a dose-dependent decrease in Oct4 and Sox2 mRNA expression in both the HO8910 and the SKOV3 OCSC-like cells. After DAPT treatment, the mRNA levels of Oct4 in the HO8910 and the SKOV3 were reduced by 35.9% and 71.7%, respectively(P<0.05), whereas the mRNA levels of Sox2 were reduced by 38.1% and 67%, respectively(P<0.05) (Figure 4C). A reduction in Oct4 and Sox2 protein expression following DAPT treatment was also detected in both the HO8910 and the SKOV3 sphere-forming OCSC-like cells. Moreover, the decrease in the Sox2 protein was more significant (Figure 4B).
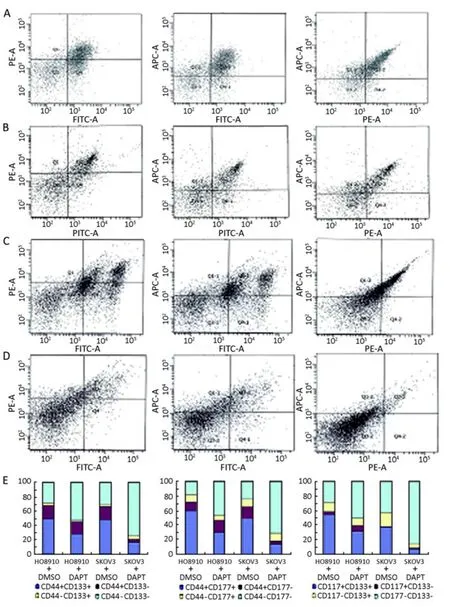
Figure 3.Surface marker expression of OCSC-like cells treated with DAPT or DMSO. All cells were incubated with FITC-anti-CD44 antibody, APC-anti-CD117 antibody, and PE-anti-CD133 antibody, and then analyzed by flow cytometry. (A,C) HO8910 and SKOV3 sphere-forming, stem-like cells were treated with DMSO alone for 24 h. in HO8910 cells, the percentage of CD44+CD133+, CD44+CD117+, and CD117+CD133+ cells were 50.4%, 60.1%, and 55.2%, respectively (A). In SKOV3 cells, the percentage was 46.9%, 48.6%, and 37.1%, respectively (C). (B<D) HO8910 and SKOV3 sphere-forming, stem-like cells were treated with DAPT for 24 h. in HO8910 cells, the percentage of CD44+CD133+, CD44+CD117, and CD117+CD133+ cells decreased to 28.0%, 29.7%, and 30.8%, respectively. In SKOV3 cells, the percentage decreased to 16.3%, 13.6%, and 7.1%, respectively. DAPT significantly inhibited the expression of these specific surface markers and reduced the proportion of double positive cells in OCSC-like cells (P<0.05). (E) The percentage of double positive, single positive, and double negative cells in HO8910 and SKOV3 stem-like cells with or without DAPT treatment.
DISCUSSION
Cancers can be viewed as abnormal organ in which tumor growth is driven by a subpopulation of CSCs[13]. Targeting CSCs, especially targeting key signaling pathway that regulate CSCs, is a promising therapeutic strategy against most aggressive and metastatic cancers[24]. The Notch signaling pathway is a highly conserved cell signaling system. Although the intracellular transduction of the Notch signal is remarkably simple, with no secondary messengers, this pathway functions in an enormous diversity of developmental processes and its dysfunction is implicated in many cancers[15,25]. Given that the Notch target genes, HES and HES-related repressor protein represses the transcriptionof tissue-specific transcription factors, Notch signal activation results In the maintenance of stem or progenitor cells through the inhibition of differentiation[26]. The Notch signaling pathway can be blocked by γ-secretase inhibitors. DAPT, a cell-permeable dipeptide that inhibits all four Notch receptors, is a promising treatment for many malignancies[27].

Figure 4.DAPT decreased Oct4 and Sox2 protein, as well as mRNA expression. (A) Immunofluorescent assays in both the HO8910 (top) and the SKOV3 OCSs (bottom), Oct4 protein (left two) and Sox2 protein (right two) were overexpressed. Nuclei were stained with DAPI. (B) Protein expression of NICD, HESI, Oct4 and Sox2 in the HO8910 and SKOV3 OCSC-like cells were detected b y western blot. Lane a corresponds to cells treated with DMSO for 24 hours; lanes b and c correspond to cells treated with 2 μg/ml and 5 μg/ml DAPT for 24 hours, respectively. DAPT treatment dose-dependently decreased NICD, HESI, Oct4, and Sox2 protein expression. (C) After treatment with various doses of DAPT, HESI, Oct4, and Sox2 mRNA expression were detected by real-time RT-PCR. DAPT treatment significantly decreased HESI, Oct4, and Sox2 mRNA expression in dose-dependent manners in HO8910 and SKOV3 OCSC-like cells (P<0.05).
Using stem cell-selective culture method, OCSC-like cells from ovarian cancer HO8910 and SKOV3 cell lines were enriched and grown. These cells could form OCCS and overexpress both the Oct4 and the Sox2 proteins in serum-free medium. DAPT treatment blocks Notch signaling pathway by preventing NICD and HES1 protein. DAPT significantly inhibits the formation of OCCS, as well as decreases the size and the number of the cell spheres, which suggests that Notch signaling is critical and plays an important role in the self-renewal of OCSCs.
In advanced ovarian malignancies, CD44, CD117, and CD133 are overexpressed[28,29]. CD44 is expressed in a large number of tumors and is a marker for CSCs in breast, prostate, and colorectal tumors. CD117 is frequently expressed by several stem cells. Zhang et al.[4]demonstrated that CD44+CD117+ cells are ovarian cancer-initiating cells. CD133 is also a surface marker for CSCs, including those in the brain, prostate, pancreas, liver, colon, and skin cancers[30,31]. Some recent studies indicated that CD133 expression defined a tumor-initiating cell population in human ovarian cancer[8,32]. In this study, in OCSC-like cells treated with DAPT, the expression of these specific surface markers is reduced. DAPT downregulates expression of these surface molecules, indicating that DAPT inhibits OCSCs stemness maintenance, decreases the subpopulation of OCSCs, and increases the subpopulation of differentiated cells. Notch signaling is critical for stemness maintenance of OCSCs.
Transcription factors Oct4 and Sox2 play important roles in the maintenance of embryonic stem cell pluripotency, and are essential to converting human somatic cells to pluripotency and establishing induced pluripotent stem cellsin vitro[33]. Oct4 and Sox2 are also involved in the regulation of cancer stem cell self-renewal, maintenance of their stem cell characteristics, and resistance to chemotherapy[34,35]. Consistent with the evidence of surface markers, DAPT also downregulates the Oct4 and Sox2 protein and mRNA expression in HO8910 and SKOV3 OCSC-like cells. High levels of Notch signaling pathway components correspond to high levels of Oct4 and Sox2 mRNA and protein expression, whereas decreasing levels of Notch signaling pathway components correspond to low levels of Oct4 and Sox2 mRNA and protein expression. These suggest that Notch signaling contributes to the regulation of transcriptionand translation of Oct4 and Sox2 and is associated with the stemness maintenance of OCSCs. Blocking Notch signaling is a potentially effective treatment for targeting OCSCs.
Notch activation or blockade may have a profound effect on many aspects of ovarian cancer. A Notch blockade may generate a net-like cascade reaction, which ultimately determines the fate of OCSCs. To effectively target OCSCs, improve treatment efficacy, and prevent recurrence of ovarian cancer, γ-secretase inhibitors and the Notch signaling pathway require further investigation.
In summary, inhibition of Notch signaling with the γ-secretase inhibitor DAPT markedly inhibits OCSC-like cells from self-renewing and proliferating, significantly downregulates the expression of OCSC-specific surface markers, and reduces protein and mRNA expression of Oct4 and Sox2 in OCSC-like cells. Our results suggest that Notch signaling is critical for the self-renewal and proliferation of OCSCs and for the stemness maintenance of OCSCs. Notch pathway components may serve as important molecular diagnostic and therapeutic targets for ovarian cancer patients. γ-Secretase inhibitors are a promising treatment targeting OCSCs and may be applied as anti-cancer drugs for ovarian cancer.
REFERENCES
1. Pecorelli S, Favalli G, Zigliani L, et al. Cancer in women. Int J Gynaecol Obstet 2003; 82: 369-79.
2. Dick JE. Stem cell concepts renew cancer research. Blood 2008; 112: 4793-807.
3. Clarke MF, Dick JE, Dirks PB, et al. Cancer Stem Cells--Perspectives on Current Status and Future Directions: AACR Workshop on Cancer Stem Cells. Cancer Res 2006; 66: 9339-44.
4. Zhang S, Balch C, Chan MW, et al: Identification and Characterization of Ovarian Cancer-Initiating Cells from Primary Human Tumors. Cancer Res 2008; 68: 4311-20.
5. Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol 2007; 18: 460-6.
6. Bapat SA, Mali AM, Koppikar CB, et al. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res 2005; 65: 3025-9.
7. Burleson KM, Casey RC, Skubitz KM, et al. Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol Oncol 2004; 93: 170-81.
8. Curley MD, Therrien VA, Cummings CL, et al. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells 2009; 27: 2875-83.
9. Wani AA, Sharma N, Shouche YS, et al. Nuclear-mitochondrial genomic profiling reveals a pattern of evolution in epithelial ovarian tumor stem cells. Oncogene 2006; 25: 6336-44.
10. Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci USA 2006; 103: 11154-9.
11. Mimeault M, Hauke R, Mehta PP, et al. Recent advances in cancer stem/progenitor celll research: therapeutic implications for overcoming resistance to the most aggressive cancers. J Cell Mol Med 2007; 11: 981-1011.
12. Leong KG, Karsan A. Recent insight into the role of Notch signaling in tumorigenesis. Blood 2006; 107: 2223-33.
13. AI-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene 2004; 23: 7274-82.
14. Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch Signaling: Cell Fate Control and Signal Integration in Development. Science 1999; 284: 770-6.
15. Androutsellis-Theotokis A, Leker RR, Soldner F, et al. Notch signaling regulates stem cell numbersin vitroandin vivo. Nature 2006; 442: 823-6.
16. Li JL, Harris AL. Notch signaling from tumor cells: a new mechanism of angiogenesis. Cancer Cell 2005; 8: 1-3.
17. Hallahan AR, Pritchard JI, Hansen S, et al. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res 2004; 64: 7794-800.
18. Sjölund J, Johansson M, Manna S, et al. Suppression of renal cell carcinoma growth by inhibition of Notch signalingin vitroandin vivo. J Clin Invest 2008; 118: 217-28.
19. Kimura K, Satoh K, Kanno A, et al. Activation of Notch signaling in tumorigenesis of experimental pancreatic cancer induced by dimethylbenzanthracene in mice. Cancer Sci 2007; 98: 155-62.
20. Rappa G, Mercapide J, Anzanello F, et al. Growth of cancer cell lines under stem cell-like conditions has the potential to unveil therapeutic targets. Exp Cell Res 2008; 314: 2110-22.
21. Bez A, Corsini E, Curti D, et al. Neurosphere and neurosphere-forming cells: morphological and ultrastructural characterization. Brain Res 2003; 993: 18-29.
22. Dontu G, Abdallah WM, Foley JM, et al.In vitropropagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 2003; 17: 1253-70.
23. Bisson I, Prowse DM. WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res 2009; 19: 683-97.
24. Reya T, Morrison SJ, Clarke MF, et al. Stem cells,cancer,and cancer stem cells. Nature 2001; 414: 105-11.
25. Bray SJ. Notch signaling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 2006; 7: 678-89.
26. Katoh M, Katoh M. Notch signaling in gastrointestinal tract. Int J Onco 2007; 30: 247-251.
27. Shih IeM, Wang TL. Notch Signaling, gamma-Secretase Inhibitors, and Cancer Therapy. Cancer Res 2007; 67: 1879-82.
28. Raspollini MR, Amunni G, Villanucci A, et al. c-KIT expression and correlation with chemotherapy resistance in ovarian carcinoma: an immunocytochemical study. Ann Oncol 2004; 15: 594-7.
29. Saegusa M, Machida D, Hashimura M, et al. CD44 expression in benign, premalignant, and malignant ovarian neoplasms: relation to tumour development and progression. J Pathol 1999; 189: 326-37.
30. Shmelkov S, Clair RSt, Lyden D, et al. AC133/CD133/Prominin-1. Int J Biochem Cell Biol 2005; 37: 715-9.
31. Mimeault M, Hauke R, Mehta PP, et al. Recent advances in cancer stem/progenitor cell research: therapeutic implications for overcoming resistance to the most aggressive cancers. J Cell Mol Med 2007; 11: 981-1011.
32. Baba T, Convery PA, Matsumura N, et al. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene 2009; 28: 209-18.
33. Huangfu D, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol 2008; 26: 1269-75.
34. Ben-Porath I, Thomson MW, Carey VJ, et al. An embryonic stem cell–like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet 2008; 40: 499-507.
35. Nicolis SK. Cancer stem cells and “stemness” genes in neuro-oncology. Neurobiol Dis 2007; 25: 217-29.
10.1007/s11670-011-0140-1
2011-01-7;Accepted2011-03-25
This work was supported by a grant from the Heilongjang Province Science and Technology Commission of China (No. GB07C32304)
*Corresponding author
E-mail: dcotorzheng52@yahoo.com.cn
©Chinese Anti-Cancer Association and Springer-Verlag Berlin Heidelberg 2011
 Chinese Journal of Cancer Research2011年2期
Chinese Journal of Cancer Research2011年2期
- Chinese Journal of Cancer Research的其它文章
- DNA Methylation Profiles of Protease Nexin 1 (SERPINE2) Gene in Human Cell Lines
- Procyanidins Inhibit Tumor Angiogenesis by Crosslinking Extracellular Matrix
- Cytochrome P450 2E1 RsaI/PstI and DraI Polymorphisms Are Risk Factors for Lung Cancer in Mongolian and Han Population in Inner Mongolia
- Phase I Study to Determine MTD of Docetaxel and Cisplatin with Concurrent Radiation Therapy for Stage III Non-SmallCell Lung Cancer
- Breast Cancer Subtypes and Survival in Chinese Women with Operable Primary Breast Cancer
- Over-expression of Metastasis-associated in Colon Cancer-1 (MACC1) Associates with Better Prognosis of Gastric Cancer Patients
