Pancreaticoduodenectomy: a comparison of superior approach with classical Whipple's technique
Kashmir, India
Pancreaticoduodenectomy: a comparison of superior approach with classical Whipple's technique
Omar Javed Shah, Mushtaq A Gagloo, Irfan Jan Khan, Rayees Ahmad and Saleema Bano
Kashmir, India
BACKGROUND:Pancreaticoduodenectomy (PD) is the standard procedure for resecting tumors arising from the periampullary area and the pancreatic head. Nevertheless this procedure is inherently diff i cult and associated with high morbidity and mortality. Besides, the technique applied for exposing the portal and superior mesenteric veins is time-consuming, diff i cult and associated with the risk of major venous injury. Recently we have introduced a modif i ed approach for making this part of the procedure quick, safe and bloodless, which constitutes the subject of this study.
METHODS:Patients who underwent pylorus preserving pancreaticoduodenectomy (PPPD) either by superior approach technique (group 1) or by classical Whipple's technique (group 2) were retrospectively identif i ed. Age-sex composition, body mass index (BMI), total operative time, operative blood loss, intraoperative blood transfusion requirement, morbidity, mortality and length of hospital stay were compared between the two groups.
RESULTS:Between January 1997 and December 2011, 72 patients underwent PPPD by the superior approach technique (group 1) and 38 underwent PPPD by the classical Whipple's technique (group 2) at our institution. Statistically signif i cant differences were observed in operative time (208.1±46.3 minutes in group 1 vs 322.0±33.8 minutes in group 2), operative blood loss (601.0±250.3 mL in group 1 vs 1371.5±471.8 mL in group 2), and intraoperative blood transfusion requirement [10 (13.9%) patients in group 1 and 24 (63.2%) in group 2]. Among 18 (16.4%) obese patients, signif i cant differences in operativetime, operative blood loss and intraoperative blood transfusion requirement were observed between groups 1 and 2. There was no signif i cant inter-group difference in complication rate among obese patients, but comparing obese patients with normal weight patients revealed higher rates of complications like pancreatic fi stula (27.8% vs 6.5%), delayed gastric emptying (16.7% vs 5.4%), and infective complications like wound infection and intra abdominal collection (44.4% vs 5.4%).
CONCLUSIONS:On the basis of analytical data, we conclude that the superior approach technique is effective for PD compared with the classical Whipple's technique. It allows fast, safe and virtually bloodless dissection for exposure of the superior mesenteric and portal veins during early steps of PD. PD is normally a diff i cult and tedious procedure carrying a remote risk of major venous injury leading to substantial blood loss.
(Hepatobiliary Pancreat Dis Int 2013;12:196-203)
superior approach technique; Whipple's technique; pylorus preserving pancreaticoduodenectomy
Introduction
Pancreaticoduodenectomy (PD), fi rst introduced by Codivilla and Kausch,[1]is considered a complex operative technique for the treatment of periampullary and pancreatic head tumors. This technique was eventually ref i ned and popularized by Whipple et al.[2]Over the years, several modif i cations of the Whipple's technique have been reported, including the pylorus preserving pancreaticoduodenectomy (PPPD) described by Watson in 1944,[3]which was reintroduced by Traverso and Longmire in the late 1970s.[4]Since the initial reports regarding the indications and technique of PD, this procedure has been widely accepted and the Whipple's procedure has been modif i ed.[1,2,4-6]
The mortality after PD which was as high as 25%-30% in the beginning has recently fallen to below 5% in some surgical centers.[7,8]Despite an appreciable decrease in the mortality, the morbidity after PD remains high. This is mostly due to hemorrhage and pathological changes of the remnant pancreatic stump. Technical modif i cations have been made to control the morbidity. These modif i cations are related to the pancreatic anastomosis. Furthermore, new operative modif i cations contributing to the substantial decrease of operative time and operative blood loss have decreased the operative risk. But exposure of the portal vein and superior mesenteric vein (SMV) remains diff i cult and time-consuming in PD. The superior approach originally introduced by Cameron et al[9]allows a quick, safe and bloodless exposure of the portal vein and SMV. Since the original description, no comparison study of this method with the conventional technique has been attempted to justify the relevance of the superior approach. Our study is an attempt in this direction.
Methods
Study design and data collection
The patients suspected of pancreatic or periampullary cancer at the Department of Surgical Gastroenterology, Sher-i-Kashmir Institute of Medical Sciences, Srinagar from January 1997 to December 2011 were subjected to a thorough preoperative diagnostic workup. Of these cases, only conf i rmed resectable cases were included in this study.
During this period, 110 consecutive patients underwent PPPD by a group of pancreatic surgeons. All patients were included in a retrospective comparison of PD by the superior approach and classical Whipple's technique with respect to the intraoperative and postoperative courses.
Early ligation and division of the gastroduodenal and the inferior pancreaticoduodenal arteries (IPDAs) prior to transection of the pancreatic neck was also undertaken in the patients where superior approach technique was applied.
Three patients who had two stages of PD were excluded from this study and no patient underwent neoadjuvant therapy.
Contrast enhanced CT scan of the abdomen supported by various other relevant investigations was performed. In some cases, endoscopic retrograde cholangiopancreatography (ERCP) was also performed; CT angiography and MRI were optional procedures. Patients with distant metastasis or local unresectable tumors as indicated by preoperative workup and intraoperative fi ndings were excluded.
Data were collected retrospectively from our institution's electronic medical records and from paper charts prior to introduction of electronic medical records. Various parameters such as personal characteristics (age, sex), body mass index (BMI), intraoperative data (type of technique, operative time, operative blood loss, red blood cell transfusion, pancreatic texture (soft, intermediate or fi rm), pancreatic duct diameter, intraoperative complication, histological diagnosis, patient outcome, postoperative length of hospital stay, morbidity and mortality were recorded in both groups of patients for comparative analysis. The criteria for red blood cell transfusion included hemoglobin (Hb) value less than 8.0 g/dL and a change in such vital signs as low blood pressure and tachycardia during the surgical procedure. Morbidity was def i ned as any perioperative complication including biliary, septic, pulmonary, cardiac or wound complications. Pancreatic fi stula was def i ned according to the International Study Group on Pancreatic Fistula (ISGPF) classif i cation.[10]
Delayed gastric emptying was adopted as described by the International Study Group of Pancreatic Surgery (ISGPS).[11]It was def i ned as a need for nasogastric tubing for 3 days or a need for the reinsertion after the third postoperative day or inability to tolerate solid food after the seventh postoperative day. Hospital mortality was def i ned as death during the hospital stay.
Surgical technique
Classical Whipple's procedure is quite similar in most of the surgical centers around the world as it has been well described elsewhere.[4,7,12]
In the superior approach, the hepatic fl exure of the colon was mobilized and pushed down. The duodenum was fully mobilized by dissecting the attachment of ligament of Treitz; the SMV was identif i ed at its entrance inferiorly near the neck of the pancreas. The pancreatic head was pulled towards the upper-left side over the right renal vein and behind the neck of the pancreas. After the pulsations of the superior mesenteric artery were felt, a 4-cm dissection was continued along its right margin to disclose the IPDA. The root of the IPDA was exposed and ligated as described earlier (Fig. 1).[13]The pyloric sphincter along with two centimeters of the fi rst part of the duodenum were preserved and the rest of the duodenum was cut off.
After cholecystectomy, the common hepatic duct was cut just above its conf l uence with the cystic duct. Using the distal ligated common hepatic bile duct as lateral traction, the space between the common bile duct (CBD) and the portal vein was opened by the tip of the leftindex fi nger (Fig. 2). Subsequently, the left index fi nger was directed medially to dissect the vessels lying on the superior border of the fi rst part of the duodenum within the hepatoduodenal ligament. This maneuver was used to identify the gastroduodenal artery which takes off from and lies inferiorly to the common hepatic artery (Fig. 3). Further dissection was directed at the ligation and division of the gastroduodenal artery. Once this vessel was divided, the left index fi nger was gradually pushed down in the space between the CBD and the portal vein, facilitating easy and quick separation of the anterior surface of the portal vein from the posterior surface of the neck of the pancreas (Figs. 4, 5). In this way, the anterior surface of the portal vein was easily separated from the neck of the pancreas, creating eventually a tunnel behind the neck of the pancreas. The rest of the procedure was continued as usual. In all patients, reconstruction wasundertaken with an isoperistalitic limb of the jejunum in a retrocolic manner and anastomosis was made in a dunking fashion (end to end) to the pancreas, followed by an end to side hepaticojejunostomy and an antecolic end to side pylorojejunostomy. A closed suction drain was routinely put across the sites of pancreaticojejunostomy and hepaticojejunostomy. No prophylactic octreotide was used.

Fig. 1.A sketch showing the location of the inferior pancreaticoduodenal artery (IPDA) after the duodenum (Duo) is kocherized and the pancreatic head is pulled towards the upper left side. IVC: inferior vena cava; AO: abdominal aorta; LRV: left renal vein; SMA: superior mesenteric artery; Pan: pancreas.

Fig. 2.Operative photograph demonstrating an initial step in the superior approach, wherein lateral traction of the ligated lower part of the common hepatic duct (CHD) facilitates exploration of the potential space between the CHD and the portal vein by the tip of the left index fi nger, which is later directed medially for locating the gastroduodenal artery within the hepatoduodenal ligament (HDL). Duo: duodenum.
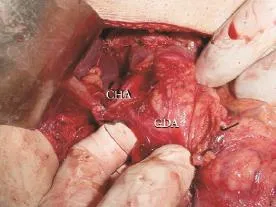
Fig. 3.Operative photograph showing dissection for isolating the gastroduodenal artery (GDA) within the hepatoduodenal ligament. CHA: common hepatic artery.
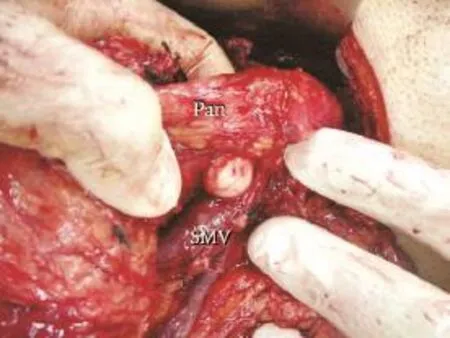
Fig. 4.Operative photograph showing the dissection with the tip of the left index fi nger for creating a tunnel between the pancreatic neck (Pan) and the anterior surface of the portal vein and superior mesenteric vein (SMV) by using the superior approach.

Fig. 5.A sketch demonstrating the direction of dissection using the superior approach for I) ligation of the gastroduodenal artery (a) and II) separation of the common hepatic, common bile duct and pancreatic neck from the portal and superior mesenteric veins (b). GB: gallbladder; CHD: common hepatic duct; CBD: common bile duct; Duo: duodenum; CHA: common hepatic artery; GDA: gastroduodenal artery; PV: portal vein; SMV: superior mesenteric vein; Pan H: pancreatic head.

Table 1.Information of patients in groups 1 and 2
Statistical analysis
Data analysis was performed using the Statistical Package for Social Sciences (SPSS) version 11.5 software (SPSS Inc., Chicago, IL., USA). The Chi-square test was used to assess the statistical signif i cance of differences in the frequency distribution of categorical variables, unless the expected cell size was less than fi ve or ten, when Fisher's exact test was used. Continuous variables were compared using Student'sttest; a non-parametric method like the Mann-WhitneyUtest was used if the data were not normally distributed.Pvalues were calculated by the two-tailed test;P<0.05 was considered statistically signif i cant.
Results
Seventy-two patients who underwent PPPD by thesuperior approach constituted group 1 and 38 matched patients who underwent PPPD by the classical approach constituted group 2. The matching variables between the two groups are presented in Table 1. Jaundice, abdominal pain and weight loss were the main presenting symptoms. Hepatomegaly and palpable gallbladder were the predominant clinical signs detected in both groups. The operative details in the two groups are shown in Table 2. Compared to group 2, the mean blood loss (601.0±250.3 vs 1371.5±471.8 mL) was lesser, and the mean operative time (208.1±46.3 vs 322.0±33.8 minutes) was also shorter in group 1. The mean operative time taken from the start of the procedure to the transection of the neck of the pancreas was also shorter in group 1 as against that in group 2 (60.3±10.7 vs 106.1±11.8 minutes). Moreover, intraoperative blood transfusion was required differently in the two groups (13.9% vs 63.2%), and the mean volume of blood transfused (1.1 vs 1.8) in group 1 was signif i cantlylower than in group 2 (P=0.000, 0.003, respectively).
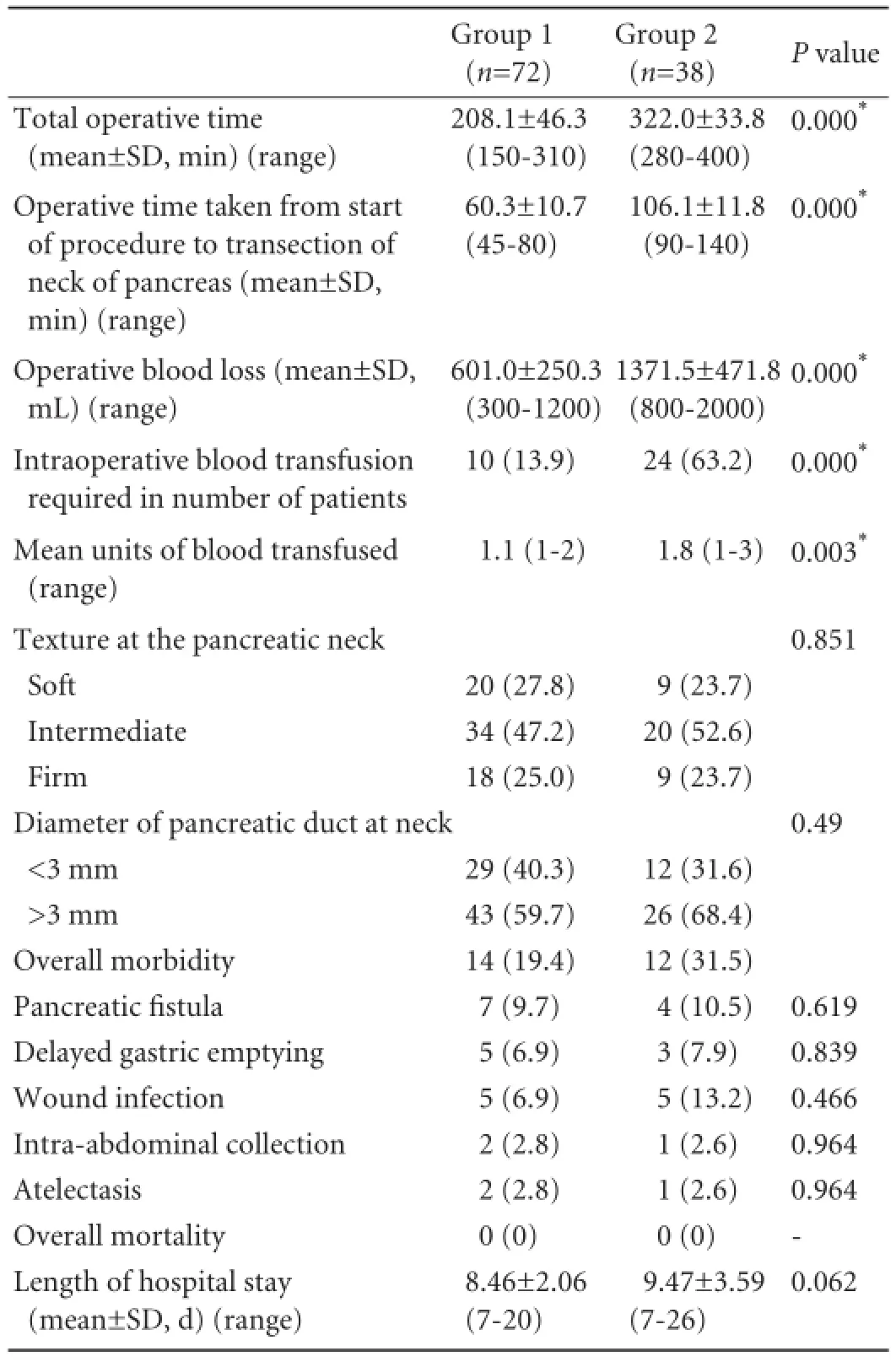
Table 2.Operative and postoperative details of patients in groups 1 and 2 (n, %)
There were 92 (83.6%) normal weight and 18 (16.4%) obese patients (def i ned as BMI ≥25 kg/m2). In the obese patients, signif i cant differences were identif i ed in operative time, operative blood loss and intraoperative blood transfusion between groups 1 and 2 (Table 3).
Although no signif i cant inter-group differences were noted in complication rates among the obese patients (Table 4), while comparing normal weight patients with obese patients, higher complication rates were found for pancreatic fi stula (6.5% vs 27.8%), delayed gastric emptying (5.4% vs 16.7%) and infections like wound infection and intra-abdominal collection (5.4% vs 44.4%) (Table 5). The right hepatic artery originating from the superior mesenteric artery was seen in 15 patients (9 patients in group 1 and 6 in group 2) and the common hepatic artery (Fig. 6) arising from the superior mesenteric artery was detected in 3 patients (2 patients in group 1 and 1 in group 2). These vessels were preserved except one right hepatic artery was injured and ligated in a patient of group 2. In group 2, 3 patients suffered from injury to tiny veins at the lower margin of the pancreatic neck as these entered the anteromedial aspect of the SMV. This accident occurred during dissection for making the tunnel between the SMV and pancreatic neck. No such intraoperative accident was observed in group 1. However no signif i cant differences between the two groups were observed in respect of the location and type of tumor, TNM status (Table 6) and staging, grading and tumor vascular invasion. No postoperative deaths occurred in either group. Thirtytwo postoperative complications were observed in 26 (23.6%) patients: 15 patients (20.8%) in group 1 and 11 (28.9%) in group 2. Pancreatic fi stula as def i ned by ISGPF developed in 9.7% of the patients in group 1 and 10.5% of the patients in group 2; all of them were managed conservatively and closed spontaneously. Delayed gastric emptying occurred in 5 (6.9%) patients in group 1 and 3 (7.9%) in group 2. The mean length ofhospital stay was 8.46±2.06 days in group 1 and 9.47± 3.59 days in group 2; there is no statistically signif i cant difference between the two groups (Table 2).

Table 3.Operative details of 18 obese patients (BMI ≥25 kg/m2)
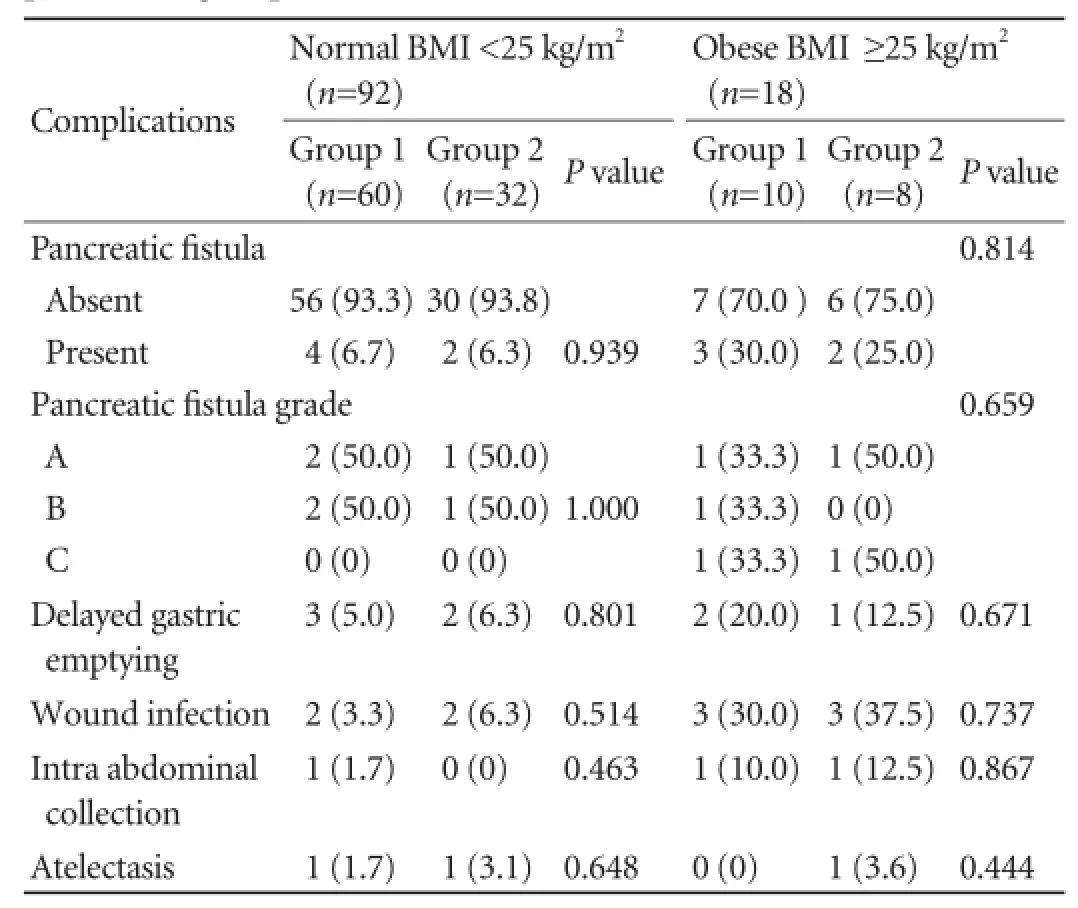
Table 4.Postoperative complications of normal weight and obese patients of groups 1 and 2 (n, %)

Table 5.Postoperative complications of normal weight and obese patients (n, %)

Fig. 6.Operative photograph demonstrating an anomalous common hepatic artery (CHA) lying in the space between the common hepatic duct (CHD) and anterior portion of the portal vein (PV). Such aberrations are identified early and preserved safely by the superior approach.
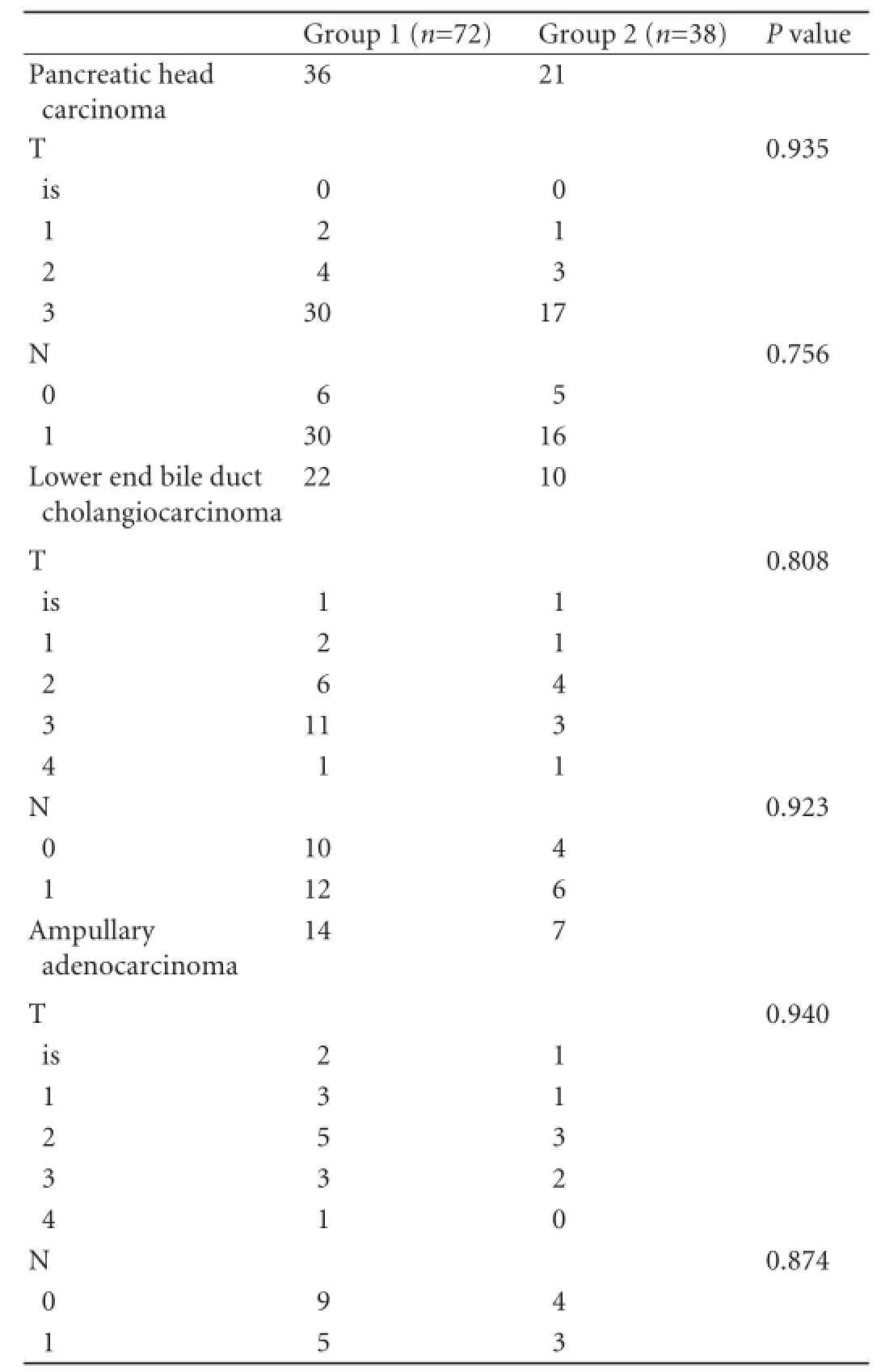
Table 6.TNM classif i cation of tumor (T) and lymph node (N) with respect to tumor origin in groups 1 and 2
Discussion
Since its inception, Whipple's procedure for PD has become the standard operation for tumors arising from the periampullary area or the pancreatic head. However, with the passage of time, many modif i cations of the original Whipple's procedure have been made. Yet PD remains a time-consuming procedure associated with substantial blood loss. One of the principal components of PD is the making of a tunnel between the neck of the pancreas and the superior mesenteric and portal veins. This is carried out in close proximity of the superior mesenteric and portal vein, with a risk of venous injury. By creating a tunnel between the pancreatic neck and these veins, the superior approach allows a rapid and safe exposure of these veins. Extensive blood loss occurs during separation of the pancreatic head from the superior mesenteric vessels and portal vein while performing PD. Blood loss is experienced especially at the fi nal stage of separation of the pancreatic head from the superior mesenteric vessels, when venous congestion occurs until the IPDA is ligated. Thus if early ligation of the IPDA is undertaken before the dissection of the pancreatic head from the superior mesenteric vessels and portal vein, intraoperative bleeding should be reduced.
An interesting and signif i cant difference in the present matched study was related to operative time and intraoperative blood loss. Interestingly, the superior approach reduced operative time and intraoperative blood loss more signif i cantly than the classical method.
Even in experienced centers, high blood loss ranging between 700 and 1500 mL is usually seen with the classical method.[7,14]In our study, the mean blood loss in the patients treated with the classical method was signif i cantly higher than that in the patients treated with the superior approach, which was due to early ligation of the inferior pancreaticoduodenal and gastroduodenal arteries performed by the superior approach. Similar results have been reported in other studies.[15,16]Horiguchi et al[15]described a modif i ed technique of PD, in which the IPDA and gastroduodenal artery are ligated before ligating the corresponding afferent veins of the pancreatic head. They reported a signif i cant reduction in blood loss during PD via the IPDA (678 mL) as compared with standard PD (1225 mL), which is similar to our result in group 1 patients. However, the difference between Horiguchi's study and our study is the signif i cantly reduced operative time in group 1 (208.1 ± 46.3 minutes) against 322.0±33.8 minutes in group 2. Using the preoperative computed image-assessed ligation of the IPDA, Kawai et al[16]found a signif i cant reduction in operative blood loss and operative time. Although intraoperative blood loss is considered a risk factor contributing to high morbidity after PD, the postoperative morbidity was not signif i cantly different between the two groups in our study.
The presence of obesity has been considered a risk factor for surgical outcomes of patients undergoing abdominal surgery.[17-19]The presence of excessive fat tissue inside and outside the visera has often increased operative time and blood loss while affecting surgical results. There are reports conf i rming greater risk for leakage and postoperative complication in obese patients as compared with normal weight patients.[17,18]In our series, operative time, operative blood loss and the volume of blood transfused were signif i cantly lower in obese patients of group 1 than in those of group 2 (Table 3). Reports[17,18]documented higher rates of complications like pancreatic fi stula, wound infectionand mortality in obese patients undergoing PD; these were also observed in our series. But no signif i cant differences were observed in the postoperative complications between the two techniques. In obese patients, the superior approach is not superior to the conventional technique with regard to complications, but it is advantageous in lowering operative time, operative blood loss and intraoperative blood transfusion. The factors making the superior approach a quick and safe procedure with a minimal loss of blood are as follows:
(a) Early division of the duodenum allows faster and controlled dissection of the neck of the pancreas from the portal vein and SMV.
(b) Absence of Gillison's fascia over the portal vein allows easy separation of the CBD.
(c) Besides reducing the blood loss, early ligation and division of the gastroduodenal artery disengages the relatively fi xed pancreatic neck making it more pliable. The tip of the left index fi nger is used to separate the anterior surface of the portal vein from the back side of the neck of the pancreas in a faster and atraumatic manner.
(d) The presence of a potential avascular space between the CBD and duodenum laterally, portal vein medially and posterosuperior part of the head of the pancreas inferiorly facilitates a faster separation of the lower part of the CBD and pancreatic neck from the anterior surface of the portal vein with less time and loss of minimal blood.
(e) The early ligation of the gastroduodenal artery and IPDA reduces the intraoperative blood loss when pancreatic head area is isolated from the portal vein and SMV.
A learning curve for PD has been demonstrated, and outcomes as def i ned by blood loss, operative time and length of hospital stay improve as surgeons progress along the curve and gain experience.[20]Thus many factors are undoubtedly responsible, but a substantial decrease in operative time and operative blood loss suggests that the superior approach is at least in part responsible.
This procedural variant allows early identif i cation and preservation of a replaced right hepatic artery arising from the superior mesenteric artery in 15% to 20% of patients.[21]This vessel is usually placed lateral to and behind the portal vein and enters the hepatoduodenal ligament posterolateral to the bile duct. In the classical procedure it is possible to injure this vessel inadvertently, which may lead to ischemia of the bilioenteric anastomosis. The variation of the right hepatic artery was observed in 15 patients in our study (9 patients in group 1 and 6 in group 2). Such a vessel may course behind, within or along the ventral side of the pancreas. The other anatomic variant of the common hepatic artery originating from the superior mesenteric artery instead of the coelic axis may also be encountered during the separation of the CBD from the anterior part of the portal vein as was observed in 3 patients in our study (2 patients in group 1 and 1 in group 2). This vessel passes anteriorly to the portal vein and lies at the point where the portal vein passes behind the fi rst portion of the duodenum and the neck of the pancreas. After the common hepatic duct is divided above the cystic duct and then the gastroduodenal artery is separated, the lateral traction of the bile duct allows excellent exposure of the suprapancreatic portal vein. Thus such aberrant vessels can be easily identif i ed and preserved safely under direct vision.
In the majority of cases, the superior approach allows a rapid and clear separation of the neck of the pancreas from the anterior aspect of the portal vein and SMV. Though rarely, however, a coronary vein or a superior pancreaticoduodenal vein (the vein of Belcher) may originate from the anterior surface of the portal vein.
With Cameron's technique,[9]the space between the CBD and duodenum (laterally), the posterosuperior part of the pancreatic head (inferiorly) and the anterior aspect of the portal vein (medially) is entered laterally between the CBD and the second part of the duodenum. With our approach, this space is approached superiorly between the common hepatic duct and portal vein (Fig. 7). Another different feature is that we apply lateral traction instead of caudal retraction of the distal CBD in our approach. Thus our approach makes the dissection quicker and virtually bloodless. With Cameron's technique, the small vessels between the lower CBD and the duodenum may tear off easily and lead to troublesome bleeding. Further, with our approach early ligation of the IPDA allows to decrease blood loss while isolating the pancreatic head from the portal and superior mesenteric vessels.

Fig. 7.A sketch showing the difference between the superior approach (A) and Cameron's technique (B) wherein the potential space between bile ducts (CHD, CBD), duodenum (Duo), pancreatic neck (Pan) and anterior portion of the portal vein (PV) and superior mesenteric vein is explored. GB: gallbladder.
Complete tumor resection with negative margins is a necessary condition to ensure a potentially curative treatment; however in a small number of patients with localized pancreatic disease, negative margins are only obtained with portal vein resection. Machado et al[22]described an alternative technique in which the posterior dissection of the superior mesenteric artery from the pancreatic head is performed as an initial step. Further, the mobilization of the jejunum and duodenum behind the mesenteric trunk eventually completely liberates the superior mesenteric artery from the uncinate process leaving the pancreas attached only to the portal vein. It is suggested that the superior approach as described herein would not only aid in the faster and better dissection of the suprapancreatic portal vein but would also allow its complete vascular control.
In conclusion, the superior approach coupled with early ligation of the IPDA could reduce operative time and operative blood loss more signif i cantly than the conventional Whipple's technique. Moreover it allows early identif i cation and preservation of various hepatic arterial aberrations. Hopefully, this approach may also be helpful in achieving a good vascular control in patients whose tumor invasion of the superior mesenteric and portal veins necessitates a vascular resection and reconstruction.
Contributors:SOJ proposed and performed all surgeries. GMA and KIJ wrote the fi rst draft. AR and BS collected and analyzed the data. All authors contributed to the design, interpretation of the study and to further drafts. SOJ is the guarantor.
Funding:None.
Ethical approval:The study received hospital approval for auditing of morbidity and mortality.
Competing interest:No benef i ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Kausch W. Das Carcinom der papilla duodeni und seine radikale Entfeining. Beitr Z Clin Chir 1912;78:439-486.
2 Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of Vater. Ann Surg 1935;102:763-779.
3 Watson K. Carcinoma of the ampulla of Vater. Successful radical resection. Br J Surg 1944;31:368-373.
4 Traverso LW, Longmire WP Jr. Preservation of the pylorus in pancreaticoduodenectomy. Surg Gynecol Obstet 1978;146: 959-962.
5 Ferrone CR, Brennan MF. The Kausch-Whipple Pancreatectomy. In Berger HG, Matsuno S and Cameron JL (eds). Diseases of the Pancreas- Current Surgical Therapy. Springer; 2008:567-579.
6 Howard JM. Historical aspects and the future of Pancreatoduodenectomy. In Hanyu F and Takasaki K (eds). Pancreatoduodenectomy. Springer; 1997:3-10.
7 Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg 2006;244: 10-15.
8 Gouma DJ, van Geenen RC, van Gulik TM, de Haan RJ, de Wit LT, Busch OR, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg 2000;232:786-795.
9 Cameron JL. Rapid exposure of the portal and superior mesenteric veins. Surg Gynecol Obstet 1993;176:395-398.
10 Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fi stula: an international study group (ISGPF) def i nition. Surgery 2005;138:8-13.
11 Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested def i nition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142: 761-768.
12 Bachellier P, Tierris J, Wener Jc, Pai M. Current practice in Pancreatic Surgery. In Habib NA and Canelo R (eds). Liver and Pancreatic Diseases Management; 2006:112-115.
13 Ohigashi H, Ishikawa O, Eguchi H, Yamada T, Sasaki Y, Noura S, et al. Early ligation of the inferior pancreaticoduodenal artery to reduce blood loss during pancreaticoduodenectomy. Hepatogastroenterology 2004;51:4-5.
14 Büchler MW, Friess H, Wagner M, Kulli C, Wagener V, Z'Graggen K. Pancreatic fi stula after pancreatic head resection. Br J Surg 2000;87:883-889.
15 Horiguchi A, Ishihara S, Ito M, Nagata H, Shimizu T, Furusawa K, et al. Pancreatoduodenectomy in which dissection of the efferent arteries of the head of the pancreas is performed fi rst. J Hepatobiliary Pancreat Surg 2007;14:575-578.
16 Kawai M, Tani M, Ina S, Hirono S, Nishioka R, Miyazawa M, et al. CLIP method (preoperative CT image-assessed ligation of inferior pancreaticoduodenal artery) reduces intraoperative bleeding during pancreaticoduodenectomy. World J Surg 2008;32:82-87.
17 Williams TK, Rosato EL, Kennedy EP, Chojnacki KA, Andrel J, Hyslop T, et al. Impact of obesity on perioperative morbidity and mortality after pancreaticoduodenectomy. J Am Coll Surg 2009;208:210-217.
18 Noun R, Riachy E, Ghorra C, Yazbeck T, Tohme C, Abboud B, et al. The impact of obesity on surgical outcome after pancreaticoduodenectomy. JOP 2008;9:468-476.
19 Sledzianowski JF, Duffas JP, Muscari F, Suc B, Fourtanier F. Risk factors for mortality and intra-abdominal morbidity after distal pancreatectomy. Surgery 2005;137:180-185.
20 Tseng JF, Pisters PW, Lee JE, Wang H, Gomez HF, Sun CC, et al. The learning curve in pancreatic surgery. Surgery 2007; 141:694-701.
21 Pessaux P, Varma D, Arnaud JP. Pancreaticoduodenectomy: superior mesenteric artery fi rst approach. J Gastrointest Surg 2006;10:607-611.
22 Machado MC, Penteado S, Cunha JE, Jukemura J, Herman P, Bacchella T, et al. Pancreatic head tumors with portal vein involvement: an alternative surgical approach. Hepatogastroenterology 2001;48:1486-1487.
Received February 16, 2012
Accepted after revision October 27, 2012
AuthorAff i liations:Department of Surgical Gastroenterology, Sher-i-Kashmir Institute of Medical Sciences, Srinagar, Kashmir, India (Shah OJ, Gagloo MA, Khan IJ, Ahmad R and Bano S)
Omar Javed Shah, MD, Department of Surgical Gastroenterology, Sher-i-Kashmir Institute of Medical Sciences, Srinagar, Kashmir, India (Tel: 0194-2463774; Fax: 0194-2471898; Email: omarjshah@ yahoo.com)
© 2013, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(13)60031-4
 Hepatobiliary & Pancreatic Diseases International2013年2期
Hepatobiliary & Pancreatic Diseases International2013年2期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Values of circulating GPC-3 mRNA and alpha-fetoprotein in detecting patients with hepatocellular carcinoma
- HBV recurrence lowered by lamivudine/HBIG combination therapy in liver transplant patients: ten-year experience
- Short-term entecavir versus lamivudine therapy for HBeAg-negative patients with acute-onchronic hepatitis B liver failure
- Management hepatolithiasis with operative choledochoscopic FREDDY laser lithotripsy combined with or without hepatectomy
- Risk factors of choledocholithiasis formation after liver transplantation
- Technical note on complete excision of choledochal cysts
