Sonochemical Synthesis of Different Morphological CaF2Microstructures
WANG Miao CHEN Ting-Ting TANG Yan-Feng JIANG Guo-Qing SHI Yu-Jun
(School of Chemistry and Chemical Engineering,Nantong University,Nantong,Jiangsu 226019,China)
Sonochemical Synthesis of Different Morphological CaF2Microstructures
WANG Miao CHEN Ting-Ting TANG Yan-Feng JIANG Guo-Qing SHI Yu-Jun*
(School of Chemistry and Chemical Engineering,Nantong University,Nantong,Jiangsu226019,China)
Different morphological CaF2crystals (cubes,flowers,polyhedra)were synthesized via a mild sonochemical route from an aqueous solution of CaCl2and NaBF4or K2SiF6.The crystal structures and morphologies of the products were characterized by using XRD,SEM and TEM.The results show that the asprepared products are cubic structure and with high crystallinity.Uniform microcubes and polyhedra can be easily synthesized from NaBF4and K2SiF6,respectively.In the presence of complex agent Na2EDTA,uniform 3D flower-like CaF2hierarchical structures were prepared and assembled by numerous nanoflakes.Some reaction parameters (fluoride source,molar ratio and chelating reagent)were investigated during the process of obtaining different morphological CaF2.Furthermore,a possible reaction mechanism for the growth of CaF2is proposed.
CaF2;microstructure;sonochemical route,Na2EDTA
0 Introduction
Ultrasound is a fast,efficient and unique heating method,therefore,sonochemicalroute has been developed into a powerful method for synthesis of micro/nanomaterials.When liquids are irradiated by high-intensity ultrasound,high temperatures,pressures and cooling rates can be achieved upon the collapse of the bubbles[1].The remarkable environments provide a unique platform for the growth of nanomaterials.Up to now,a variety of inorganic materials,including metal oxides,sulfides,hydrates have been prepared via sonochemical method[2-7].However,little work has been conducted for the fabrication of inorganic fluorides[8-9].
As an important fluoride,nanoscale CaF2has attracted increasing interest with respect to UV lithography,UV-transparent optical lenses,the surface conditioning of glass,promoting agents for bone/teeth reconstruction, and biocompatible luminescent markers[10-12].Up to now, a series of CaF2nano/microstructures with different morphologies,such as cubes[13],plates and polyhedras[14], wires[15],hollow spheres[16-18]and flowers[19]have been fabricated by a variety of methods.The synthesis of CaF2nano/microstructures with wet chemical approaches involving polyol method[12],hydrothermal methods[13,17,19],thermolysis[14],coprecipitation[15-16]and reversed micelle[18].However,to the best of our knowledge,few studies have focused on the synthesis of highly mono-dispersed CaF2microstructures by complex fluorides via sonochemical route.Herein,by employing NaBF4or K2SiF6as fluoride sources,we present a simple,facile and effective sonochemical route to synthesize different morphological CaF2.It is found that chelating reagent,fluoride source and molar ratio of reactants play crucial roles in the formation of products with different morphologies.
1 Experimental
1.1 Preparation
CaCl2,NaBF4,K2SiF6and Na2EDTA (AR)were purchased from Shanghai Chemical Reagent Corporation and used without further purification.In a typical procedure,1 mmol of CaCl2was first added to 30 mL distilled water in a 50 mL flask at room temperature,after magnetic stirring for 5 min,0.5 mmol Na2EDTA was introduced under stirring and was continuously stirred for 10 min to form a calcium complex,then followed by addition of 1 mmol of complex fluoride NaBF4.The above mixture solution was irradiated by ultrasonic wave with 50 Hz at the ultrasonic power of 300 W for 1 h.At the end of the sonication,a temperature of about 40℃ was reached under ambient air without cooling.Finally,the white precipitate was centrifuged,washed with distilled water and ethanol,and dried at 70°C for 3 h.
1.2 Materials characterization
The crystalline phases of the products were analyzed by XRD on a Shimadzu XRD-6000 powder X ray diffractometer(Cu Kα radiation,λ=0.154 18 nm), employing a scanning rate of 4.00°·min-1,in the 2θ range from 20°to 80°.The operation voltage and current were maintained at 40 kV and 30 mA, respectively.The sizesand morphologiesofthe resulting products were studied by transmission electron microscopy (TEM,TECNAI F20S-TWIN)at 200 kV and field emission scanning electron micros copy(FE-SEM,HITACHI S-4800)at 15 kV.
2 Results and discussion
2.1 Structure characterization
The XRD patterns shown in Fig.1a are for the sample in a typical experiment,with the fixed molar ratio of CaCl2/EDTA/NaBF4at 1∶0.5∶1,all diffraction peaks can be indexed to cubic structured CaF2(PDF No.04-0864).No peak of impurities is observed, confirming the formation of pure CaF2.The strong and sharp diffraction peaks indicate that the as-obtained products are well-crystalline ones.
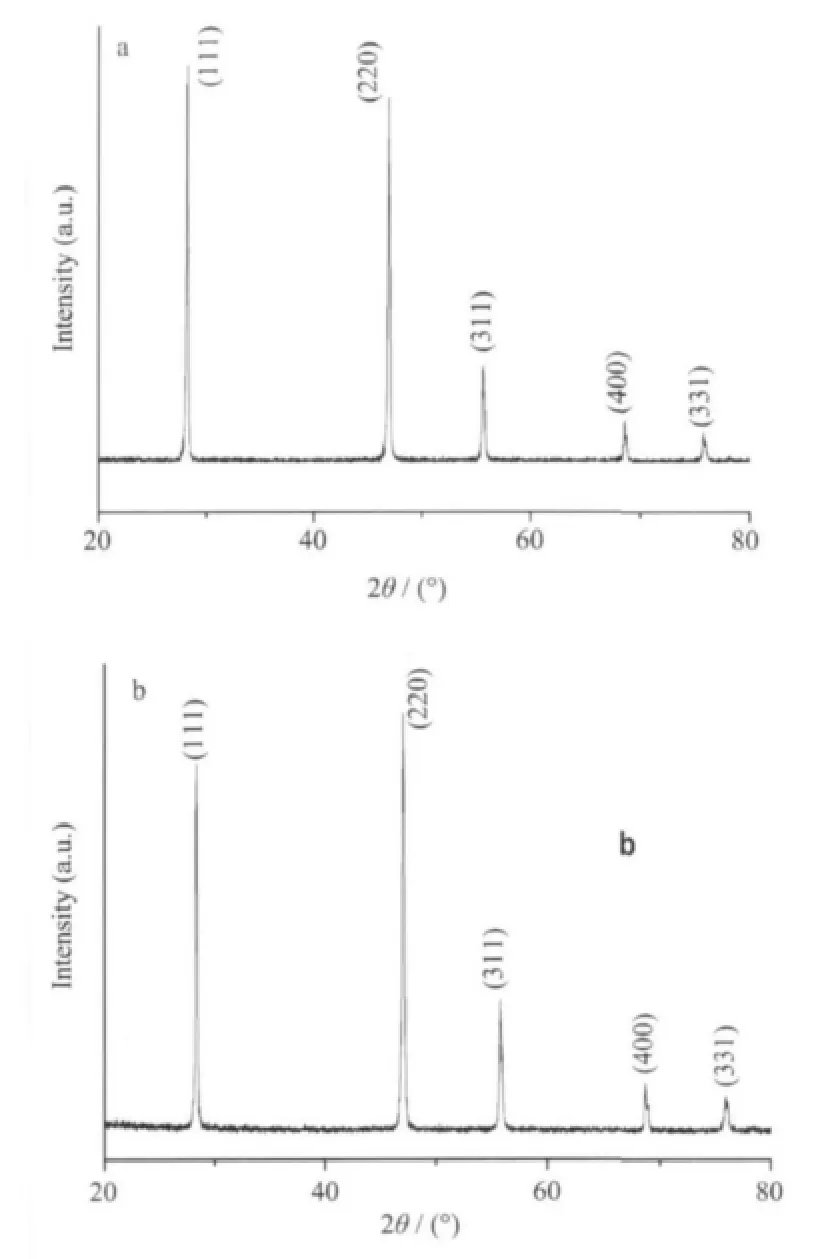
Fig.1 XRD patterns for the CaF2with 0.5 mmol Na2EDTA added in(a)NaBF4;(b)K2SiF6
2.2 Morphology characterization
Fig.2a is the low-magnification SEM image of the sample obtained in a typical procedure.Cubic phased CaF2products are primarily composed of uniform flower-like structures,indicating that high yield 3D hierarchical structures can be obtained by this method.The magnified SEM image(Fig.2b)reveals that the CaF2flower-like structures are assembled by many nanoflakes and the flakes are interweaving together.The TEM images also imply that the flower-like CaF2structures are self-assembled from slightly bending nanoflakes(Fig.2c-d).The SAED pattern(inset in Fig.2c)reveals the single-crystalline nature of the CaF2nanoflakes.
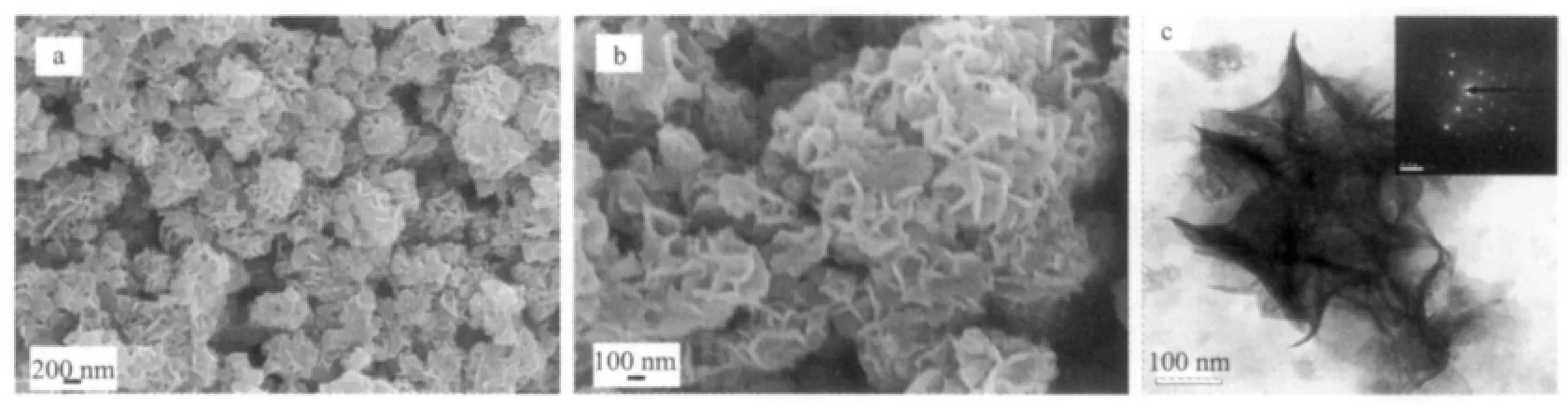
Fig.2 SEM and TEM images for the product prepared in a typical procedure
2.3 Effect of reaction parameters
Fig.3a is the SEM image for the product prepared in the absence of Na2EDTA.It is found that the morphology of the as-prepared CaF2consists of highly dispersed cubes with edge length of 150~400 nm.The size of the cubes is different from each other.This observation indicates that Na2EDTA plays a key role in controlling the morphology and size of the products.When 1 mmol Na2EDTA is added,similar 3D flowerlike CaF2hierarchical structures are formed,however, the edge of the flake is not clear as the former one (Fig.3b).When the amount of Na2EDTA is increased to 2 mmol(Fig.3c),rodlike aggregates are formed.The SEM image reveals that the surfaces of the rod aggregates are notsmooth and made ofsmall nanocrystals.When we further increase the amount of Na2EDTA to 5 mmol,only microrods are observed (Fig.3d).Therefore,the morphologies of the CaF2microstructures are sensitive to the amountof Na2EDTA.
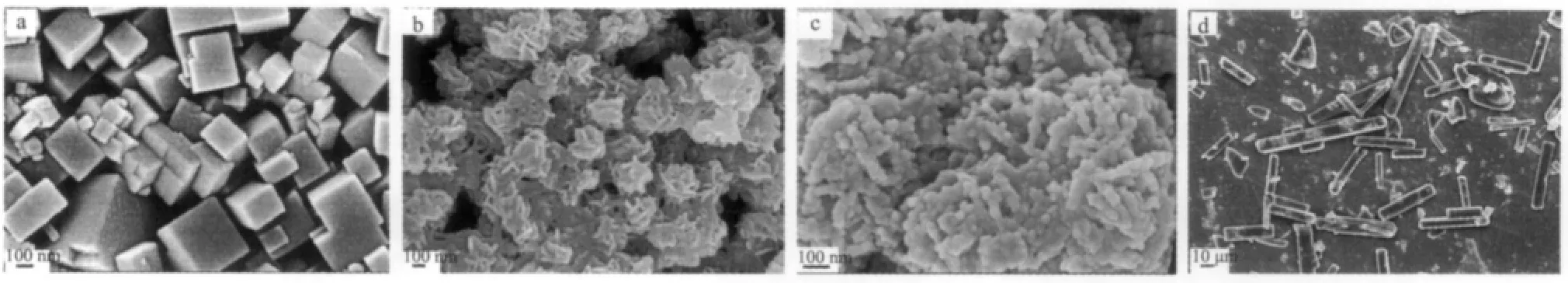
Fig.3 SEM images for the products prepared from NaBF4with different amounts of Na2EDTA
In order to investigate the effect of fluoride source, K2SiF6was used instead of NaBF4to synthesize CaF2by an identical procedure.Fig.1b shows the XRD patterns of the product prepared from 0.5 mmol Na2EDTA in the mixture of 1 mmol CaCl2and 1 mmol K2SiF6.All diffraction peaks of the product are perfectly indexed to the cubic phase of CaF2(PDF No.04-0864).It clearly reveals that the variation of fluoride source has no much effect on the crystalline phase.Fig.4 shows the SEM images for the products prepared from K2SiF6with different amounts of Na2EDTA.Fig.4a is the SEM image of the product prepared in the absence of Na2EDTA.The morphology of the as-prepared CaF2consists of irregular polyhedra.When 1 mmol Na2EDTA is used,the as-prepared CaF2are all polyhedra,but the edge is much clear than former one (Fig.4b).Further increasing the amount of Na2EDTA from 1 mmol,2 mmol to 5 mmol,the shape of the product changes from polyhedra,urchinlike spheres to aggregated spheres.These results indicate that the chelating reagents have a remarkable impact on the morphologies of the final products.
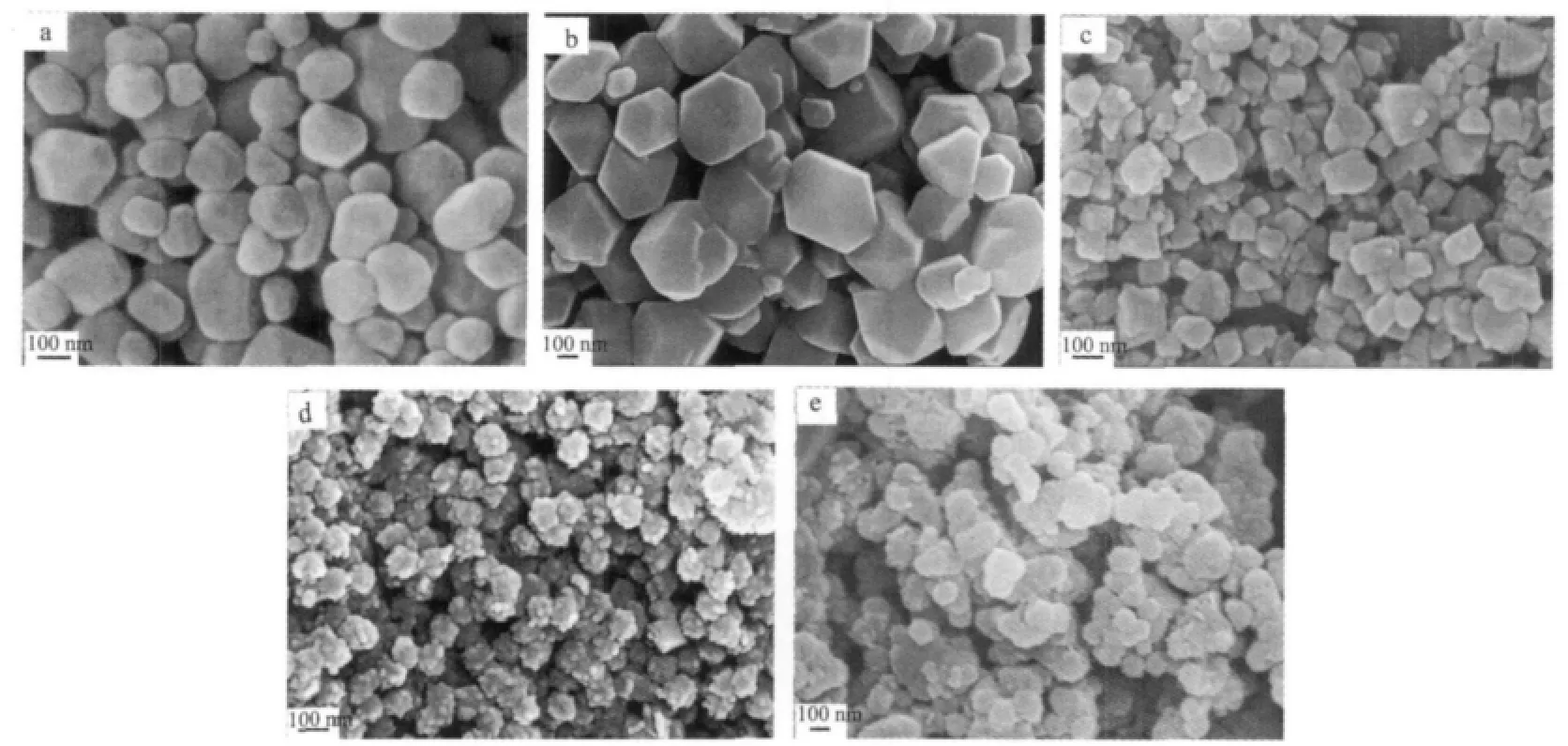
Fig.4 SEM images for the products prepared from K2SiF6with different amount of Na2EDTA
It is noted that the molar ratio of starting materials also plays an important role in the formation of different morphological CaF2.A series of contrastive experiments were conducted to fabricate CaF2when the molar ratio of Ca2+/BF4-or Ca2+/SiF62-varied while other reaction conditions kept constant.It should be mentioned that varying the molar ratio of the reactants does not change the phase of the CaF2products in our present work.All the samples involved in this study are characterized as pure cubic phased CaF2.Here we only take CaF2as a typical example to explain the morphology of the products.Fig.5 shows the SEM images of the products prepared from different molar ratios of Ca2+/NaBF4and Ca2+/K2SiF6,respectively.It is clearly indicated that the as-prepared CaF2are all cubes,however,with the increasing of the amount of NaBF4,more and more cubes aggregate together and many small particles coexist when Ca2+/NaBF4is fixed at 1∶4.As shown in Fig.5d~e,changing the molar ratio of Ca2+/K2SiF6has no obvious effect on the shape of the CaF2.
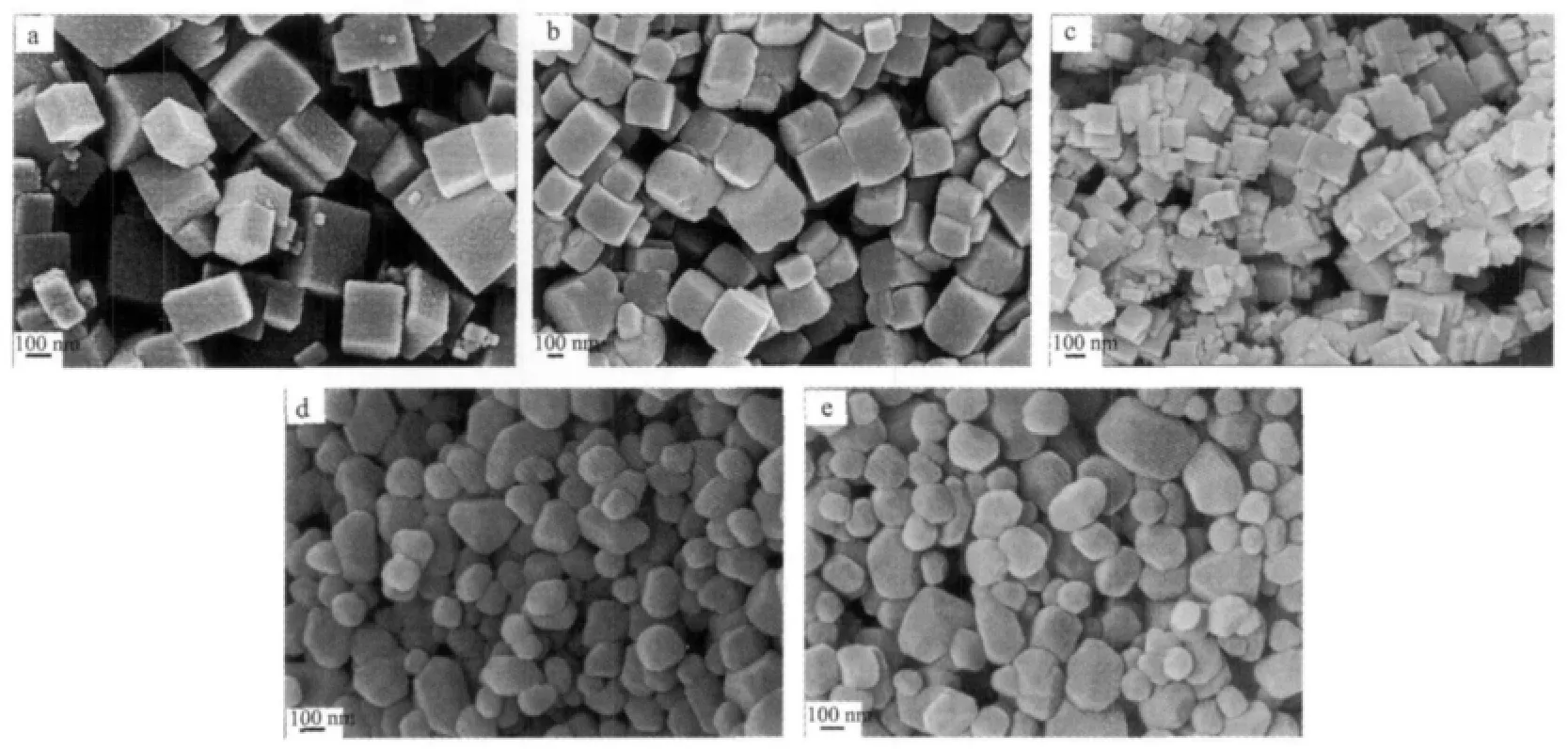
Fig.5 SEM images of the products prepared from different molar ratios of Ca2+/NaBF4as(a)1∶0.5,(b)1∶2, (c)1∶4 and Ca2+/K2SiF6as(d)1∶0.5,(e)1∶2
The ultrasonic irradiation plays an important role in the formation of different morphological CaF2in our current experimental conditions.In order to validate it, contrastive experiments were carried out to fabricate CaF2through stirring for 1 h without ultrasonication at room temperature.The XRD results indicate that the as-prepared products are pure cubic phased CaF2.However,the shape of the corresponding product is irregular,as shown in Figs.6a and 6b.In the results presented here,the instantaneous high-temperature and high-pressure field developed during ultrasonic irradiation will help to control the particle growth of CaF2,possibly because of the short collapse between a few nucleation centers in collapsing bubbles.Therefore, the ultrasonic irradiation is a key factor in the growth of CaF2.
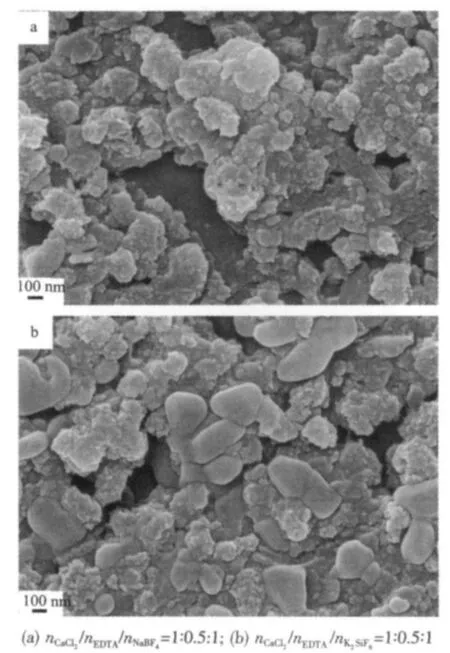
Fig.6 SEM images of the CaF2prepared through stirring for 1 h without ultrasonication at room temperature
2.4 Possible formation mechanism
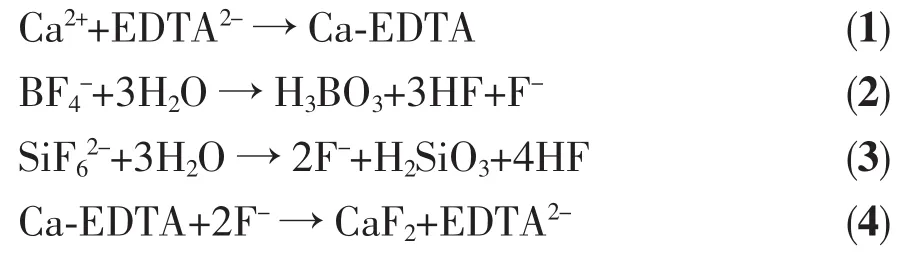
Based on the above experimental results,the probable reaction process for the formation of CaF2may be summarized as Eqs(1~4).In the current study,Ca2+and EDTA2form Ca-EDTA complexes at the beginning.It is known that NaBF4(or K2SiF6)yields F by hydrolysis of BF4(or Si).The Eqs(2~3)were reported earlier[20-21].The F-ions released from NaBF4(or K2SiF6)then react with the Ca2+ions(released from the Ca-EDTA complex)toform CaF2nanoparticles.OncetheCaF2nanoparticles formed,the EDTA molecules and the decomposed products from NaBF4and K2SiF6can be selectively adsorbed onto certain crystallographic planes of the CaF2seeds.In a further crystallization process,the unequal growth rates of different crystal facets(i.e.,anisotropic growth),will lead to the preferential growth of some specific crystalline planes[19].As a result,CaF2microstructures with different morphologies are synthesized via a mild sonochemical route.The ultrasonic waves may accelerate the hydrolysis of NaBF4or K2SiF6,which is presumably helpful to the nucleation and growth of CaF2microstructures.Furthermore,the cavitation and shock wave created by the ultrasound can accelerate solid particles to high velocities,leading to the interparticle collision and effective fusion at the point of collision.
3 Conclusions
Cubic phased CaF2microcrystals with different shapes(cubes,flowers,polyhedra)were synthesized by tuning the reaction parameters via a simple sonochemical route.The fluoride sources,molar ratio and chelating reagent are all key factors in the formation of products with different morphologies.
[1]Suslick K S,Choe S B,Cichowlas A A,et al.Nature,1991, 353:414-416
[2]Zhu J J,Xu S,Zhu J M,et al.Adv.Mater.,2003,15:156-159
[3]Ni Y H,Li H,Jin L N,et al.Cryst.Growth Des.,2009,9: 3868-3873
[4]Li H,Ni Y H,Cai Y F,et al.J.Mater.Chem.,2009,19:594-597
[5]Deng J G,Zhang L,Dai H X,et al.J.Phys.Chem.C,2010, 114:2694-2700
[6]Liu J K,Luo C X,Yang X H,et al.Mater.Lett.,2009,63: 124-126
[7]Feng L D,Chen X B,Mao C J.Mater.Lett.,2010,64:2420-2423
[8]GUO Qi(郭琦),GENG Jun(耿珺),JIANG Li-Ping(姜丽萍), et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010, 26:2121-2125
[9]Ni Y H,Li G Y,Hong J M.Ultrasonic Sonochem.,2010,17: 509-516
[10]Kinsman B E,Hanney R.Adv.Mater.Opt.Electron.,1995, 5:109-115
[11]Moon H J,Kim K N,Kim K M,et al.J.Biomed.Mater. Res.Part A,2005,74A:497-502
[12]Feldmann C,Roming M,Trampert K.Small,2006,2:1248-1250
[13]Sun X M,Li Y D.Chem.Commun.,2003:1768-1769
[14]Du Y P,Sun X,Zhang Y W,et al.Cryst.Growth Des.,2009, 9:2013-2019
[15]MaoYB,ZhangF,WongSS.Adv.Mater.,2006,18:1895-1899
[16]Wang W S,Zhen L,Xu C Y,et al.ACS Appl.Mater. Interfaces,2009,1:780-788
[17]Zhang C M,Li C X,Peng C,et al.Chem.Eur.J.,2010,16: 5672-5680
[18]Guo F Q,Zhang Z F,Li H F,et al.Chem.Commun.,2010, 46:8237-8239
[19]Hou S Y,Zou Y C,Liu X C,et al.CrystEngComm., 2011,13:835-840
[20]WANG Miao(王淼),SUN Tong-Ming(孙同明),SHI Yu-Jun (石玉军).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao), 2010,26:274-278
[21]WANG Miao(王淼),SHI Yu-Jun(石玉军),JIANG Guo-Qing (江国庆).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao), 2009,25:1785-1790
超声化学法制备不同形貌的CaF2微米晶
王 淼 陈婷婷 汤艳峰 江国庆 石玉军*
(南通大学化学化工学院,南通 226019)
采用超声化学法,以CaCl2与不同氟源(NaBF4、K2SiF6)在溶液中反应,制得了不同形貌的CaF2微米晶(立方体、花状、多面体)。用XRD、SEM及TEM对产物晶相及形貌进行了表征。XRD结果显示所有产物均为结晶良好的立方相CaF2。SEM及TEM结果表明由NaBF4制得的产物形貌为均匀的立方体微米晶,而由K2SiF6制得的产物为多面体。在添加配体Na2EDTA的情况下,由NaBF4得到的产物为纳米片组成的花状结构。本文详细讨论了氟源种类、反应物比例、配体等反应参数对产物CaF2形貌的影响,并提出了可能的反应机理。
CaF2;微结构;超声法;Na2EDTA
O614.23+1;O613.41
A
1001-4861(2012)01-0185-06
2011-06-17。收修改稿日期:2011-08-24。
国家自然科学基金(No.20906052),江苏省自然科学基金(No.BK2010281,No.2009610),南通大学校级课题(No.09ZY003;10ZY004)资助项目。*
。E-mail:wangmiao@ntu.edu.cn
- 无机化学学报的其它文章
- 纳米结构CuO为敏感电极的阻抗谱型NO2传感器
- Synthesis,Crystal Structure and Fluorescence Property of Cu(Ⅱ)Coordination Polymer with Pyridylimidazolidinone and Bipyridine
- Tumor-Imaging Core-Shell Nano-Models for Catalase
- Hydrothermal Synthesis,Crystal Structure and Catalytic Properties of a Polyoxovanadate Organicamine
- Syntheses and Crystal Structures of Nickeand CopperComplexes with Schiff Base Ligand of 5-Chlorosalicylaldehyde
- S2-控制剂对Ag纳米产物的形貌及光学性能的影响

