Metabolism and thermoregulation between Mrs Hume’s Pheasant (Syrmaticus humiae) and Elliot’s Pheasant (S. ellioti)
LUO Ying, YU Tai-Lin, HUANG Cheng-Ming, ZHAO Tong, LI Han-Hua, LI Chang-Jian
(1. Department of Life Science and Chemistry, University of Science and Engineering, Yongzhou 425600, China; 2. College of Life Science, Guangxi Normal University, Guilin 541004, China; 3. Institute of Zoology, the Chinese Academy of Sciences, Beijing 100101, China)
Metabolism and thermoregulation between Mrs Hume’s Pheasant (Syrmaticus humiae) and Elliot’s Pheasant (S. ellioti)
LUO Ying1, YU Tai-Lin2,*, HUANG Cheng-Ming3,*, ZHAO Tong2, LI Han-Hua2, LI Chang-Jian1
(1. Department of Life Science and Chemistry, University of Science and Engineering, Yongzhou 425600, China; 2. College of Life Science, Guangxi Normal University, Guilin 541004, China; 3. Institute of Zoology, the Chinese Academy of Sciences, Beijing 100101, China)
To understand metabolic adaptations, the basal metabolic rate (BMR) of Mrs Hume’s Pheasant (Syrmaticus humiae) and Elliot’s Pheasant (Syrmaticus ellioti) were investigated. Metabolic rate (MR), body temperature (Tb) and thermal conductance (C) were determined in both species at a temperatrue range of 5 − 35 ℃, respectively. Oxygen consumption was measured with a closed circuit respirometer. The thermal neutral zones (TNZ) were 24.5 − 31.6℃, and 23.0 −29.2 ℃, respectively. With a temperature range of 5 − 35 ℃, Mrs Hume’s Pheasant and Elliot’s Pheasant could maintained stable Tbat a mean of (40.47±0.64) and (40.36±0.10) ℃, respectively. Mean BMRs within TNZs were (1.36±0.84) mLO2/(g·h) for Mrs Hume’s Pheasant and (2.03±0.12) mLO2/(g·h) for Elliot’s Pheasant, which were 77% and 86% of the expected value based on their body mass, respectively. Thermal conductance of Mrs Hume’s Pheasant and Elliot’s Pheasant were (0.12±0.01) and (0.17±0.01) mLO2/(g·h·℃), below the lower critical temperature, respectively, which were 119% and 124% of the expected value based on their body mass, respectively. The ecophysiological characteristics of these species were low metabolic rate, high body temperature, and high thermal conductance, which allow both species to better adapt to the warmer climate environment in south China.
Syrmaticus humiae;Syrmaticus ellioti; Body temperature; Basal metabolic rate; Thermal conductance
Metabolism is one of the most basic animal characteristics, going with energy flowing and information communion in the course of substance metabolism. Metabolism is a major factors in all life processes, including energy utilization, and important part of life history (Williams & Tieleman, 2000). Bird metabolism affects distribution and abundance, which are considered major survival countermeasures (Weathers, 1997; Lovegrove, 2003). Basal metabolic rate (BMR) is the rate of energy transformation in a rested, awake and fasted state in the absence of thermal stress, and is the minimum metabolic rate of animals maintaining normal physiological function. It is important parameters of energy metabolism comparison (Jessen, 2001). The use of BMR as an index of energy expenditure has received a great deal of attention from environmental physiologists, ecophysiologists and comparative physiologists (Reynolds & Lee, 1996).
Comparative physiological ecology is important for developing general rules about birds through comparison. Most basal rate variation in bird metabolism can be explained by the combined influences of body size, phylogeny, climate condition, activity and feeding habits (McNab, 2000; McKechnie & Wolf, 2004; Canterbury, 2002; Weathers, 1979). Comparing small birds from different habitats and with different habits highlights, the ecological significance of BMRs (Rezende et al, 2002) is shown. Take the small-sized birds living in cold environments as an example, though feather growth is limited due to body size, they can resist the cold by increasing heat production (Stokkan, 1992; Liknes et al, 2002); birds living in high latitude temperate zones remain active in winter, mainly through behavioral, formal and physiology mechanical changes (Corp et al, 1997); and birds retain hypothermia in tropical areas (Weathers, 1997).
Mrs Hume’s Pheasant (Syrmaticus humiae) and Elliot’s Pheasant (Syrmaticus ellioti) are threatenedSymmaticus,Phasianidae,Galliformesspecies (Baillie et al, 2004), within Cenwanglaoshan Nature Reserve in China, they are National level protected animals (Zhang et al, 2003). In China, Mrs Hume’s Pheasant is only found in Yunnan and Guangxi provinces, but are also found in Northeast India, Northwest Thailand, and the west, north and east Burma. It is a typical species for the southwest subregion mountain areas of the Oriental Realm (Liu et al, 2008). Mrs Hume’s Pheasant mainly inhabit broad-leaved forest at an altitude of 780−1 800 m, mixed coniferous broad-leaved forest, scrub woodland, grassland and forest edge areas. It is omnivorous, mainly eating acorns, berries, seeds, roots, leaves, buds and other plant food. It also eats insects and other animalbased food, and occasionally targets cultivated crops at the forest edge (Mackinnon et al, 2000). Elliot’s Pheasant, a species peculiar to China, is distributed in Zhejiang, Anhui, Fujian, Jiangxi, Hubei, Hunan, Guangdong, Guangxi, and Guizhou provinces. It is a typical species of eastern hilly subregion in Central China of the Oriental Realm (Shi & Zheng, 1997). Elliot’s Pheasant mainly inhabit rugged mountains and the jungle of valleys at an altitude of 200 − 1 500 m, more commonly in mixed coniferous broad-leaved forests. It can also be found in dense bamboo and understory. It is omnivorous, mainly eating plant leaves, stems, buds, flowers, fruits, seeds and other plant food crops, athough it also eats insects and other animal-based food (Mackinnon et al, 2000). While limited work has been done on habitat selection and artificial propagation of the two species, no research has been conducted on their energy metabolism. The purpose of this study was to measure the BMR of the two endangered species and research their adaptability to the warm wet climate of Southeast and Southwest China.
1 Materials and Methods
1.1 Animals
Six Mrs Hume’s Pheasants (3 males, 3 females) and 6 Elliot’s Pheasants (3 males, 3 females) hatched in May 2010 were housed at the Biological Park of Guangxi Normal University. The birds were kept in a closed aviary (95.0 cm × 45.0 cm × 45.0 cm) where the temperature was maintained at approximately 38°C in the first two weeks of their life, and at approximately 35°C in the following two weeks. After one month of age, the birds spent most of the day in open aviaries (5.4 m×2.8 m×1.9 m) , which allowed free movement and feeding (food and water suppliedadlimbitum). The experiments were carried out in July 2010 when the birds were 80 days old, the mean body mass of Mrs Hume’s Pheasant and Elliot’s Pheasant were (398.83±22.93) g and (388.25±14.58) g, respectively.
1.2 Metabolic trials
Oxygen consumption was measured using a closed circuit respirometer (Górecki, 1975). Temperatures in a water bath inside the animal chambers were measured and maintained at a constant level (to ± 0.5 °C). Volume of the metabolic chamber was 5.8 L. Oxygen consumption rates were measured over a temperature range of 5 − 35 °C, with each trial conducted 45 min after the animals had been in the metabolic chamber for 1 h to stabilize its environment. Food was removed 15 h before each test to minimize the heat increment of feeding and animals were weighed to the nearest ± 0.1 g. Both H2O and CO2were absorbed by silica gel and NaOH. Recording of oxygen consumption due to animal activity in the chamber were discarded when computing the metabolic rate of each individual. All measurements were made daily between 8:00 and 15:00. The metabolic rates (MR) of birds were measured in the rest phase under natural light conditions. The birds were wrapped with gauze to restrict their activities. Reading interval of O2consumption was 5 min. Two consecutive, stable and minimum recording were used to calculate metabolic rates as mLO2/(g·h). Body temperatures (Tb) of all individuals were recorded before and after each measurement, and Tbwas measured with a digital thermometer (Beijing Normal University Instruments Co.) in the cloaca at a depth of 1.5 cm. Body mass was measured before and after the experiments.
1.3 Thermal conductance
Over thermal conductance (C, mLO2/g·h·°C) was calculated at temperature below the thermal neutral zone using the formula as:
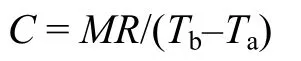
Where MR is metabolic rate (mLO2/g·h), Tais ambient temperature (°C),Tbis body temperature (°C). This formula was suggested by Aschoff (1981) for calculating conductance at any givenTa.
Aschoff & Pohl (1970) reviewed the BMRs of bird species, and obtained the allometric equations for birds. Expectation ration ofBMRandCpredicted by the appropriate equation of Aschoff & Pohl (1970 ) and Aschoff (1981), respectively, uses the following formulas:

1.4 Statistics
Data were analyzed using the SPSS11.5 statistical package. Differences between temperature treatments were determined by repeated measures ANOVA andP<0.05 is taken to be statistically significant. All results were expressed as mean±SE, and linear regression analysis was used to analyze the relationship between energetic parameters andTa.
2 Results
2.1 Mrs Hume’s Pheasant
Mean Tbof Mrs Hume’s Pheasant ranged from a mean of (40.6±0.07) ℃ at 24.5 ℃ to (41.4±0.11) ℃ at 35 ℃. The Tbof Mrs Hume’s Pheasant remained almost constant from 5 to 35℃ Tas, with a mean value of (40.47±0.64) ℃ (Fig. 1a).

Fig. 1 Changes in body temperature(a), metabolic rate(b) and thermal conductance(c) with ambient temperature in Mrs Hume’s Pheasant
There was no significant difference for metabolic rates between 24.5 ℃ and 31.6 ℃ (Fig. 1b). The thermal neutral zone (TNZ) was from 24.5℃ to 31.6 ℃. Mean BMR was (1.36±0.84) mLO2/(g·h) (n= 24). Metabolic rates between 20 ℃ and 24.5 ℃ showed a significant difference (t= 4. 148,df= 28,P= 0.000<0.0001), with the difference between 31.6 ℃ and 35 ℃ also significant (t= 6.473,df= 28,P= 0.000<0.0001). The metabolism rate was variable at temperatures below 24.5℃ and dependent onTa.

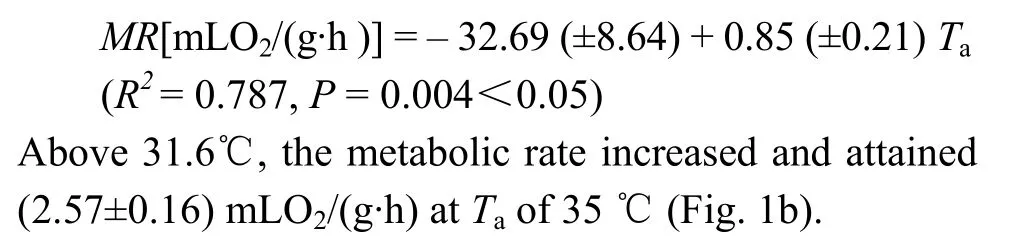
Thermal conductance (C) was calculated as (0.12± 0.01) mLO2/(g·h·℃) which was 119% of the predicted value by Aschoff (1981). Within and above the TNZ,Cincreased significantly withTa, and reached to (0.37± 0.03) mLO2/(g·h·℃) at 35 ℃ (Fig. 1c).
2.2 Elliot’s Pheasant
Elliot’s Pheasant maintained stableTbs withinTarange of 5 − 35℃, at which the meanTbwas (40.36± 0.10) ℃ (Fig. 2a).
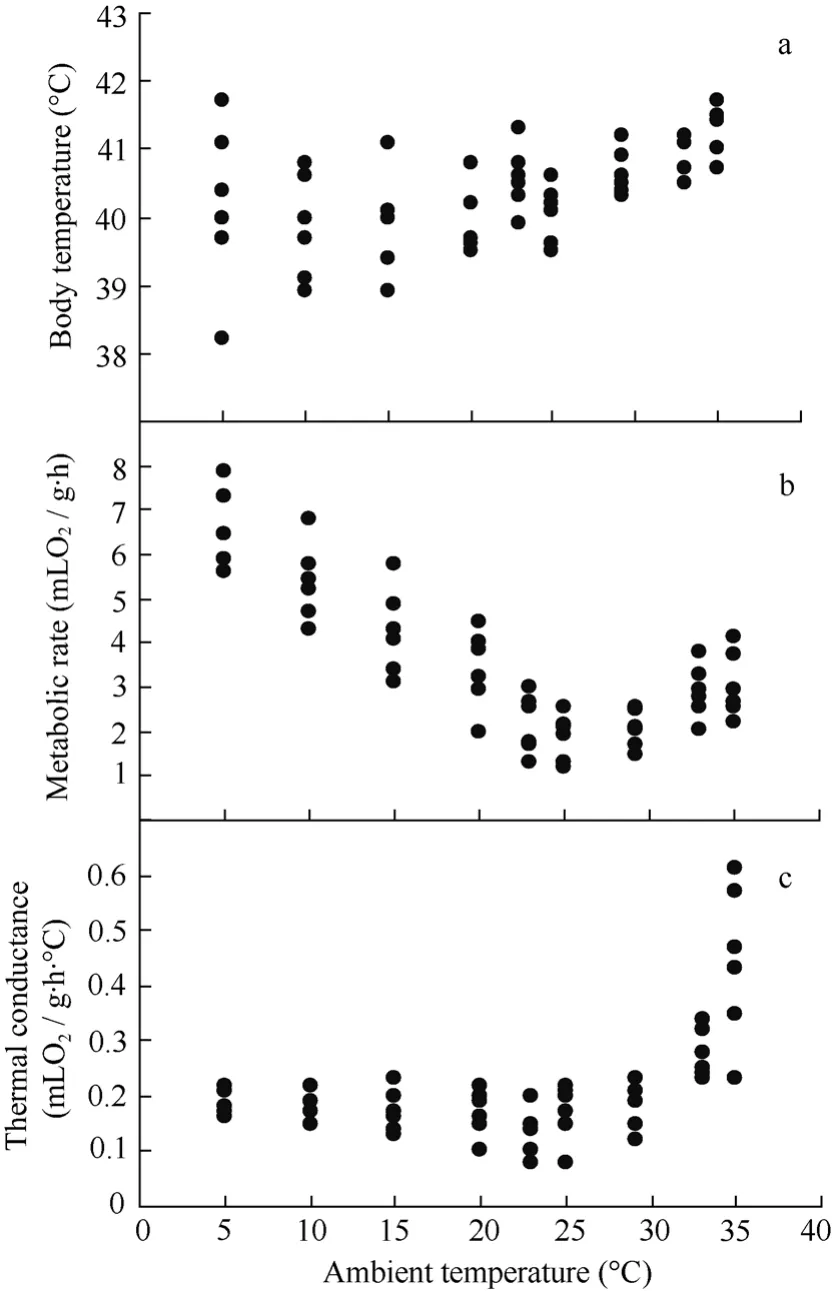
Fig. 2 Changes in body temperature(a), metabolic rate(b) and thermal conductance(c) with ambient temperature in Elliot’s Pheasant
There was no significant difference for metabolic rates between 23 and 29.2℃ (Fig. 2b). The thermal neutral zone (TNZ) was from 23 to 29.2 ℃. MeanBMRwas (2.03±0.12) mLO2/(g·h) (n= 18). Metabolic rates between 20 and 23 ℃ showed a significant difference (t= 4.690,df= 22,P= 0.000<0.0001), with the difference between 29.2 ℃ and 33 ℃ also significant (t= 3.407,df= 22,P= 0.03<0.05). Rate of metabolism was variable at temperature below 23℃ and dependent onTa.
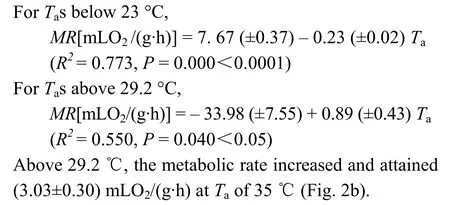
Thermal conductance (C) was calculated as (0.17± 0.01) mLO2/(g·h·℃), which was 124% of the predicted value by Aschoff (1981). Within and above the TNZ,Cincreased significantly withTa, and reached to (0.44± 0.06) mLO2/(g·h·℃) at 35 ℃ (Fig. 2c).
3 Discussion
3.1 Body temperature
The samples collected in this experiment were relatively small. According to Zhao (2009), the body temperature of Mrs Hume’s Pheasant and Elliot’s Pheasant nestings changes with ages. Specifically at 60 days of age the body temperature of the two nestlings fluctuates, while after 60 days of age body temperature stabilizes close to the adult level. This shows that the ability of chemical thermoregulation of Mrs Hume’s Pheasant and Elliot’s Pheasant is developed to stablity level after being hatched for a certain age, usually about 60 days, which is consistent with previous studies onTragopan caboti(Li et al, 1993). Therefore, we measured the metabolites of 80-day-old Mrs Hume’s Pheasant and Elliot’s Pheasant.
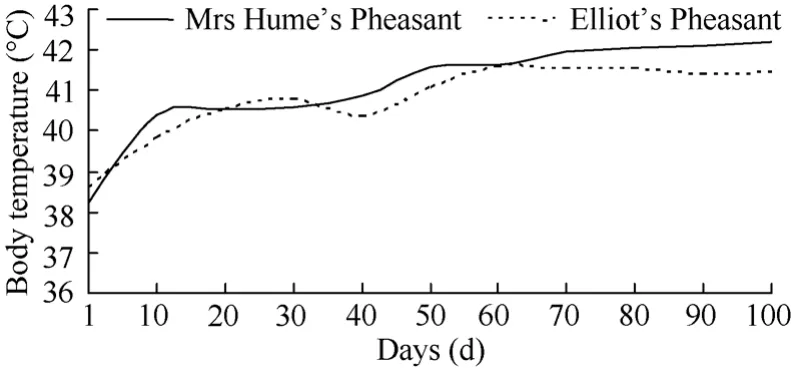
Fig. 3 Changes in body temperature with age in nestling of Mrs Hume’s Pheasant and Elliot’s Pheasant
Compared with mammals, birds have relatively high Tbs, due to higher energy metabolism needed for bird flight (Prinzinger et al, 1991). Birds can usually maintain body temperature at 40 − 42 ℃, and in the TNZ, most birds have a Tbof 38.4 ℃. In this study, the Tbs of Mrs Hume’s Pheasant (40.47 ℃) and Elliot’s Pheasant (40.36℃) were higher thanCrossoptilon mantchuricum(38.7℃) andLyruruste trix baikallensis(39.5 ℃)(Jia et al, 2003; Zhang et al, 2001), similar toCoturnix coturnix(41.5 ℃) andAcridotheres cristatellus(41.4 ℃)(Wang & Zhang, 1986; Lin et al, 2010), but lower thanErythrura gouldiae(42.7 ℃)(Burton & Weathers, 2003). This has great adaptive significance because the high Tbincreases the temperature difference between the body and their environments and increases the ability to dissipate heat in summer (McNab, 2000).
3.2 Basal metabolic rate (BMR)
Many factors affect basal metabolism in birds, such as body mass, climate condition, food habits, season, activity and feeding habits (AL-Mansour, 2004; Weathers, 1979; Liknes et al, 2002; McKechnie &Wolf, 2004; McNab, 1988). In this study, the BMR of Mrs Hume’s Pheasant and Elliot’s Pheasant were 77% and 86% of the expected value from Ashoff & Pohl (1970), respectively (Tab. 1). The BMRs of Mrs Hume’s Pheasant and Elliot’s Pheasant were lower thanPrunella rubeculoides(115%)(Deng & Zhang, 1990),Prunella montanella(168%)(Liu et al, 2004a), andFringilla montifringilla(135%)(Liu et al, 2004b); but close toErythrura gouldiae(81%)(Burton & Weathers, 2003),Estrida melpodaandChloebia gouldiae(82% and 80% respectively)(Marschall & Prinzinger, 1991), andPycnonotus sinensisandSturnus sericeus(79% and 90% respectively) (Zhang et al, 2006).Prunella rubeculoideslive in the Qinghai-Tibet Plateau, which experience an average summer temperature of 8.7 ℃;Prunella montanellaandFringillamontif ringillabreed in Siberia and other northern regions, whose heat regulation has obvious high altitudes and cold animals metabolic characteristics.Erythrura gouldiaelives in California and other hot and humid regions, where the average winter temperature is 24.4℃;Estrida melpodaandChloebia gouldiaelive in the humid tropical environment;Pycnonotus sinensisandSturnus sericeuslive mainly in the tropical south and southern subtropical monsoon zone of China, and exhibit energy metabolism characteristics typical of tropical birds. The lower BMR of tropical birds is an adaptation to heat stress and water maintenance (Williams & Tieleman, 2000; Tieleman et al, 2002). The BMRs of Mrs Hume’s Pheasant (77%) were lower than Elliot’s Pheasant (86%), which may relate to the geographical and latitudinal distribution of the two birds as generally a 1℃ increase with latitude causes a 1% higher average metabolic rate (Zhang et al, 2001). Mrs Hume’s Pheasant and Elliot’s Pheasant show characterisitcs of southern humid zone animals, with their lower metabolic levels a strategy for adapting to the environment. In addition, these two different experimental birds were able to maintain constant body temperatures, that is, when the ambient temperature increased, the body temperature did not increase. To maintain a constant body temperature in a high-temperature environment is one reason why the metabolic rate was relatively low (Rozman et al, 2003; Schleucher, 2002).
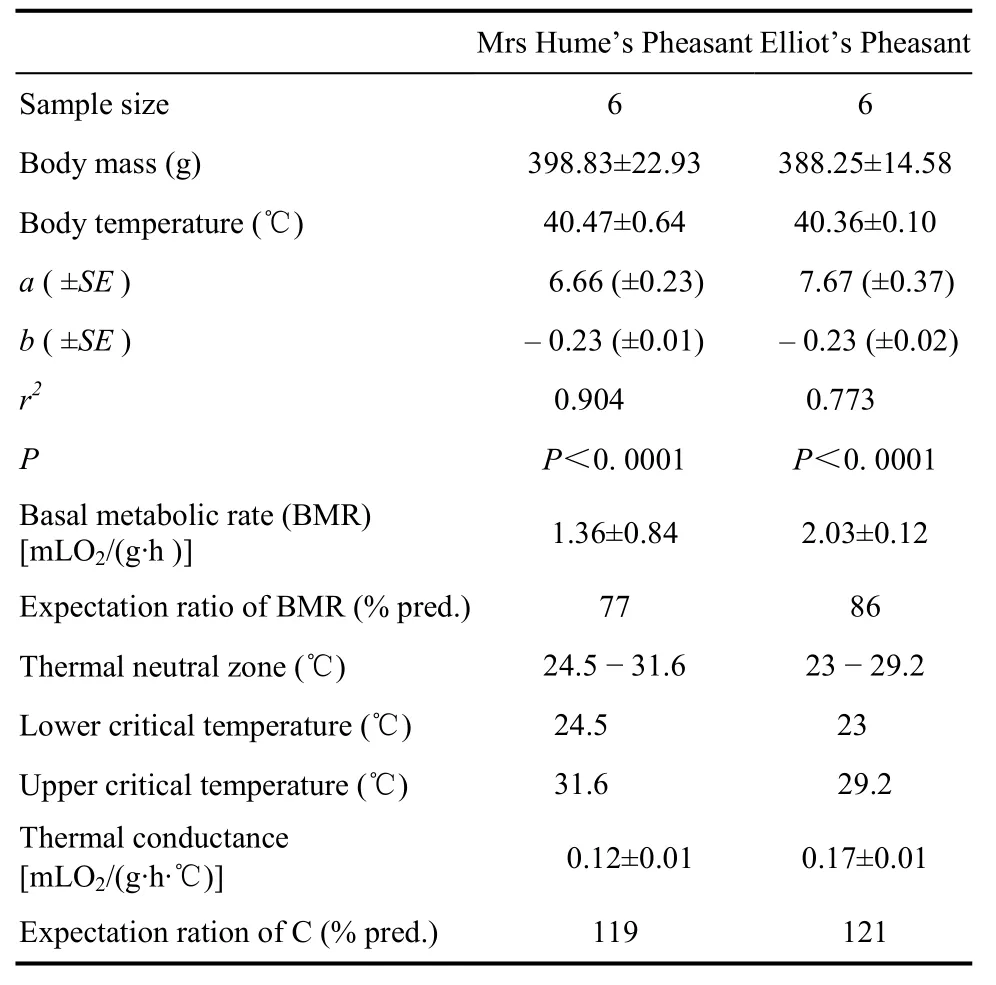
Tab. 1 Parameters of energetics in Mrs Hume’s Pheasant and Elliot’s Pheasant
3.3 Thermal neutral zone
The thermal neutral zone (TNZ) is difineas as the range of temperatrues where production of surplus heat is sufficient to compensate for heat loss, without regulatory changes in metabolic heat production or evaporative heat loss (McNamara et al, 2004). In the TNZ, metabolic rate is independent of Taand animals can regulate temperature by controlling heat loss instead of metabolic heat production and evaporative heat regulation (Schmidt-Nielsen, 1997). This study showed that the TNZ for Mrs Hume’s Pheasant was 24.5 − 31.6 ℃ and for Elliot’s Pheasant was 23.0 − 29.2 ℃ (Tab. 1). The lower critical temperatures of both these special were higher thanCrossoptilon mantchuricum(20 ℃)(Jia et al, 2003),Lyruruste trix baikallensis(20 ℃)(Zhang et al, 2001),Bombycilla garrulusandEmberiza spodocephala(18 ℃and 20 ℃ respectively)(Li et al, 2005); and similar toCoturnix coturnix(25℃)(Wang & Zhang, 1986), but were lower thanErythrura gouldiae(31.7 ℃)(Burton & Weathers, 2003), andAlaemon alaudipes(32.7℃) (Tieleman et al, 2002). A birds’ high thermal conductance and high temperature can increases both lower and the upper critical temperatures, thus reducing evaporative water loss and reduce energy consumption (Burton & Weathers, 2003). Both Mrs Hume’s Pheasant and Elliot’s Pheasant had high thermal conductivity and narrow TNZ, which is conducive to the protection of water evaporation and loss and it is an adaptive characteristics to help survive in hot and humid environments.
3.4 Thermal conductance
Overall conductance depends on body weight because of the size dependent changes in the ratio of surfacevolume, and the dependence of plumage thichness on size (Aschoff, 1981). In the present study, conductance of Mrs Hume’s Pheasant and Elliot’s Pheasant were (0.12±0.01) mLO2/(g·h·℃) and (0.17±0.01) mLO2/ (g·h·℃) (Tab. 1), which were 119% and 124% of the expected (Aschoff, 1981) values respectively. As the birds were 80 days old in the experiment and were still in the long feathers period, insulation was relatively poor, and therefor heat dissipation and thermal conductivity were relatively high. In addition, thermal conductivity (C) of birds in tropical areas is relatively high but is relatively low for bird in cold regions (Weathers, 1997) . For Mrs Hume’s Pheasant and Elliot’s Pheasant, the high C observed in summer is adaptation to the hot environment as it is conductive for high heat dissipation to avoid overheating.
In short, the ecological characteristics of Mrs Hume’s Pheasant and Elliot’s Pheasant are in accordance with the metabolic characteristics of southern birds, that is higher body temperature, lower metabolic rate, and higher thermal conductivity. They can better adapt to hot and humid environments through good physical and chemical regulation.
Acknowledgements:We are grateful to Professor LIU Jin-Song of Wenzhou University for providing valuable suggestions and references during this experiment.
AL-Mansour MI. 2004. Seasonal variation in basal metabolic rate and body composition within individual sanderling birdCalidris alba[J].J Biol Sci, 4: 564-567.
Aschoff J. 1981. Thermal conductance in mammals and birds: its dependence on body size and circadian phase[J].Comp Biochem Physiol, 69A: 611-619.
Aschoff J, Pohl H. 1970. Metabolism at rest of birds as function of time of day and body size[J].Ornithol, 111: 38-47.
Baillie JEM, Hiltorr Taylor C, Stuart SN. 2004. 2004 IUCN Red List of Threatened Species: A Globe Species Assessment[M]. Switzerland: IUCN.
Burton CT, Weathers WW. 2003. Energetics and thermoregulation of the Gouldian finchErythrura gouldiae[J].Emu, 103: 1-10.
Canterbury G. 2002. Metabolic adaptation and climatic constraints on winter birds distribution[J].Ecology, 83: 946-957.
Corp N, Goman ML, Speakman JR. 1997. Seasonal variation in the resting metabolic rate of male wood miceApodemus sylvaticusfrom two contrasting habitats 15km apart[J].J Comp Physiol, 167: 229-239.
Deng HL, Zhang XA. 1990. Standard metabolic rate in several species of passerine birds in alpine meadow[J].Acta Zool Sin, 36(4): 377-384. (in Chinese)
Górecki A. 1975. Kalabukhov-Skvortsov. Respirometer and Resting Metabolic Rate Measurement[M] // Grodziński W. IBP Handbook, No. 24: Methods for Ecological Energetics. Oxford: Blackwell, 309-313.
Jessen C. 2001. Temperature Regulation in Humans and other Mammals[M]. New York: Springer - Verlag Berlin Heidelberg, 1-193.
Jia F, Wu YF, Wu ML, Guo SB, An CL, Pang XB. 2003. Study on the resting metabolic rate (RMR) for the caged female brown eared pheasant (Crossoptilon mantchuricum)[J].Chn J Zool, 38(6): 52-56. (in Chinese)
Li J, Li QF, Zheng GM. 1993. Studies on the resting metabolic rate of the yellow-bellied tragopan[J].Zool Res, 14(4): 341-345. (in Chinese)
Li M, Liu JS, Han HL, Zhang HJ, Fang H. 2005. Metabolism and thermoregulation in waxwingsBombycilla garrulousand blackfaced buntingsEmberiza spodocephala[J].Zool Res, 26: 287-293. (in Chinese)
Liknes ET, Scott SM, Swanson DL. 2002. Seasonal acclimatization in the American goldfinch revisited: to what extent do metabolic rates vary seasonally[J].Condor, 104: 548-557.
Lin L, Wang LH, Liu JS. 2010. Metabolism and thermoregulation in Crested Mynas (Acridotheres cristatellus)[J].Chn J Zool, 45(5): 47-53. (in Chinese)
Liu JS, Chen MR, Wang Y, Wang XH, Song CG. 2004a. Metabolic thermogenesis of Siberian accentor (Prunella montanella) [J].Zool Res, 25(2): 117-121. (in Chinese)
Liu JS, Wang DH, Wang Y, Chen MH, Song CG, Sun RY. 2004b. Energetics and thermoregulation of theCarpodacus roseus,Fringilla montifringillaandAcanthis flammea[J].Acta Zool Sin, 50: 357-363.
Liu Z, Zhou W, Zhang Q, Li JX, Ling N, Zhang RE. 2008. Selection and plant community characteristics of foraging sites for Hume’s Pheasant (Syramticus humiae) in Nanhua part of Ailaoshan National Nature Reserve[J].Zool Res, 29(6): 464-452. (in Chinese) Lovegrove BG. 2003. The influence of climate on the basal metabolic rate of small mammals: a slow-fast metabolic continuum[J].J Comp Physiol, 173: 87-112.
Mackinnon J, Phillipps, K, He FQ. 2000. A Field Guide toThirds of China[M]. Oxford University Press, 35: 15-30.
Marschall U, Prinzinger R. 1991. Verleichende okophysiologie von funf prachtfinkenarten (Estrididae) [J].Fur Orni, 132: 319-323.
McKechnie AE, Wolf BO. 2004. The allometry of avian basal metabolic rate: good predictions need good data[J].Physiol Biochem Zool, 77: 502-521.
McNab BK. 1988. Food habits and the basal rate of metabolism in birds[J].Oecologia, 77: 343-349.
McNab BK. 2000. The influence of body mass, climate, and distribution on the energetic of south pacific pigeons[J].Comp Biochem Physiol. 127A: 309-329.
McNamara JM, Ekman J, Houston AI. 2004. The effect of thermoregulatory substitution on optimal energy reserves of small birds in winter[J].Oikos, 105: 192-196.
Prinzinger R, Prebmar A, Schleucher E. 1991. Body temperature in Birds[J].Comp Biochem Physiol, 89: 499-506.
Reynolds PS, Lee RM. 1996. Phylogenetic analysis of avian energetics Passerines and non-passerines do not differ[J].Am Nat, 147: 735-759.
Rezende EL, Swanson DL, Novoa FF. 2002. Passerines versus nonpasserines: so far, no statistical differences in the scaling of avian energetics[J].J Exp Biol, 205: 101-107.
Rozman J, Runciman D, Zann RA. 2003. Seasonal variation in body mass and fat of Zebra Finches in south-eastern Australia[J].Emu, 103: 11-19.
Schleucher E. 2002. Metabolism, body temperature and thermal conductance of fruit-doves (Aves: Columbidae, Treronidae) [J].Comp Biochem Physiol,131: 417-428.
Schmidt-Nieisen K, 1997. Animal Physiology [M]. 5th ed. London: Cambridge University Press. 169-214.
Shi JB, Zheng GM. 1997. The seasonal changes of habitats of Elliot’s pheasant[J].Zool Res, 18(3): 275-283. (in Chinese)
Stokkan KA. 1992. Energetics and adaptation to cold in ptarmigan in winter[J].Ornis Scandinavica, 22: 366-370.
Tieleman BI, Willians JB, Buschur ME. 2002. Physiological adjustments to arid mesic environments in larks (Alaudidae) [J].Physiol Biochem Zool, 75: 305-313.
Wang PC, Zhang P. 1986. Resting metabolic rates and homoeothermic level of different aged Eastern Ouill[J].J East China Normal Univ:Natural Science Ed, 4: 108-112. (in Chinese)
Weathers WW. 1979. Climatic adaptation in avian standard metabolic rate[J].Oecologia, 42: 81-89.
Weathers WW. 1997. Energetics and thermoregulation by small passerines of the humid, lowland tropics[J].Auk, 114: 341-353.
Williams JB, Tieleman BI. 2000. Flexibility in basal metabolic rate and evaporative water loss among hoopoe larks exposed to different environmental temperature[J].J Exp Biol, 203(20): 3153-3159.
Zhang LQ, Yang ZC, Wu YF, Li CQ, Sun RY. 2001. Study on the resting metabolic rate (RMR) of caged black grouse (Lyrurus tetrix baikallensis)[J].J Hebei Normal Univ:Nat Sci Ed, 25(3): 381-384. (in Chinese)
Zhang YP, Liu JS, Hu XJ, Yang Y, Chen LD. 2006. Metabolism and thermoregulation in two species of passerines from south-eastern China in summer[J].Acta Zool Sin, 52(4): 641-647. (in Chinese)
Zhang ZW, Ding CQ, Ding P, Zheng GM. 2003. The current status and a conservation strategy for species of Galliformes in China[J].J Biodiver Sci, 11: 414-421. (in Chinese)
Zhao T. 2009. Comparison of Nestlings Growth betweenSyrmaticus elliotiandSyrmaticus humiaein Captivity[D]. Ph.D. College of Life Science, Guangxi Normal University. (in Chinese)
笼养黑颈长尾雉和白颈长尾雉代谢产热特征及体温调节
骆 鹰1, 庾太林2,*, 黄乘明3,*, 赵 彤2, 李汉华2, 李常健1
(1.湖南科技学院 生命科学与化学工程系,湖南 永州425100; 2.广西师范大学 生命科学学院,广西 桂林541004; 3.中国科学院动物研究所,北京100101)
采用封闭式流体压力呼吸仪, 在5~35 ℃的环境温度范围内测定了黑颈长尾雉(Syrmaticus humiae)和白颈长尾雉(Syrmaticus ellioti)的代谢率(MR)、热传导(C) 和体温(Tb)等指标, 探讨了其代谢产热特征。结果显示:黑颈长尾雉和白颈长尾雉的热中性区(TNZ)分别为24.5~31.6 ℃和23.0~29.2 ℃。在5~35 ℃的温度范围内, 黑颈长尾雉和白颈长尾雉能保持稳定的体温, 分别为(40.47±0.64)和(40.36±0.10) ℃; 在热中性区内, 黑颈长尾雉和白颈长尾雉的平均基础代谢率(BMR)分别为(1.36±0.84)和(2.03±0.12 ) mLO2/(g·h),分别是体重预期值的77 %和86%。在下临界温度以下, 黑颈长尾雉和白颈长尾雉的最小热传导分别是(0.12±0.01)和(0.17±0.01) mLO2/(g·h·℃), 分别是体重预期值的119%和124%。这两种鸟的生理生态学特征是:黑颈长尾雉和白颈长尾雉都具有较低的代谢率, 较高的体温和热传导, 能较好地适应南方湿热的气候特征。
黑颈长尾雉; 白颈长尾雉; 体温; 基础代谢率; 热传导
Q959.725; Q958.112.4
A
0254-5853-(2011)04-0396-07
2011-01-17;接受日期:2011-05-19
骆鹰(1979-),男,讲师,硕士研究生。研究方向:动物生理生态学
10.3724/SP.J.1141.2011.04396
date: 2011-01-17; Accepted date: 2011-05-19
s: This research was funded by the National Natural Science Foundation of China (30760039), the Key Laboratory of Ecology of Rare and Endangered Species and Environmental Protection (Guangxi Normal University), Ministry of Education, China, and the projects of Science and Technology Bureau of Yongzhou, Hunan (201019)
*Corresponding authors (通信作者), E-mail: yutail@163.com; cmhuang@ioz.ac.cn
- Zoological Research的其它文章
- 悬尾应激对小鼠空间记忆及其反转学习的损伤效应
- Behavioral migration diversity of the Yangtze River Japanese Eel, Anguilla japonica, based on otolith Sr/Ca ratios
- Visual modeling reveals cryptic aspect in egg mimicry of Himalayan Cuckoo (Cuculus saturatus) on its host Blyth’s Leaf Warbler (Phylloscopus reguloides)
- Afferent and efferent pathways in the visual system of the freshwater snail Planorbarius corneus
- Notch signaling dependent differentiation of cholangiocyte-like cells from rhesus monkey embryonic stem cells
- Localization of stationary pronuclei during conjugation of Paramecium as indicated by immunofluorescence staining

