Synergistic Multilayer Adsorption for Low Concentration Dyestuffs by Biomass
Keith Kim Hung Choy* and Gordon Mckay
Department of Chemical and Biomolecular Engineering, the Hong Kong University of Science and Technology,Hong Kong, China
1 INTRODUCTION
Increasing government legislative requirements for effluent discharges emphasize the need for effective treatment methods. Coloured organic compounds generally impart a considerable organic load in wastewaters; furthermore, their deep colour is easily detectable and detracts from the aesthetic value of rivers and receiving waters. In addition, the presence of colour reduces photosynthetic processes and some dyestuffs, particularly some azo dyes, are carcinogenic and toxic [1, 2]. Large volumes of aqueous effluent are discharged by the clothing sector, including textiles,leather and dyeing—70% of commercial dyes produced are azo dyes [3].
Adsorption is gaining prominence as an effective and cost effective method for the removal of pollutants from wastewaters [4]. The mechanism is based on the ability of porous solids, adsorbents, to concentrate and hold solute molecules, such as dyestuffs, on their surfaces. In recent years extensive research has been undertaken to identify suitable adsorbents for dyes.
Adsorption of acid dyes has been studied using peat [5-8], activated carbon [9, 10], orange pith, banana pith and bagasse sugar cane pith [11-16], fuller’s earth[17, 18], and wood [19, 20]. Other dye-adsorbent systems have also demonstrated commercial potential,including hardwood [20-23], waste red mud [24-26],agricultural byproducts [27-29]. Yoshida et al. [30-32]studied the adsorption of various dyes on chitosan fibers, and the use of chitin/chitosan has been assessed in various studies [33-38].
Due to the high cost of adsorbents, such as active carbons, and high costs of thermal regeneration in recent years, much research has focused on the search for cheaper adsorbents which, after saturation, could be combusted and used as fuels. Consequently, bamboo and pallet wood have been selected and tested in the present study to adsorb a basic dye, Methylene Blue, and an acidic dye, Acid Blue 117. In addition,their chars and activated carbons were prepared and tested also using the same dyes. The adsorption capacity results of all these six materials will be compared with the performance of the commercial water treatment activated carbon F400.
Three of these adsorbents were selected for binary layer adsorption to check the multilayer concept and the potential application for better adsorbent usage, that is, after adsorbing the basic dye, adsorption of acidic dye on top of the basic dye loaded adsorbent was tested, thus increasing its capacity and service time. The two cheapest adsorbents, bamboo and wood are compared with the commercial activated carbon F400. All three systems had some ability to multilayer adsorption
2 ADSORPTION THEORY
The equilibrium sorption capacity curves of adsorbents can be obtained by measuring the sorption isotherm of the dyes onto adsorbents. Different sorption equilibrium models have been proposed in describing the adsorption behavior and in this study, two isotherm models, namely, the Langmuir isotherm and Freundlich isotherm were applied to correlate the adsorption process.

Langmuir isotherm describes the monolayer adsorption of homogeneous surfaces [39]. It is based on the kinetic principle in which the rate of adsorption is equal to the rate of desorption from the surface at equilibrium. The constant KLand aLare the Langmuir equilibrium constant, andKL/aLgives the theoretical monolayer saturation capacity,q0.
Freundlich suggested a model to describe the adsorption properties of heterogeneous systems [40],which is characterized by the heterogeneity factor 1/n.It is derived by assuming an exponential decay energy distribution function inserted into the Langmuir equation and not restricted to the formation of monolayer.This model has been widely used in heterogeneous adsorption systems especially for organic compounds and highly interactive species.
3 EXPERIMENTAL METHODS AND MATERIALS
3.1 Adsorbents
Seven kinds of adsorbents were used for single component adsorption in this study: bamboo, wood,bamboo/wood charcoal (activated and non-activated),and commercial activated carbon F-400 (Calgon Carbon, USA). The raw material adsorbents used in this study were waste wood and bamboo, obtained from local carpentry workshop, sawdust and construction site respectively. The wood dust was sieved into a discrete particle size range from 355 to 710 μm and bamboo was cut into pieces and sieved into 500 to 710 μm. Both of the waste materials were cleaned with deionized water and dried at 70 °C in an oven overnight.
To compare with raw materials, carbonized wood and bamboo (charcoal) were produced by placing the prepared raw material into an electric furnace for heating to 500 °C at a speed of 5 °C·min-1with the nitrogen flow rate of 400 ml·min-1. The materials were maintained at this temperature for 0.5 h and then cooled to obtain charcoal.
To increase the pore volume of the adsorbents,activated bamboo/wood charcoal were made by the following steps: first mix the raw material with sulfuric acid (1.84 mg·L-1) with the mass ratio of 1︰3, and then left in the furnace overnight at the temperature of 160 °C. After that, the sample was carbonized in the furnace at the temperature of 750 °C using a heating rate of 5 °C·min-1and nitrogen flow rate of 450 ml·min-1. The materials were maintained at this temperature for 0.5 h and then cooled to obtain activated charcoal. Tests were done to determine the surface area of the produced activated charcoals, and the BET for bamboo charcoal (activated) is 518.6 m2·g-1and for wood is 623.8 m2·g-1.
For the multi-component isotherm, bamboo and wood raw materials as well as AC (F-400) were used.
3.2 Adsorbates
Two commercially available textile dyestuffs were used in the study,i.e. Acid Yellow 117 and Methylene Blue. The dye information is shown in Table 1.Stock solutions of the dyes were prepared in 2 L volumetric flasks with the concentration of 500 mg·L-1and then diluted to make a series of standard solutions to be used for the equilibrium isotherm studies.
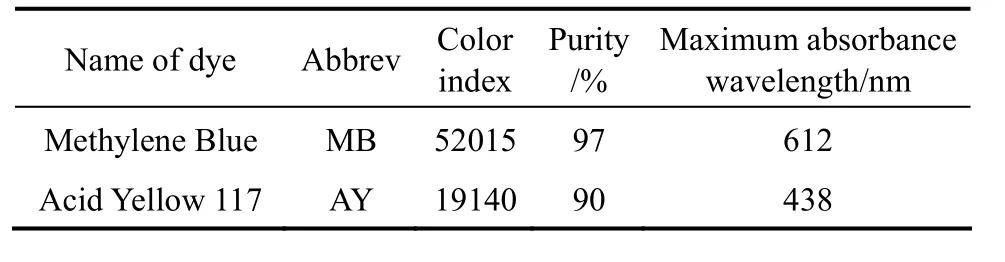
Table 1 Physical and chemical characteristics of dyestuffs
3.3 Methods
3.3.1Equilibrium sorption studies
For the single component adsorption, series of fixed volumes (250 ml) of AY solution and MB solution with predetermined initial dye concentration were prepared and brought into contact with a fixed mass of each kind of adsorbent. The jars were sealed and agitated on the shaker for 15 days when equilibrium was reached. Nine runs of experiments were carried out in the isotherm equilibrium studies:
(1) Acid Yellow—wood-0.02 g;
(2) Acid Yellow—bamboo-0.02 g;
(3) Methylene Blue—wood-0.02 g;
(4) Methylene Blue—bamboo-0.02 g;
(5) Methylene Blue—AC-0.015 g;
(6) Methylene Blue—wood charcoal-0.015 g;
(7) Methylene Blue—bamboo charcoal-0.015 g;
(8) Methylene Blue—activated wood charcoal-0.012 g;
(9) Methylene Blue—activated bamboo charcoal-0.012 g.
For the multi-component isotherm, bamboo and wood raw materials as well as AC (F-400) were used.They were tested for single component adsorption first,until saturated with the dye on their surface. The stock solution of Methylene Blue (800 mg·L-1) was prepared and 900 ml infused into each of the three cone flasks with three types of adsorbents respectively:i.e.bamboo (1.8 g), wood (1.8 g) and activated carbon(0.9 g). The materials were mixed with magnetic stirrers for approximately 15 days until the solution concentration was stable.
Three adsorbents saturated with Methylene Blue were then brought into contact with 260 ml Acid Yellow solutions individually with predetermined initial concentration. And the jars were also sealed and agitated on the shaker for another 15 days when equilibrium was reached. Three runs of experiments were carried out for the multi-component isotherm equilibrium studies:
(1) Acid Yellow—wood (saturated with MB)-0.05 g;
(2) Acid Yellow—bamboo (saturated with MB)-0.05 g;
(3) Acid Yellow—AC (saturated with MB)-0.012 g.
At timet=0 and equilibrium, the dye concentrations of the solutions were measured with a UV/VIS spectrophotometer. These data were used to calculate the adsorption capacity,qeof the adsorbent. Finally,diagrams of adsorption capacity,qeagainst equilibrium concentrationCe, were plotted for various systems.
3.3.2Concentration measurement and calibration
For single component systems, in order to calculate the concentration of the sample from the optical density value, a dye calibration curve was first prepared for the two dyes, three concentrations (5, 10, 20 mg·L-1) of standard dye solutions were prepared to determine the calibration curve. Each sample taken was analyzed using a Varian Cary 1E spectrophotometer by measuring the optical density (OD) of the dye at their respective maximum absorbance wavelengths (438 nm for Acid Yellow and 612 nm for Methylene Blue). From these results, the concentration of the dye samples can be calculated with the following equations and the constants for calculations are summarized in Table 2.Acid Yellow:
dye concentrationC=optical density (OD)/kAYMethylene Blue:
dye concentrationC=optical density (OD)/kMBwherekAYandkMBare calibration constants for the Acid Yellow and Methylene Blue respectively, at their maximum absorbance wavelength.
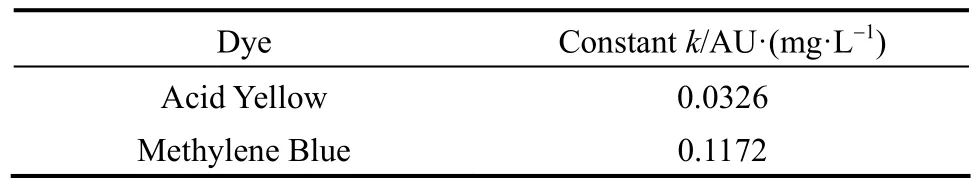
Table 2 Constants for calculations of dye concentrations
The multi-component system consisting dye components A and B, measured at the maximum absorbance wavelengthsλ1andλ2respectively gave optical densities (OD) ofd1andd2. The respective single component concentrations for dyes A and B are given by the following equations:
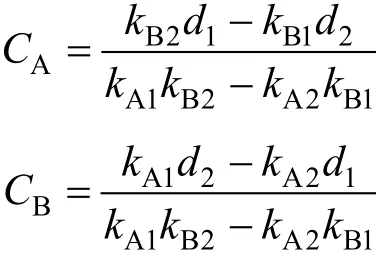
wherekA1,kB1,kA2andkB2are the calibration constants for components A and B at the two wavelengthsλ1andλ2respectively. The optical density values for the two dyes at two wavelengths are shown in Table 3.
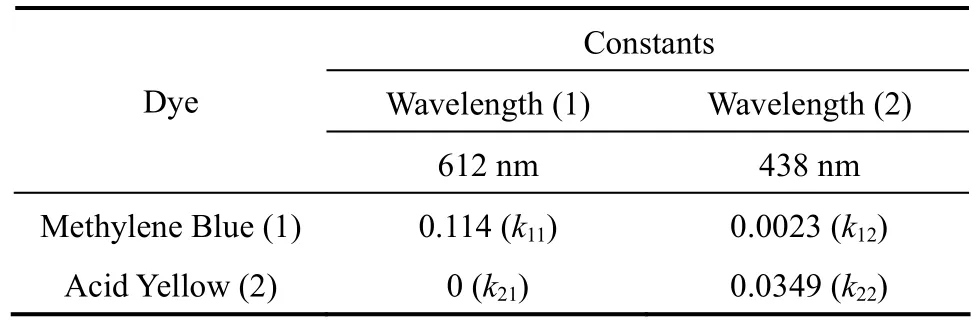
Table 3 Constants for calculations of dye concentrations in mixtures from optical density values
Finally, the dye concentration at equilibrium,qewas calculated from
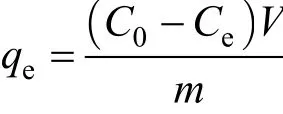
whereqe=the dye concentration in the adsorbent equilibrium (mg dye per g adsorbent),C0=the initial dye concentration in the liquid phase (mg dye per L),Ce=the liquid-phase dye concentration at equilibrium(mg dye per L),V=the total volume of dye solution used (L), andm=the mass of adsorbent used (g).
4 RESULTS AND DISCUSSION
4.1 Single component adsorption
The experimental results of single component systems show that the wood and bamboo show preference for Methylene Blue and cannot adsorb the Acid Yellow dye. The experimental data of the Methylene Blue on seven adsorbentsi.e. bamboo, wood, bamboo/wood charcoal (activated and non-activated), and AC is shown in Fig. 1, analyzed according to the Langmuir isotherm and Freundlich isotherm. The adsorption constants for the two models as well as the sum of square of errors are listed in Table 4 and Table 5. Based on the Langmuir isotherm analysis, the monolayer adsorption capacities of Methylene Blue were determined to be 57.16 mg per g bamboo; 73.88 mg per g wood; 75.41 mg per g bamboo charcoal; 81.25 mg per g wood charcoal; 112.97 mg per g activated bamboocharcoal; 121.00 mg per g activated wood charcoal;and 366.61 mg per g AC. By comparison with the sum of square errors of the two isotherm analysis, the Freundlich isotherm equation was found to better predict the adsorption for the seven adsorbents.
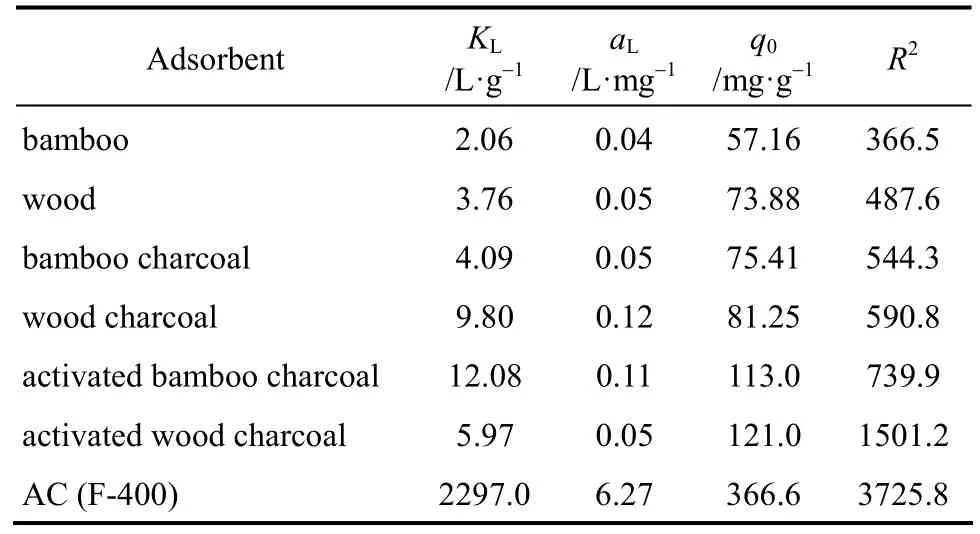
Table 4 Langmuir sorption isotherm constants for seven adsorbents
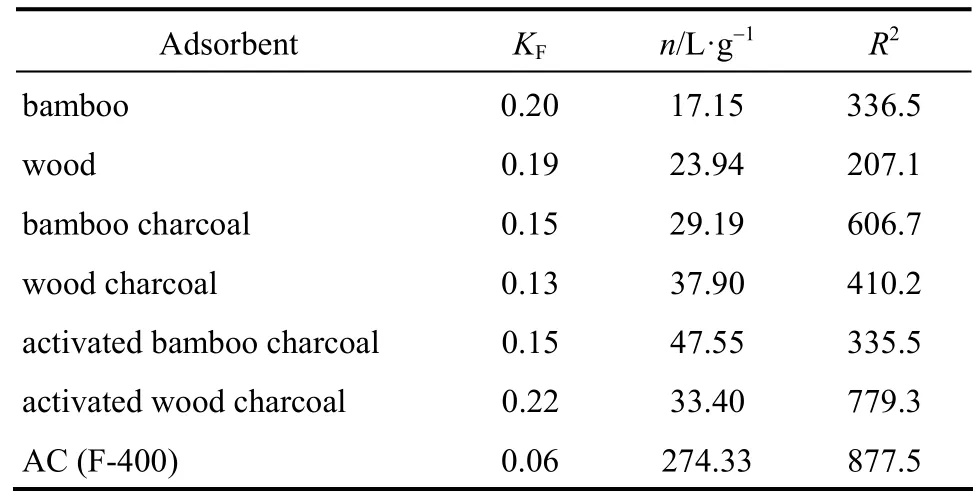
Table 5 Freundlich sorption isotherm constants for seven adsorbents
From their performance, it can be seen that all the adsorbents can adsorb the dye of Methylene Blue and their saturation capacity derived from Langmuir isotherm are in the order of bamboo<wood<bamboo charcoal<wood charcoal<activated bamboo charcoal<activated wood charcoal<AC. This proves that the raw material bamboo and wood have the ability to remove Methylene Blue from effluents, and with carbonization(better after activation) treatments, the charcoal produced from the raw materials can have nearly one third the adsorption capacity as that of commercial activated carbon.
4.2 Multi-layer adsorption
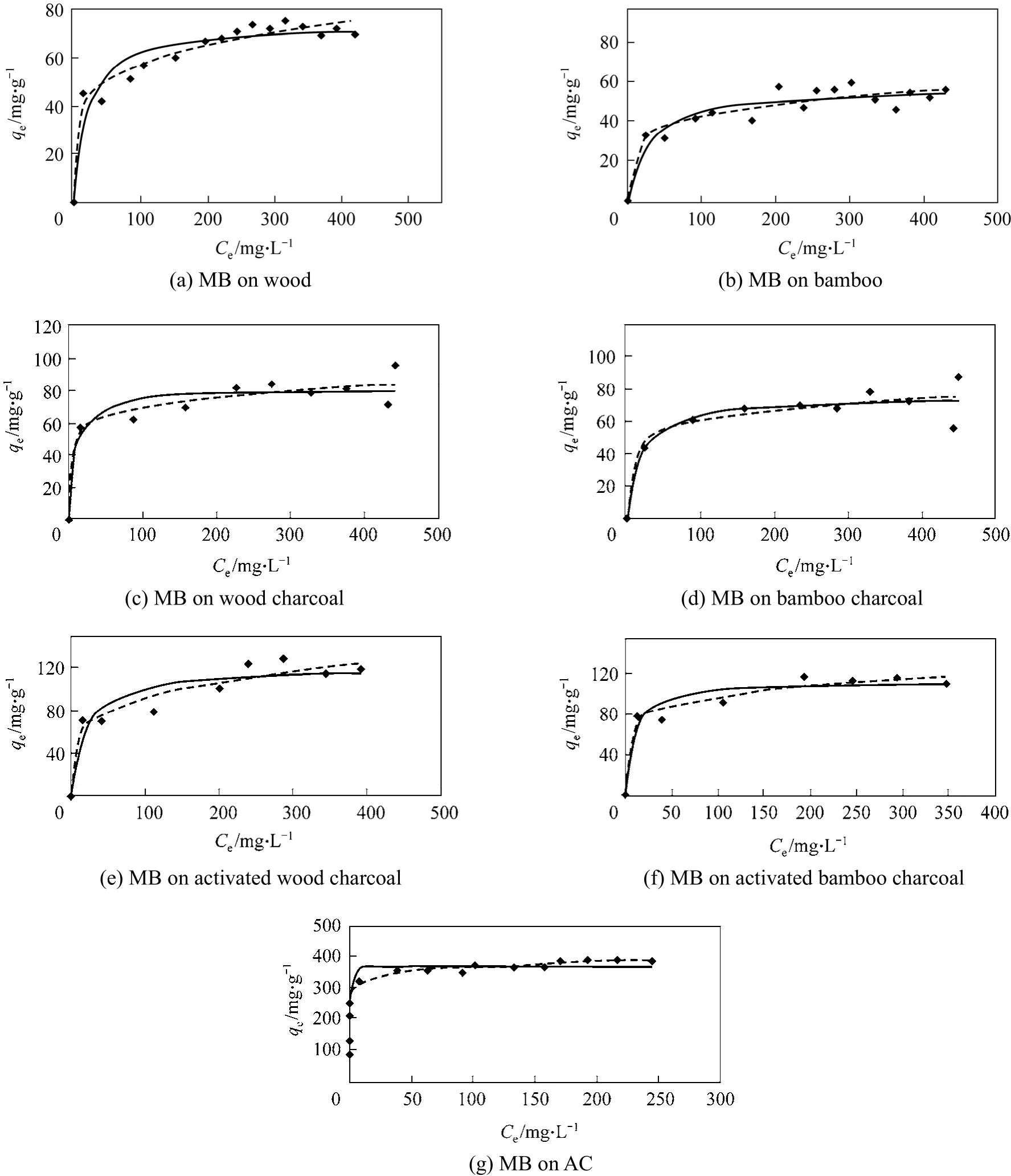
Figure 1 Adsorption of Methylene Blue onto different adsorbents◆ exp;Lang; Freund
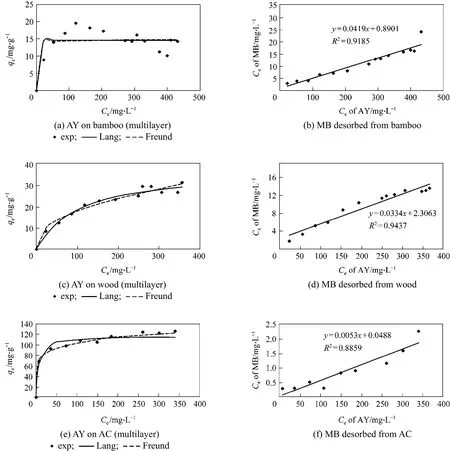
Figure 2 Multi-component adsorption of Acid Yellow and Methylene Blue on different adsorbents
The experimental results of multi-layer systems are shown in Fig. 2 and Tables 6, 7. Langmuir and Freundlich isotherm were applied to predict the adsorption data. Based on the Langmuir isotherm analysis, the second layer adsorption capacities of Acid Yellow were determined to be 14.55 mg per g bamboo;37.95 mg per g wood; and 116.54 mg per g AC. The Freundlich isotherm equation was found to be better in predicting the adsorption, especially for AC.
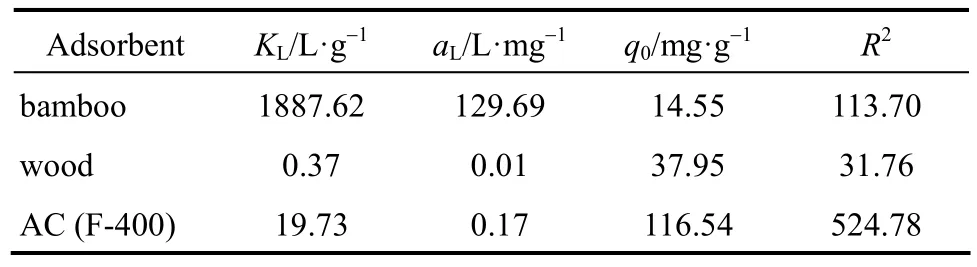
Table 6 Langmuir sorption isotherm constants for three adsorbents (multilayer)
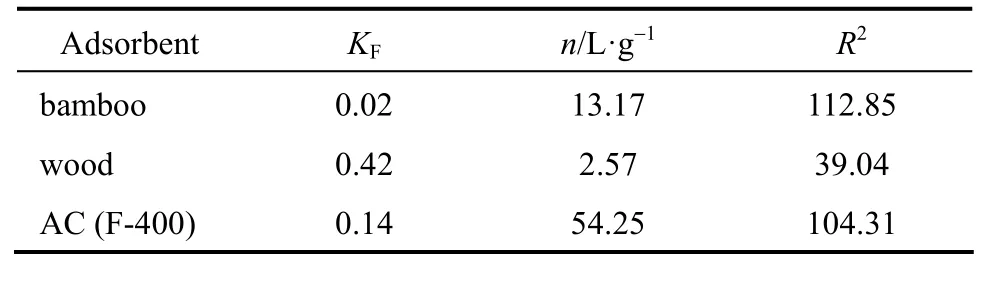
Table 7 Freundlich sorption isotherm constants for three adsorbents (multilayer)
Wood and bamboo and their chars and carbons were shown not to adsorb Acid Yellow 117 in the single component experiment; however, with a saturated layer of Methylene Blue, the adsorbents could adsorb the dye of Acid Yellow, which indicates that interaction has taken place between the two kinds of dye molecules and it is the bond between the basic dye and acid dye that attracts the Acid Yellow molecular on the Methylene Blue layer surface. That is, a synergism multilayer adsorption has formed on the waste surface.The same phenomenon happened for the AC and the Acid Yellow adsorbed on the second layer (116.54 mg per g AC) was approximately half the quantity for the single component adsorption, but less than 2.5 mg·L-1of MB was desorbed from the AC. The binary layer adsorption data of Acid Yellow on bamboo loaded with Methylene Blue show more deviations than the other systems; this was probably due to the fact that it was difficult to collect and dry the bamboo particles after the first adsorption of Methylene Blue, because the system became slightly colloidal making the separation process of the Methylene Blue loaded particles from the solution difficult and giving a larger error in the bamboo mass readings for the second adsorption.
Also it was noticed that wood has a better adsorption capacity of Acid Yellow on the second layer than bamboo, and amounts to nearly one third of the capacity of AC. The multi-component experiment gives the potential of applying the waste wood and bamboo to treat effluents with Acid Yellow and Methylene Blue at the same time. Since the layers of the two dyes overlap, rather than competing for the sites on the adsorbent surface, the adsorbents are utilized with a better efficiency.
5 CONCLUSIONS
The textile industry discharges large volume of colored effluents which can be treated by adsorption to remove the dyes. Due to the high cost of adsorbents,such as active carbons, and high costs of thermal regeneration in recent years, much research has focused on the search for cheaper adsorbents which could even be burned as fuel after use. This paper presents a further extension to this application by attempting to re-use the spent adsorbent to adsorb a second layer of dye of different but compatible characteristics, namely,an oppositely charged dye ion.
Several cheap adsorbents and derived adsorbents have been shown to be successful in adsorbing single component basic and/or acidic dyes.
Three of the adsorbents were selected for binary layer adsorption to check the multilayer concept and the potential application for better adsorbent usage.The two cheapest adsorbents, bamboo and wood and these are compared with the commercial activated carbon F400. All three systems had some ability to multilayer and only using two layers the adsorption capacities increased by 20% to 50%.
Hence, by arranging the flow of dye effluents from textile dyeing companies, they can extend the use of their adsorbents using binary layer or possibly multilayer adsorption.
1 Gottlieb, A., Shaw, C., Smith, A., Wheatley, A., Forsythe, S., “Toxicity of textile reactive azo dyes after hydrolysis and decolorisation”, J.Biotechnol., 101, 49-56 (2003).
2 Mathura, N., Bhatnagara, P., Nagarb, P., Bijarnia, M.K.,“Mutagenicity assessment of effluents from textile/dye industries of Sanganer, Jaipur (India): A case study”, Ecotoxicol. Environ. Safety,61 (1), 105-113 (2005).
3 Carliell, C.M., Barclay, S.J., Shaw, C., Wheatley, A.D., Buckley, C.A,“The effect of salts used in textile dyeing on microbial decolourisation of a reactive azo dye”, Environ. Technol., 19 (11), 1133-1137(1998).
4 McKay, G., Use of Adsorbents for the Removal of Pollutants from Wastewaters, CRC Press, Boca Raton, FL, USA (1995).
5 Poots, V.J.P., McKay, G., Healy, J.J., “The removal of basic dye from effluent using Naturally occurring adsorbents (I) Peat”, Water Res.,10, 1061-1066 (1976).
6 Walker, G.M., Weatherley, L.R., “Adsorption of acid dyes on to granular activated carbon in fixed beds”, Water Res., 31 (8), 2093-2101(1998).
7 Ho, Y.S., McKay, G., “Sorption of dye from aqueous solution by peat”, Chem. Eng. J., 70 (2), 115-124 (1998).
8 Ho, Y.S., McKay, G., “The kinetics of sorption of divalent metal ions onto sphagnum moss moss”, Water Res., 34 (3), 735-742 (1999).
9 Davies, R.A., Kaempf, H.J., Clemens, M.M., “Granular carbon for the treatment of textile effluents”, Chem. Ind., 827-831 (1973).
10 McKay, G., “Adsorption of dyestuffs from aqueous solutions with activated carbon (I) Equilibrium and batch contact time studies”, J.Chem. Technol. Biotechnol., 32, 759-772 (1982).
11 McKay, G., El Geundi, M.S., Nassar, M.M., “Equilibrium studies during the removal of dyestuffs from aqueous solutions using bagasse pith”, Water Res., 21 (12), 1513-1518 (1987).
12 Namasivayam, C., Muniasamy, N., Gayatri, K., Rani, M., Ranganathan, K., “Removal of dyes from aqueous solutions by cellulosic waste orange peel”, Biores. Technol., 57, 37-42 (1996).
13 Namasivayam, C., Prabha, D., Kumutha, M., “Removal of direct red and acid brilliant blue by adsorption on to banana pith”, Bioresour.Technol., 64 (1), 77-79 (1998).
14 Chen, B., Hui, C.W., McKay, G., “Pore-surface diffusion modelling for dyes from effluents on pith”, Langmuir, 17, 740-748 (2001).
15 Namasivayam, C., Radhika, R., Suba, S., “Uptake of dyes by a promising locally available agricultural solid waste: Coir pith”,Waste Manage., 21 (4), 381-387 (2001).
16 Namasivayam, C., Kavitha, D., “Removal of Congo Red from water by adsorption onto activated carbon prepared from coir pith, an agricultural solid waste”, J. Dyes Pigments, 54 (1), 47-58 (2002).
17 McKay, G., Otterburn, M.S., Sweeney, A.G., “Fuller’s earth and fired clay as adsorbents for dyestuffs—Equilibrium and rate studies”, Water, Air, Soil Poll., 24, 307-313 (1985).
18 Atun, G., Hisarli, G., Sheldrick, W. S., Muhler, M., “Adsorptive removal of methylene blue from colored effluents on fuller’s earth”, J.Colloid Interface Sci., 261 (1), 32-39 (2003).
19 Poots, V.J.P., McKay, G., Healy, J.J., “Removal of acid dye from effluent using natural adsorbents (2) Wood”, Water Res., 10 (12),1067-1070 (1976).
20 Asfour, H.M., Nassar, M.M., Fadali, O.A., Elgeundi, M.S., “Color removal from textile effluents using hardwood sawdust as an adsorbent”, 35 (1), 28-35 (1985).
21 McKay, G., Ho, Y.S., “ Kinetic models for the sorption of dye from aqueous solution by wood”, Trans. I. Chem. E., 70, 115-124 (1998).
22 Ho, Y.S., McKay, G., “Pseudo-second order model for sorption processes”, Process Biochem., 34 (5), 451-465 (1998).
23 Elgeundi, M.S., “Color removal from textile effluents by adsorption techniques”, Water Res., 25 (3), 271-273 (1991).
24 Namasivayam, C., Arasi, D., “Removal of Congo red from wastewater by adsorption onto waste red mud”, Chemosphere, 34 (2),401-417 (1997).
25 Namasivayam, C., Yamuna, R.T., Arasi, D., “Removal of acid violet from wastewater by adsorption on waste red mud”, Environ. Geol.,41 (3-4), 269-273 (2001).
26 Namasivayam, C., Yamuna, R.T., Arasi, D., “Removal oforange from wastewater by adsorption on waste red mud”,(2002).
27 Marshall, W.E., Champagne, E.T., “Agricultural by-products as adsorbents for metal-ions in laboratory prepared solutions and in manufacturing waste-water”, J. Environ. Sci. Health A Environ. Sci.Eng. Toxic Hazard. Subt. Control, 30 (2), 241-261 (1995).
28 Marshall, W.E., Johns, M.M., “Agricultural by-products as metal adsorbents: Sorption properties and resistance to mechanical abrasion”, J. Chem. Technol. Biotechnol., 66 (2), 192-198 (1996).
29 Valix, M., Cheung, W.H., McKay, G., “Preparation of activated carbon using low temperature carbonisation and physical activation of high ash raw bagasse for acid dye adsorption”, Chemosphere, 56 (5),493-501 (2004).
30 Yoshida, H., Fukuda, S., Okamoto, A., Kataoka, T., “Recovery of direct dye and acid dye by adsorption on chitosan fiber—Equilibria”,Water Sci. Technol., 23 (7-9), 1667-1676 (1991).
31 Yoshida, H., Okamoto, A., Kataoka, T., “Adsorption of acid dye on cross-linked chitosan fibers—Equilibria”, Chem. Eng. Sci., 48 (12),2267-2272 (1993).
32 Yoshida, H., Takemori, T., “Adsorption of direct dye on cross-linked chitosan fiber: Breakthrough curve”, Water Sci. Technol., 35 (7), 29-37(1997).
33 Kim, C.Y., Choi, H.M., Cho, H.T., “Effect of deacetylation on sorption of dyes and chromium on chitin”, J. Appl. Polym. Sci., 63 (6),725-736 (1997).
34 Knorr, D., “Dye binding-properties of chitin and chitosan”, J. Food Sci., 48 (1): 36-41 (1983).
35 dos Anjos, F.S.C., Vieira, E.F.S., Cestari, A.R., “Interaction of indigo carmine dye with chitosan evaluated by adsorption and thermochemical data”, J. Colloid Interface Sci., 253 (2), 243-246 (2002).
36 Shimizu, Y., Kono, K., Kim, I.S., Takagishi, T., “Effects of added metal-ions on the interaction of chitin and partially deacetylated chitin with an azo-dye carrying hydroxyl-groups”, J. Appl. Polym. Sci.,55 (2), 255-261 (1995).
37 Gerente, C., Lee, V.K.C., Le Cloirec, P., McKay, G., “Application of chitosan for the removal of metals from wastewaters by adsorption—Mechanisms and models review”, Criti. Rev. Envir. Sci. Technol., 37,41-127 (2007).
38 Wong, Y.C., Szeto, Y.S., Cheung, W.H., McKay, G., “Effect of temperature, particle and percentage deacetylation on the adsorption of acid dyes on chitosan”, Adsorption, 14, 11-20 (2008).
39 Langmuir, I., “The adsorption of gases on plane surfaces of glass,mica and platinum”, J. Am. Chem. Soc., 40 (9), 1361-1403 (1918).
40 Freundlich, H., “Concerning adsorption in solutions”, Z. Physik Chem., A57 (4), 385-470 (1906).
 Chinese Journal of Chemical Engineering2012年3期
Chinese Journal of Chemical Engineering2012年3期
- Chinese Journal of Chemical Engineering的其它文章
- Experimental and Modelling Studies of Biomass Pyrolysis*
- Kinetics of Photocatalytic Degradation of Gaseous Organic Compounds on Modified TiO2/AC Composite Photocatalyst*
- 3D Numerical Study on Compound Heat Transfer Enhancement of Converging-diverging Tubes Equipped with Twin Twisted Tapes*
- Techno-economic Analysis of Distributed Hydrogen Production from Natural Gas
- Error Analysis of Adsorption Isotherm Models for Acid Dyes onto Bamboo Derived Activated Carbon
- Issues in Freeze Drying of Aqueous Solutions*
