Co3O4纳米线的丙三醇辅助合成及其电化学性质
张国梁 赵 丹 郭培志,* 位忠斌 赵修松,2
(1青岛大学化学化工与环境学院,纤维新材料与现代纺织国家重点实验室培育基地,山东青岛266071; 2School of Chemical Engineering,The University of Queensland,St Lucia,QLD 4072,Australia)
Co3O4纳米线的丙三醇辅助合成及其电化学性质
张国梁1赵 丹1郭培志1,*位忠斌1赵修松1,2
(1青岛大学化学化工与环境学院,纤维新材料与现代纺织国家重点实验室培育基地,山东青岛266071;2School of Chemical Engineering,The University of Queensland,St Lucia,QLD 4072,Australia)
以硝酸钴和丙三醇为反应物通过反应条件的改变控制制备出Co3O4纳米线.利用粉末X射线衍射(XRD),扫描电子显微镜(SEM)和透射电子显微镜(TEM)对产物的形貌与结构进行了表征.实验发现,在低扫描速率下,Co3O4纳米线电极的循环伏安(CV)曲线呈现出两对氧化还原峰.恒电流充放电实验中,氧化钴纳米线电极在1A·g-1电流密度下的电容为163 F·g-1;在1和4 A·g-1条件下,其容量随循环次数的增加先上升后下降, 1000次充放电循环后容量保持率分别在98%和80%以上,继续增加循环次数则容量下降比较明显.锂离子电池性质测试中,氧化钴纳米线的放电容量为1124 mAh·g-1,然而放电容量随循环次数增加下降较快.基于实验结果,对Co3O4纳米线的形成机理及其结构与电化学性质之间的关系进行了探讨.
电极;电容量;Co3O4;纳米线;丙三醇
1 Introduction
When the material dimensions fall into the nanometer scale, the so-called nanostructured materials may display unique magnetic,optical,catalytic,and electrochemical properties compared with those of the corresponding bulk counterparts.1-3Many methods,including the hydrothermal synthesis,template method,thermolysis,electrochemical route,and chemical vapour deposition,and so on,have been developed to synthesize various nanomaterials with controlled morphologies and architectures,such as nanocrystals,nanorods,nanowires,nanobelts, complex and hierarchical assemblages.3-9For instance,metal nanocrystals that display excellent electrocatalytic properties have been synthesized using an effective electrodeposition method.3
With the critical demand of advanced functional nanomaterials for electrochemical energy conversion and storage,nanostructures of transition metal oxides have received increasing interest due to their easy synthesis,excellent properties,and non-expensive nature.Among the inorganic oxide nanomaterials synthesized,cobalt oxide nanomaterials have been prepared due to their important applications in gas sensors,catalysts,supercapacitor,and lithium-ion batteries.10-21For example,Co3O4nanotubes that were synthesized using anodic aluminum oxide membranes as the templates showed high discharge capacity and superior cycling reversibility as well as excellent sensitivity to hydrogen and alcohol.10Mesoporous cobalt oxide aerogels displayed excellent supercapacitive properties with high specific capacitances and cycle stability.11Mesoporous Co3O4nanowire arrays can be used as anodes in lithium-ion batteries which show high capacity and rate capability.15In the solutionbased synthesis of nanostructured materials,various liquids,including water,alcohol,ethylene glycol,and glycerol have usually been used as the solvent.1,4-6,22-25In this paper,however, glycerol has been selected as the reagent to synthesize Co3O4nanostructures22-24because hydroxyl groups in the molecules can react with metal ions.It is found that the molar ratio of Co(NO3)2to glycerol in the synthesis system plays an important role in the formation of desired products.The electrochemical properties of the samples are characterized by cyclic voltammetry(CV),galvanic charge-discharge,and cycling experiments either in three-electrode systems and lithium-ion batteries.
2 Experimental
Alcohols(≥99.7%),Co(NO3)2·6H2O(≥99.0%),glycerol(≥99.0%),and KOH(≥85.0%)were purchased from Sinopharm Chemical Reagent Company.Acetylene black(99.99%,Strem Chemicals,USA),polytetrafluorothylene latex(PTFE,60%) and polyvinylidene fluoride(PVDF)were purchased from Sigma-Aldrich,USA.Double distilled water was used in all the experiments.In a typical synthesis,aqueous Co(NO3)2solution (0.2 mol·L-1,15 mL)was dropped into aqueous glycerol solution(0.6 mol·L-1,15 mL)under stirring.The mixed solution was then transferred to a 40 mL teflon-lined autoclave.Hydrothermal process was carried out in an oven at 200°C for 24 h. The solid was collected and washed thoroughly with distilled water and ethanol each,and then dried in an oven at 60°C for 6 h.Co3O4nanowires were obtained after calcination of the collected solid in a tube furnace at 500°C for 2 h in air.
X-ray diffraction(XRD)patterns were recorded on a Bruker D8 Advance X-ray diffractometer(German)equipped with graphite monochromatized Cu Kαradiation(λ=0.15418 nm) from 10°to 80°(2θ)using a solid detector.Scanning electron microscopy(SEM)images were taken with a JSM-6390LV scanning electron microscope(Japan)operated at 20 kV.Transmission electron microscopy(TEM)images were obtained with a JEM-2000EX transmission electron microscope(Japan) operated at 160 kV.Electrochemical measurements were performed on a CHI760C electrochemical workstation using a three-electrode cell with platinum wire as counter electrode and Hg/HgO electrode as reference electrode in aqueous KOH (3 mol·L-1)solutions.Electrodes for electrochemical studies were prepared by mixing Co3O4samples with acetylene carbon black and PTFE in a mass ratio of 75:20:5 and was blended to achieve a homogeneous mixture.The resulting slurry was then pressed onto a nickel foam grid(1 cm×1 cm)at 1.5×107Pa. The typical mass load of each electrode material was about 5 mg.Before measurements,the working electrodes were dipped into KOH(3 mol·L-1)solutions overnight.
The performance of Co3O4nanowires as the cathode was also evaluated by a fastener cell with a lithium metal anode.To prepare positive electrodes,a homogeneous mixture of 75% (w)Co3O4with 20%(w)acetylene black and 5%(w)PVDF was cast onto an Al foil.After placed in a vacuum oven at 120°C for 12 h,fastener cells were assembled in an argon filled glove box.The assembled cells consisted of Co3O4based composites fabricated onto Al foil,Li metal foil as the negative electrode,and Celgard 2400 separator saturated with a 1 mol· L-1LiPF6electrolytic solution in 1:1 volume ratio of ethylene carbonate to dimethyl carbonate.The galvanostatic charge/discharge experiment was performed between 3.0 and 0.1 V.
3 Results and discussion
Fig.1 shows the XRD patterns of the as-made samples.As depicted in Fig.1a,the XRD pattern of the hydrothermal precipitate can be mainly indexed to Co(OH)2phase(JCPDS No. 03-0913).If the precipitate is undergone heat treated in air,the corresponding XRD pattern(Fig.1b)can be well indexed to the pure cubic Co3O4phase(JCPDS No.74-2120).Furthermore, the peaks in Fig.1b are very sharp,indicating the existence of well crystalline Co3O4phase in the calcinated sample.
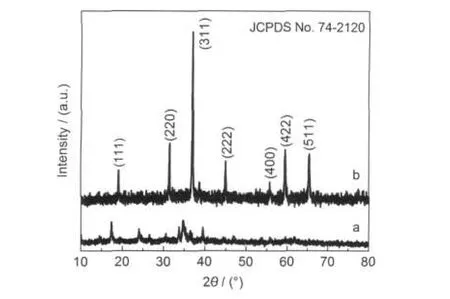
Fig.1 XRD patterns of the hydrothermal precipitate(a)and the calcinated sample(b)prepared from the systems with the Co2+/ glycerol molar ratio of 1:3 in the raw materials
The morphology and structure of the as-made samples have been characterized by SEM and TEM,as shown in Fig.2.It can be seen from Fig.2a that needle-like nanowires of Co(OH)2are obtained with the length scales more than 10 μm.TEM image of sample Co(OH)2further confirms the formation of nearly uniform wire-like nanostructures with the width of about 20 nm(Fig.2b).The inset in Fig.2b shows the selected area electron diffraction(SAED)pattern of a single Co(OH)2nanowire, indicating the polycrystalline nature of the nanowires.Clearly, wire-like nanostructures are maintained for sample Co3O4(Fig.2c)after the heat treatments of sample Co(OH)2.As can be verified by the TEM image(Fig.2d),the contour of sample Co3O4is similar to that of the precursor,which exhibits the formation of moniliform nanowires with nanoparticle decorated. The average size of Co3O4nanoparticles is about 20 nm.The SAED pattern of the sample shown in the inset of Fig.2d approves that Co3O4nanowires are not single crystalline.
In order to study the formation mechanism of sample Co(OH)2,the intermediates obtained from early stages of the hydrothermal process were investigated by SEM.It should be pointed out that no product can be obtained when the reaction time is less than 120 min.As depicted in Fig.3a,wire-like nanostructures as well as spherical aggregates are formed if the reaction time was 140 min.When the synthesis time prolonged to 160 min,disperse nanowires or bundles of nanowires can be observed except the existence of few particles as depicted in Fig.3b.With the time further up to 3 h,pure nanowire structures can be gained(Fig.3c).Finally,uniform nanowires are obtained with the reaction time extended to 24 h(Fig.2a).
It is interesting to find that no product can be obtained when glycerol was not involved in the synthesis systems at similar conditions.This indicates that Co2+ions and glycerol are indeed reacted in the hydrothermal synthesis conditions.21Our results confirm that the molar ratio between Co2+and glycerol play a key role in the formation of cobalt hydroxide.21These can be verified by the XRD and SEM results of the samples prepared with different Co2+/glycerol molar ratios of the reactants. As depicted in Fig.4a,mainly cubic phase of Co3O4(JCPDS No.74-2120)can be obtained when the Co2+/glycerol molar ratio is 1:1.Furthermore,a weak peak at 2θ of 32.5°appeared, ascribed to the(100)peak of Co(OH)2(JCPDF No.74-1057), indicating the existence of a small amount of Co(OH)2in the sample.If the Co2+/glycerol molar ratio changes to 1:1.2,besides Co3O4phase,as derived from Fig.4b,two peaks at 14.7° and 17.1°attributed to the(100)and(101)peaks of β-Co(OH)2(JCPDS No.45-0031),respectively,and one peak at 23.9°attributed to the(001)peak of Co(OH)2(JCPDS No.03-0913) are observed.With the Co2+/glycerol molar ratio increased to 1: 1.5,main β-Co(OH)2and Co(OH)2phases are gained concomitant a small amount of cubic Co3O4phase(Fig.4c).When the molar ratios are further up to 1:2,as shown in Fig.4d,the diffraction peaks of the sample are similar to those shown in Fig.1a and Co(OH)2phases are predominant in the product.
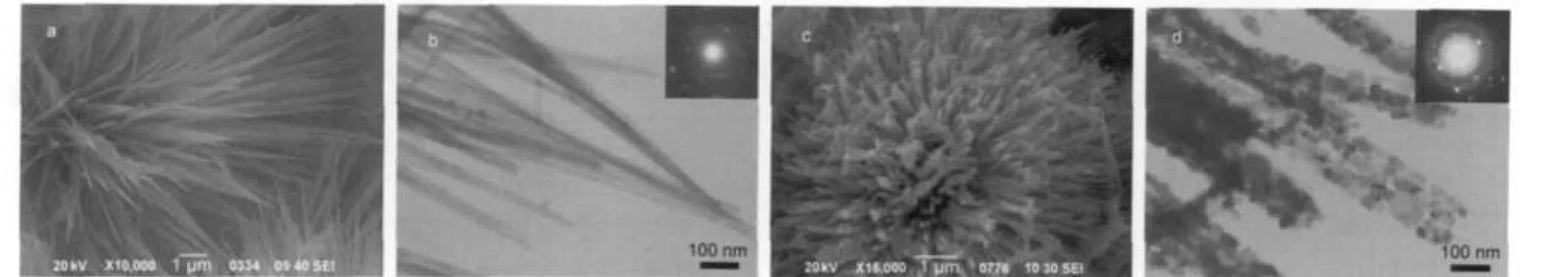
Fig.2 SEM(a,c)and TEM(b,d)images of samples Co(OH)2(a,b)and Co3O4(c,d)

Fig.3 SEM images of the intermediates after hydrothermal processes for 140 min(a),160 min(b),and 180 min(c)
Fig.5 shows the SEM images of the above samples.It is clear that the Co2+/glycerol molar ratios in the raw materials have strong effects on the shapes,and sizes of the products.As depicted in Fig.5a,micro-scale aggregates composed of irregular structures are formed with the molar ratio of 1:1.Two types of structures with micron size,namely flower and aggregate structures,can be observed when the Co2+/glycerol molar ratio is 1:1.2(Fig.5b).More micro-scale flower structures composed of nanosheets with the thickness of 20-40 nm can be seen from Fig.5c when the sample synthesized with the molar ratio of 1:1.5.However,wire-like nanostructures are observed (Fig.5d)when the Co2+/glycerol molar ratio is further increased to 1:2,of which the widths of the nanowires are much larger than the sample shown in Fig.2a.Based on these experimental results,it is suggested that at the early stage of the synthesis one Co2+ion coordinates with one glycerol molecule when the Co2+/glycerol molar ratio is 1:1.However,the Co2+-glycerol coordinated complex can be formed from one Co2+ion and two glycerol molecules when the Co2+/glycerol molar ratio is 1:2 or 1:3.Thereafter,Co(OH)2nanowires can be obtained ultimately after the proper reaction time.

Fig.4 XRD patterns of the products synthesized from the systems with the Co2+/glycerol molar ratios of(a)1:1,(b)1:1.2, (c)1:1.5,(d)1:2 in the raw materials

The electrochemical properties of Co3O4nanowires were investigated while micro-scale aggregates of Co3O4obtained from the system with the Co2+/glycerol molar ratio of 1:1 were not involved due to the existence of impurity in the sample. Fig.6 shows the CV curves of Co3O4nanowires in aqueous KOH electrolytes.It can be concluded from Fig.6a that two pairs of peaks in CV curves of Co3O4electrodes are mainly associated with the redox process,which should be attributed to cubic Co3O4phase.4,11,26The redox reactions derived from CV curves can be indexed to the following reactions:27Based on the average value of peak potential(Ep)of p1 versus p2,peak p1 appears prior to oxygen evolution due to the oxidation of Co2+to Co3+and peak p2 is for the reverse process as shown in reaction(1).27-30However,peak p3 occurs due to oxidation Co3+to Co4+and peak p4 is therefore attributed to the reduction of Co4+to Co3+as shown in reaction(2).22,27The ΔEpvalues of p1 versus p2 and p3 versus p4 are increased from 80 and 9.1 mV to 119 and 64 mV,respectively,with the scan rates varied from 2 to 20 mV·s-1.It is well-known that the theoretic ΔEpvalue for a reversible single-electron transfer process is 58 mV. These results indicate that the reaction occurs as a quasi-reversible process at a lower scan rate during the anodic potential sweep of the electrode.If the scan rate is 0.3 V·s-1or larger, the shape of CV curves for the sample changes largely due to the irreversibility of electrochemical reactions(Fig.6b).

Fig.5 SEM images of the products synthesized from the systems with the Co2+/glycerol molar ratios of(a)1:1,(b)1:1.2,(c)1:1.5,(d)1:2 in the raw materials

Fig.6 CV curves of Co3O4nanowire-based electrodes at different scan rates
It can also be derived from Fig.6 that the capacitive charac-teristic of Co3O4nanowire electrodes is mainly based on the redox mechanism.Fig.7 shows the galvanostatic charge-discharge curves of nanowire-based electrodes at different current densities under the potential range of 0-0.47 V in a three-electrode system.The specific capacitance can be calculated from the equation,C=Itd/(mΔv),31where I is the current in the charge-discharge measurement,m is the mass of the active materials,tdis the variance metric of charge or discharge time, and Δv is the variance metric of charge or discharge.It can be calculated that the capacitance of the nanowire is 163 F·g-1at a current density of 1 A·g-1.The capacitances are gradually decreased to 136,121,and 119 F·g-1at current densities of 2,3, and 4 A·g-1,respectively.These results were in good agreement with those of the CV experiments.

Fig.7 Galvanostatic charge-discharge curves of Co3O4 nanowire-based electrodes at different current densities i/(A·g-1):(a)1,(b)2,(c)3,(d)4
The cycle stability of the active material was investigated by galvanostatic charge-discharge measurements.Fig.8 displays the variations of specific capacitances at 1 and 4 A·g-1with the cycle number.It can be seen that the capacitance increases slightly at the first cycle,indicating that more active substance were excited.31When the current density is 1 A·g-1,the specific capacitance increases from 120 F·g-1at the first cycle to 135 F·g-1at the 50th cycle,retains more than 98%after 1000 cycles and then decreases rapidly to 78 F·g-1after 1600 cycles.If the current density increases to 4 A·g-1,the change of the specific capacitance is similar to that obtained at 1 A·g-1.The specific capacitance increases at the first cycle and then decreases with the cycles larger than 50.After 1000 cycles,the capacitance retains more than 80%of the origin and then reduces rapidly to 85 F·g-1after 1600 cycles.Furthermore,long-term cycle test could lead to distinctly irreversible electrochemical reactions and morphology change,14explaining similar capacity behavior of these electrodes after 1100 cycles.

Fig.8 Specific capacitances of Co3O4nanowires-based electrodes at 1A·g-1(a)and 4A·g-1(b)
The performance of Co3O4nanowires as lithium-ion battery positive electrodes is evaluated by the cell configuration Co3O4/ Li at room temperature.Fig.9 displays both the discharge curves(i.e.,voltage vs capacity)and cycle performance of the sample.The first discharge capacity at the rate of 0.1C(1C= 890 mA·g-1)is 1124 mAh·g-1(Fig.9a),with the first cycle irreversible loss of 410 mAh·g-1(Fig.9b).Furthermore,the voltage plateaus at around 1.0 V in discharge curves for these samples can be ascribed to the formation of metallic Co embedding in the Li2O matrix.15The large first discharge capacity is normally ascribed to irreversible reactions(e.g.,decomposition of electrolyte)occurring during the first discharge.The first discharge capacity of the sample decreased drastically to 631 and 368 mAh·g-1at 0.5C and 1C rates,respectively.It can also be observed from Fig.9b that the capacity decreases slowly after the second cycle similar to other reports.32-34The reason is that with the Li+insertion and extraction process,its large volume expansion/contraction and severe particle result in electrode pulverization and loss of interparticle contact and,conse-quently lead to a large irreversible capacity loss and poor cycling stability.15,35,36

Fig.9 The first discharge curves(a)and specific capacity(b)of Co3O4nanowires at different rates
4 Conclusions
Co3O4nanowires were controllable synthesized by a glycerol-assisted synthesis method using Co(NO3)2and glycerol as the reactants.The CV curves of Co3O4nanowire electrodes display two pairs of redox reactions at scan rates less than 0.2 V· s-1.Galvanostatic charge-discharge measurements in the threeelectrode system showed that specific capacitances of Co3O4nanowire electrodes were 163 F·g-1at a current density of 1 A· g-1.The electrodes showed higher cycle stability at a lower current density based on the cycle-capacitance relationship under current densities of 1 and 4 A·g-1.In lithium-ion battery measurements,Co3O4nanowire electrodes showed a discharge capacitance of 1124 mAh·g-1.The formation mechanism of Co3O4structures and the relationship between their structures and electrochemical properties were discussed based on the experimental results.
(1) Burda,C.;Chen,X.;Narayanan,R.;El-Sayed,M.A.Chem. Rev.2005,105,1025.
(2)Xia,Y.;Yang,P.;Sun,Y.;Wu,Y.;Mayers,B.;Gates,B.;Yin,Y.; Kim,F.;Yan,H.Adv.Mater.2003,15,353.
(3)Tian,N.;Zhou,Z.Y.;Sun,S.G.;Ding,Y.;Wang,Z.L.Science 2007,316,732.
(4) Xiong,S.L.;Yuan,C.Z.;Zhang,X.G.;Xi,B.J.;Qian,Y.T.
Chem.Eur.J.2009,15,5320.
(5)Guo,P.Z.;Wei,Z.B.;Wang,B.Y.;Ding,Y.H.;Li,H.L.;
Zhang,G.L.;Zhao,X.S.Colloids Surf.A 2011,380,237.
(6) Chen,C.H.;Abbs,S.F.;Morey,A.;Sithambaram,S.;Xu,L.P.; Garces,H.F.;Hines,W.A.;Suib,S.L.Adv.Mater.2008,20, 1205.
(7)Li,Y.G.;Tan,B.;Wu,Y.Y.J.Am.Chem.Soc.2006,128, 14258.
(8) Cong,H.P.;Yu,S.H.Cryst.Growth Des.2009,9,210.
(9)Chen,Y.C.;Hu,L.;Wang,M.;Min,Y.L.;Zhang,Y.G.
Colloids Surf.A 2009,336,64.
(10) Li,W.Y.;Xu,L.N.;Chen,J.Adv.Funct.Mater.2005,15,851.
(11)Wei,T.Y.;Chen,C.H.;Chang,K.H.;Lu,S.Y.;Hu,C.C.
Chem.Mater.2009,21,3228.
(12)Zhao,Z.G.;Geng,F.X.;Bai,J.B.;Cheng,H.M.J.Phys.
Chem.C 2007,111,3848.
(13) Hu,L.H.;Peng,Q.;Li,Y.D.J.Am.Chem.Soc.2008,130, 16136.
(14) Lou,X.W.;Deng,D.;Lee,J.Y.;Archer,L.A.J.Mater.Chem. 2008,18,4397.
(15) Li,Y.G.;Tan,B.;Wu,Y.Y.Nano Lett.2008,8,265.
(16)Mekhemer,G.A.H.;Abd-Allah,H.M.M.;Mansour,S.A.A. Colloids Surf.A 1999,160,251.
(17) Salabas,E.L.;Rumplecker,A.;Kleitz,F.;Radu,F.;Schueth,F. Nano Lett.2006,6,2977.
(18)Nam,K.T.;Kim,D.W.;Yoo,P.J.;Chiang,C.Y.;Meethong, N.;Hammond,P.T.;Chiang,Y.M.;Belcher,A.M.Science 2006,312,885.
(19) Li,T.;Yang,S.;Huang,L.;Gu,B.;Du,Y.Nanotechnology 2004,15,1479.
(20)Kang,Y.M.;Song,M.S.;Kim,J.H.;Kim,H.S.;Park,M.S.; Lee,J.Y.;Liu,K.H.;Dou,S.X.Electrochim.Acta 2005,50, 3667.
(21)Yang,L.X.;Zhu,Y.J.;Li,L.;Zhang,L.;Tong,H.;Wang,W. W.;Cheng,G.F.;Zhu,J.F.Eur.J.Inorg.Chem.2006,4787.
(22)Xiu,S.N.;Shahbazi,A.;Shirley,V.;Mims,M.R.;Wallace,C. W.J.Anal.Appl.Pyrol.2010,87,194.
(23)Yao,J.F.;Yu,L.;Zhang,L.X.;Wang,H.T.Mater.Lett.2011, 65,2304.
(24) Li,X.H.;Zhang,D.H.;Chen,J.S.J.Am.Chem.Soc.2006, 128,8382.
(25)Guo,P.Z.;Han,G.T.;Wang,B.Y.;Zhao,X.S.Acta Phys.-Chim.Sin.2010,26,2557.[郭培志,韩光亭,王宝燕,赵修松.物理化学学报,2010,26,2557.]
(26) Zheng,M.;Cao,J.;Liao,S.;Liu,J.;Chen,H.;Zhao,Y.;Dai, W.;Ji,G.;Cao,J.;Tao,J.J.Phys.Chem.C 2009,113,3887.
(27) Gao,Y.Y.;Chen,S.L.;Cao,D.X.;Wang,G.L.;Yin,J.L. J.Power Sources 2010,195,1757.
(28) Lin,C.;Ritter,J.A.;Popov,B.N.J.Electrochem.Soc.1998, 145,4097.
(29) Barbero,C.;Planes,G.A.;Miras,M.C.Electrochem.Commun. 2001,3,113.
(30) Xu,J.;Gao,L.;Cao,J.Y.;Wang,W.C.;Chen.Z.D. Electrochim.Acta 2010,56,732.
(31)Ye,X.G.;Zhang,X.G.;Mi,H.Y.;Yang,S.D.Acta Phys.-Chim.Sin.2008,24,1105. [叶向果,张校刚,米红宇,杨苏东.物理化学学报,2008,24,1105.]
(32) Lou,X.W.;Deng,D.;Lee,J.Y.;Feng,J.;Archer,L.A.Adv. Mater.2008,20,258.
(33)Kang,J.G.;Ko,Y.D.;Park,J.G.;Kim,D.W.Nanoscale Res. Lett.2008,3,390.
(34) Binotto,G.;Larcher,D.;Prakash,A.S.;Urbina,R.H.;Hegde, M.S.;Tarascon,J.M.Chem.Mater.2007,19,3032.
(35)Yao,W.L.;Wang,J.L.;Yang,J.;Du,G.D.J.Power Sources 2008,176,369.
(36)Wu,Z.S.;Ren,W.C.;Wen,L.;Gao,L.B.;Zhao,J.P.;Chen,Z. P.;Zhou,G.M.;Li,F.;Cheng H.M.ACS Nano 2010,4,3187.
October 11,2011;Revised:November 18,2011;Published on Web:November 24,2011.
Glycerol-Assisted Synthesis and Electrochemical Properties of Co3O4Nanowires
ZHANG Guo-Liang1ZHAO Dan1GUO Pei-Zhi1,*WEI Zhong-Bin1ZHAO Xiu-Song1,2
(1Laboratory of New Fiber Materials and Modern Textile,the Growing Base for State Key Laboratory,School of Chemistry, Chemical Engineering and Environmental Sciences,Qingdao University,Qingdao 266071,Shandong Province,P.R.China;2School of Chemical Engineering,The University of Queensland,St Lucia,QLD 4072,Australia)
Cobalt oxide(Co3O4)nanowires were controllably synthesized using glycerol and Co(NO3)2as reagents and adjustment of the experimental parameters.The morphology and structure of the asprepared products were characterized by a series of techniques such as X-ray podwer diffraction(XRD), scanning electron microscopy(SEM),and transmission electron microscopy(TEM).Electrochemical performance of the nanowires was studied by cyclic voltammetry(CV)and galvanostatic charge-discharge measurements.It was found that two pairs of redox peaks appeared in the CV curves of Co3O4nanowire electrodes at low scan rates.The specific capacitance of the Co3O4nanowire electrodes was 163 F·g-1at a current density of 1 A·g-1,according to the galvanostatic charge-discharge measurements.Cycle stability tests showed that the specific capacitance increased over the first tens of cycles and then reduced slowly. After 1000 cycles,the capacitance retention was over 98%at 1 A·g-1and 80%at 4 A·g-1;it then decreased obviously with further increase in cycle number.In Li-ion battery measurements,Co3O4nanowire electrodes showed a discharge capacitance of 1124 mAh·g-1which decreased rapidly during the cycle test. The formation mechanism and the relationship between the structure and electrochemical properties of Co3O4nanowires were discussed based on the experimental results.
Electrode;Capacitance;Co3O4;Nanowire;Glycerol
10.3866/PKU.WHXB201111241
*Corresponding author.Email:pzguo@qdu.edu.cn,guopz77@yahoo.com;Tel:+86-532-83780378.
The project was supported by the National Natural Science Foundation of China(20803037,21143006),Natural Science Foundation of Shandong Province,China(ZR2009BM013),and Foundation of Qingdao Municipal Science and Technology Commission,China(11-2-4-2-(8)-jch).
国家自然科学基金(20803037,21143006),山东省自然科学基金(ZR2009BM013)和青岛市应用基础研究项目(11-2-4-2-(8)-jch)资助
O646;O613.3;O614.8

