以1-萘乙酸、5,5′-二甲基-2,2′-联吡啶构筑的双核钐配合物的晶体结构和荧光性质
黄德乾 张 宏 盛良全 蒋雪月 刘昭第 徐华杰
(阜阳师范学院化学化工学院,阜阳 236037)
0 Introduction
The study of luminescent lanthanide metal complexes has gained great recognition over the last decade due to their superior functional properties and various potential applications[1-6]. Compared to first-row transition metals, lanthanide ions have a larger coordination sphere and more flexible coordination geometry, which makes it even more difficult to control the structures. Thus, to be able to rationally design and construct lanthanide coordination polymers with predicted geometries is still a great challenge, as many factors can affect the overall structural formation[7].It has proved that the selection of ligands containing appropriate coordination sites is crucial to build lanthanide complexes. Fortunately, the lanthanide ions have a strong preference to bond to the O-donor atoms to form, e.g., lanthanide carboxylate subunits. This provides a handle that can be utilized in the construction of new lanthanide complexes with unusual useful physical-chemical properties and intriguing structural topologies. A variety of fascinating lanthanide complexes have been constructed by carboxylate ligands and/or Ncontaining ligands as the auxiliary ligands[8-11]. 1-naphthaleneacetate (1-npac) is interesting in the field of coordination complexes due to its strong and various coordination modes. For example, Liu et al.[12]obtained two dinuclear lanthanide complexes based on 1-npac and 1,10-phenanthroline ligands. Chen also synthesized one dinuclear copper(Ⅱ)complex involving 1-npac and dimethylsulfoxide ligands[13]. As part of an on-going study related to lanthanide metal carboxylates, we report here the preparation and structural characterization of a new dinuclear lanthanide complex, Sm2(1-npac)6(dmpy)2·(H2O)3(1).The luminescent properties of 1 were also studied.
1 Experimental
1.1 Materials and measurements
All chemicals purchased were of reagent grade and used without further purification. All syntheses were carried out in 23 mL Teflon-lined autoclaves under autogenous pressure. Elemental analyses (C, H and N) were performed on a Perkin-Elmer 240 CHN elemental analyzer. Infrared spectra were recorded (4 000 ~400 cm-1) as KBr disks on Shimadzu IR-440 spectrometer. Powder XRD investigations were carried out on a Bruker AXS D8-Advanced diffractometer at 40 kV and 40 mA with Cu Kα (λ=0.154 06 nm)radiation. UV-Vis spectra were recorded at room temperature on a Shimadzu UV-160A spectrophotometer in barium sulfate based paint. Luminescence spectra for crystal solid samples were recorded at room temperature on an Edinburgh FLS920 phosphorimeter. Thermogravimetry analyses (TGA)were performed on an automatic simultaneous thermal analyzer (DTG-60, Shimadzu) under a flow of N2at a heating rate of 10 ℃·min-1between ambient temperature and 800 ℃.
1.2 Synthesis of complex 1
Complex 1 was prepared by the addition of stoichiometric amounts of Sm(NO3)3·6H2O (0.222 g,0.5 mmol) and 5,5′-Dimethyl-2,2′-bipyridine (dmpy,0.092 g, 0. 5 mmol) to a hot aqueous solution (15 mL)of 1-naphthaleneacetic acid (0.279 g, 1.5 mmol) where the pH value was adjusted to 8~9 with NaOH (0.016 g, 0.4 mmol). The resulting solution was sealed in a 23 mL Teflon-lined stainless steel autoclave and heated at 150 ℃ for 3 days under autogenous pressure. Colorless single crystals were obtained(yield: 43%, based on dmpy) upon cooling the solution to room temperature at 5 ℃·h-1. Anal. Calcd.(%) for C96H84N4O15Sm2: C, 62.19; H, 4.64; N, 3.02. Found(%):C, 62.24; H, 4.60; N, 3.05. IR (KBr, cm-1): 3 423(vs),3 043(m),2 919(m),1 597(vs),1 551(m),1 508(w),1 478(w),1 408(vs),1 381(vs),1 289(m),1 257(m),1 234(m),1 160(w), 1 041(s), 1 018(w), 982(w), 929(m), 874(w),855(w),832(s),778(vs),735(m),708(s),681(w).
1.3 Crystal structure determination
A single crystal with dimension of 0.30 mm×0.28 mm×0.23 mm was mounted on a glass fiber for data collection on a Bruker Apex ⅡCCD diffractometer operating at 50 kV and 30 mA using MoKα radiation(λ=0.07 1073 nm) at room temperature. In the range of 1.64°<θ<27.49°, a total of 33 797 reflections were collected, of which 9 258 were unique (Rint=0.033 8)and 8 063 observed ones (I>2σ(I)) were used in the succeeding structure calculations. Data collection and reduction were performed using the APEX Ⅱsoftware[14]. Multi-scan absorption corrections were applied for all the data sets using the SADABS[14]. The structure was solved by direct methods and refined by full matrix least squares on F2using the SHELXTL program package[15]. All non-hydrogen atoms were refined with anisotropic displacement parameters.Hydrogen atoms attached to carbon were placed in geometrically idealized positions and refined using a riding model. O2W is disordered and it is split into two sets of positions, with occupancy ratios of 0.5∶0.5.Water H atoms were tentatively located in difference Fourier maps and were refined with distance restraints of O-H 0.082 nm and H …H 0.139 nm, with an standard deviation of 0.001 nm, and with Uiso(H)=1.5 Ueq(O). The final R=0.0291 and wR=0.0684 (w=1/[σ2(Fo2)+(0.0400P)2+0.9700P], where P=(Fo2+2Fc2)/3) for 8063 observed reflections with I>2σ (I). S=1.057, (Δ/σ)max=0.000. Crystal parameters and details of the data collection and refinement are given in Table 1.Selected bond lengths and angles are given in Table 2. H-bonding parameters are given in Table 3.
CCDC: 898378.
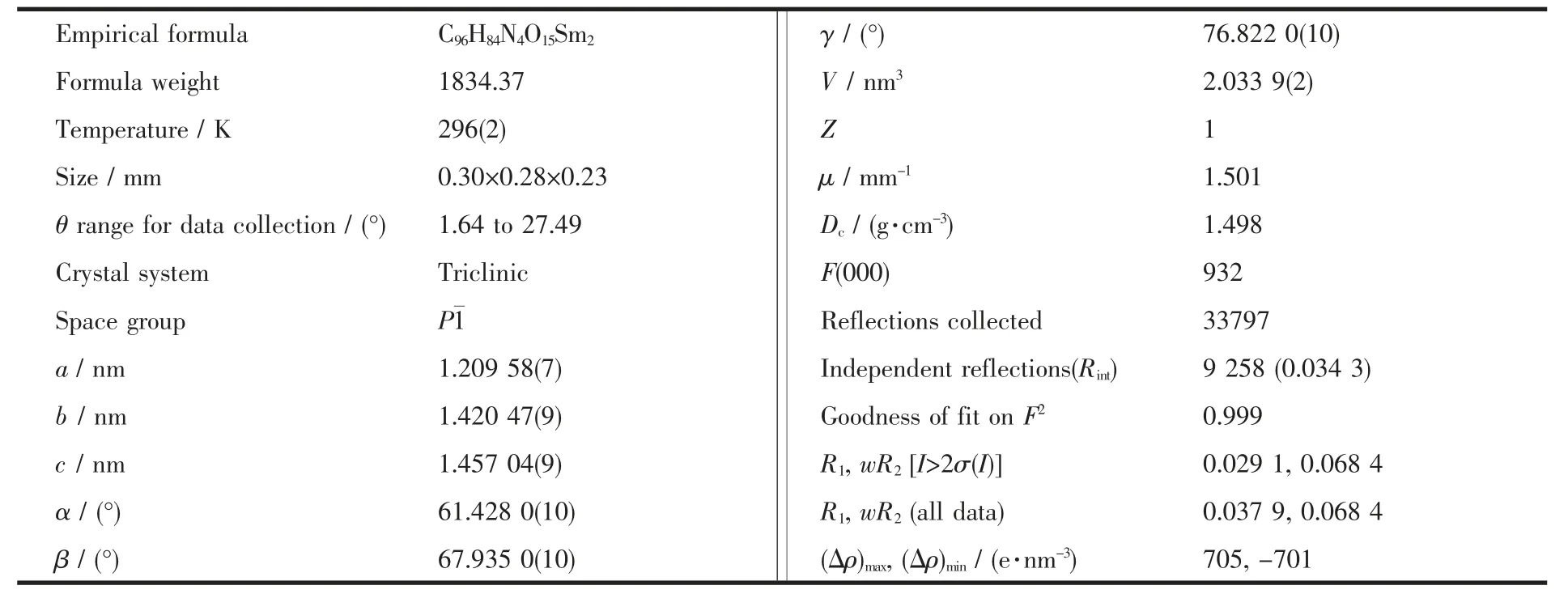
Table 1 Crystal data and structure refinements of the title complex
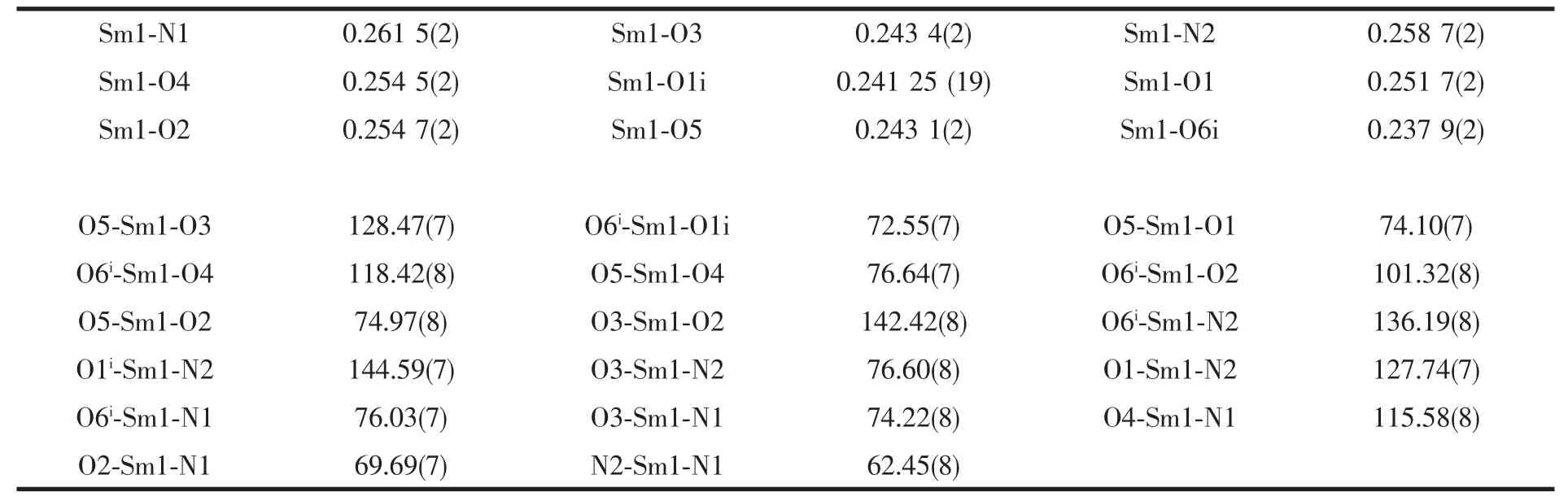
Table 2 Selected bond lengths (nm) and angles (°) for the title complex
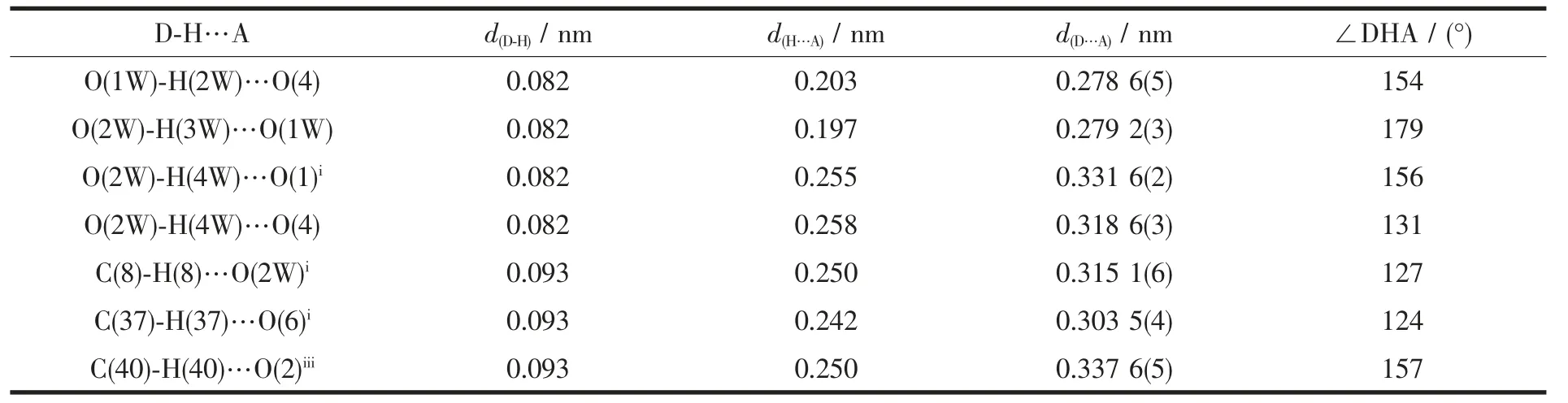
Table 3 Hydrogen bond lengths(nm) and angles (°) for the title complex
2 Results and discussion
2.1 IR spectra
The IR spectra of complex 1 (Fig.1) shows broad band at 3 423 cm-1, which may be assigned to the ν (O-H) stretching vibrations of the free water molecules. The moderate absorption band observed at 3 043 and 2 919 cm-1are attributed to the ν(Cmethyl-H)vibration of dmpy ligand. The features at 1 597 and 1 408, 1 381 cm-1are associated with the asymmetric(COO) and symmetric (COO) stretching vibrations.
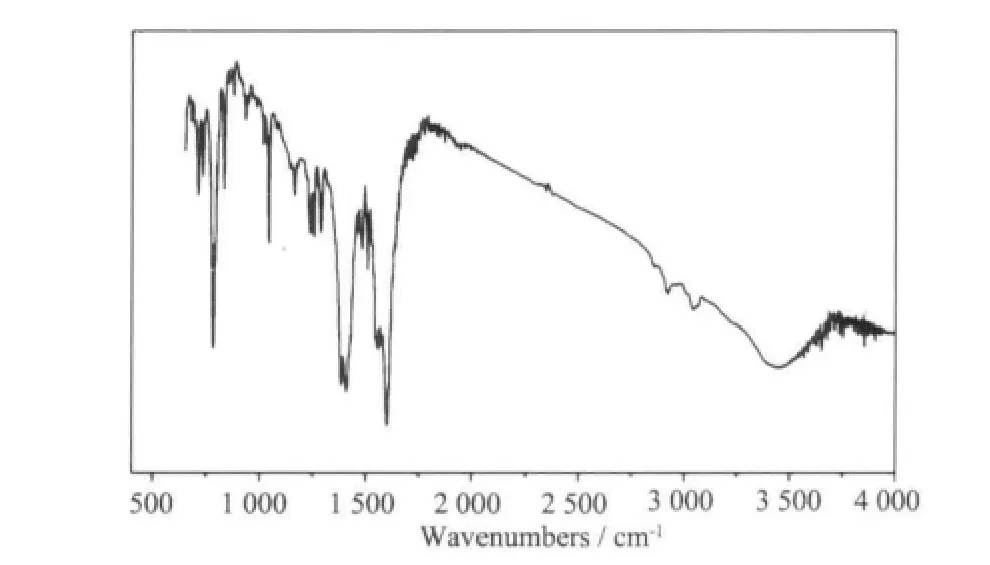
Fig.1 IR spectra of 1
2.2 Structure description
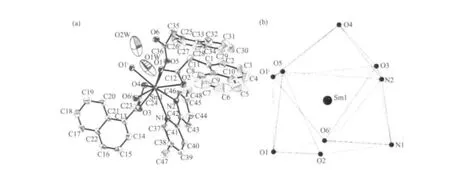
Fig.2 (a) View of the asymmetric unit of complex 1. Non-H atoms are shown as 30% probability displacement ellipsoids.(b) Tricapped trigonal prismatic (TTP) geometry surronding a Sm(Ⅲ)atom of complex 1
Single-crystal X-ray diffraction analysis reveals that complex 1 is a centrosymmetric dinuclear structure and crystallizes in triclinic system withspace group. A thermal ellipsoid plot of 1 is shown in Fig.2a. In the asymmetric unit of 1, there are one Sm(Ⅲ)ion, three 1-npac ligands, one dmpy ligand and one and a half water molecules. The structure consists of a centrosymmetric dimers Sm (Ⅲ)ions bridged by two bidentate and two terdentate corboxylato groups.The Sm(Ⅲ)is nine-coordinated by seven oxygen atoms from five different 1-npac ligands and two nitrogen atoms from one dmpy ligand. The Sm1 center can be described as having a distorted tricapped trigonal prismatic (TTP) geometry (Fig.2b). O1i, O5, O6i, O2,O3, N2 atoms form the prism and O4, O1 and N1 atoms cap the rectangular faces (symmetry code:i1-x,1-y, -z). Dihedral angles between the rectangular faces are 53.4°, 58.5° and 69.0°, respectively. The Sm-O, Sm-N bond distance and O-Sm-O, O-Sm-N bond angle ranging from 0.237 9(2) to 0.261 5(2) nm and 51.02(6)° to 151.57(7)°, respectively, all of which are within the range of those observed for other ninecoordinate Sm(Ⅲ)complexes with nitrogen and oxygen donors ligands[16-17]. The 1-npac ligands display three types of coordination (Scheme 1). One acts as a conventional bidentate bridging ligand, bonding to Sm1 through O5 and Sm1ithrough O6i. The second one is chelated to Sm1 through O2, and O1i, with O1ialso linked to Sm1i. The third one acts as a bidentate chelate, bonding to Sm1 through O3 and O4. The structure has three distinct Sm-O distances involving 1-npac ligands depending on three coordination modes; average bond length of Sm-Obridging, Sm-Ochelateand Sm-Oterdentateis 0.240 5, 0.249 0 and 0.249 2 nm,respectively. This indicates that the order of ring strain is terdentate >chelating >bridging[18]. Compared with [SmTb(1-npac)6(phen)2]2·2C3H7NO[19], average bond length is nearly the same (0.2377, 0.2466 and 0.2466 nm, respectively), which shows that different carboxylato groups with the same mode of coordination with Ln (Ⅲ) ion have essentially the same bond requirements. The separation of Sm…Sm (0.392 5 nm)in the dimer just exceeds the sum of the two ionic radii and is significantly shorter than in [SmTb(1-npac)6(phen)2]2·2C3H7NO(0.3953 nm)[19].The short separation,therefore, may be attributed to the simultaneous appearance of four-membered and eight-membered rings between the two samarium atoms. The average Sm-N bond distance is 0.260 2 nm. Structures,including the complex 1 and [SmTb(1-npac)6(phen)2]2·2C3H7NO suggest strongly that N-bidentate heterocyclic amines as ligands have stronger coordination ability for lanthanide ions than N-unidentate which are hard, and much less common. The centrosymmetric dinuclear molecules are further connected into a supramolecular structure through intermolecular O-H…O, C-H…O hydrogen bonding interactions(Fig.3,Table 3).
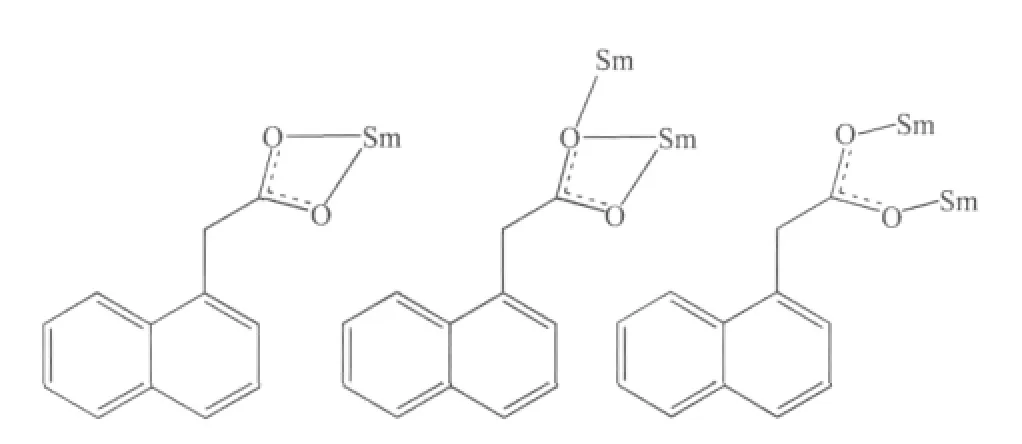
Scheme 1 Coordination mode of 1-npac ligands in the structure of complex 1
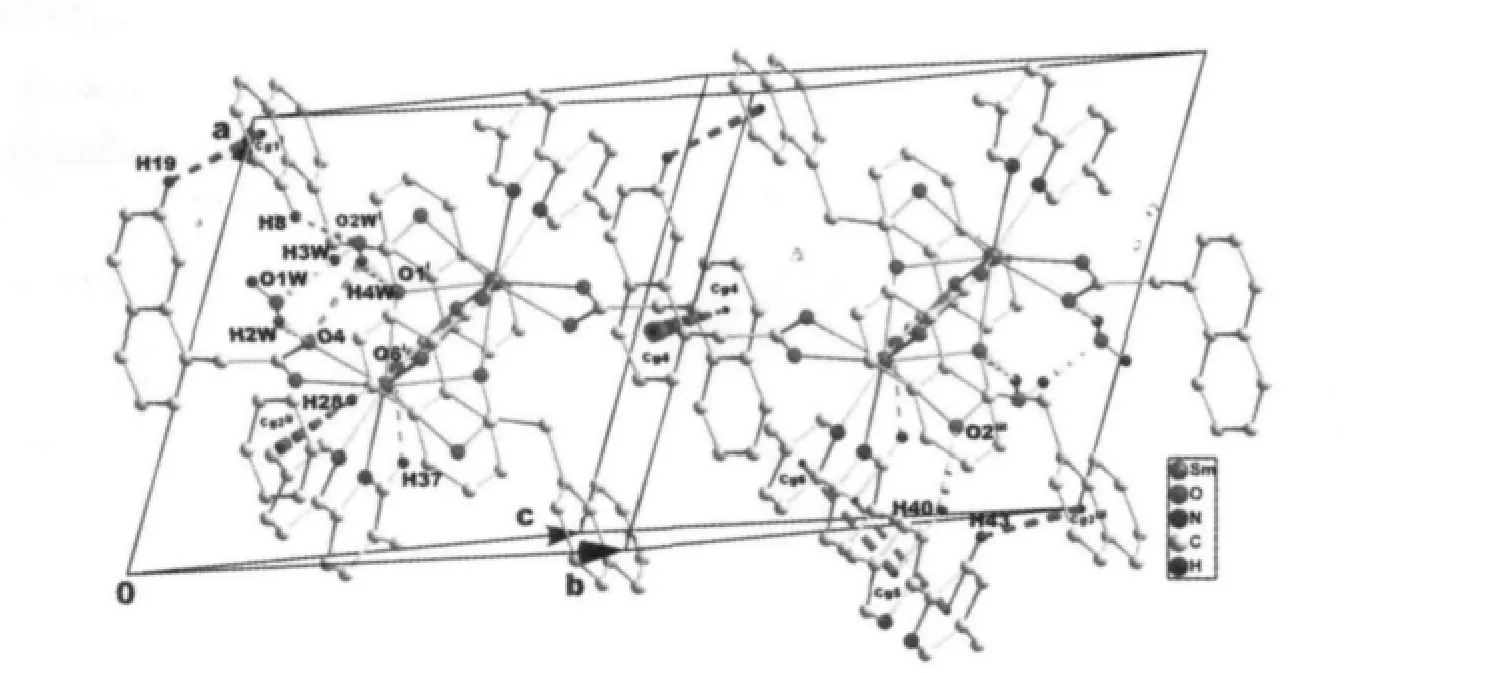
Fig.3 View of the 3D supramolecular structure of complex 1 formed by hydrogen bonds (dashed lines), C-H…π and π…π stacking interactions (dashed lines). Cg1-Cg6 are the centroid of the C5-C10 ring, C17-C22 ring,C1-C4/C9/C10 ring, C13-C16/C21/C22, C38-C41/N1, and C42-C46/N2, respectively. Symmetry codes:i 1-x,1-y,-z; ii 1-x,1-y,1-z; iii 2-x,1-y,-z.
There are also stabilized by C-H…π and π…π stacking interactions. The H-to-centroid distances of H(19)…Cg(1)i=0.292(3) nm, H(28)…Cg(2)ii=0.295(4) nm and H (43) …Cg (3)iii=0.292 (2) nm, and the C-tocentroid distances of C(19)…Cg(1)i= 0.365(2) nm, H(28)…Cg(2)ii=0.387(3) nm and H(43)…Cg(3)iii=0.363(2) nm [Cg(1), Cg(2) and Cg(3) are the centroid of the C5-C10 ring, C17-C22 ring and C1-C4/C9/C10 ring,respectively. Symmetry codes:ii1-x,1-y,1-z;iii2-x,1-y,-z]. The centroid to centroid distances involving parallel pyridyl rings of neighboring dmpy ligands(Cg5 and Cg6 are the centroid of the C37-C41/N1 ring, and the C42-C46/N2 ring, respectively) and parallel benzene rings of neighboring 1-npac ligands(Cg4 is the centroid of the C13-C16/C21/C22 ring) are Cg5…Cg6iii=0.3744(5) and Cg4…Cg4iv=0.3738(5) nm,respectively. The dihedral angles of Cg5…Cg6iiiand Cg4…Cg4ivare 12.11 (2)° and 0.00 (3)°, respectively[Symmetry code:iv1-x,2-y,-z]. Moreover, intramolecular C37-H37…O6 hydrogen bonds are also observed.
2.3 Thermal analysis
The TG curve is depicted in Fig.4, which shows three weight loss steps. The first weight loss corresponding the release of two free water molecules is observed from 50 to 120 ℃ (Obsd. 3.95%, Calcd.3.89%). The second weight loss corresponding the escape of one dmpy ligand is observed from 180 to 300 ℃ (Obsd. 20.11 %, Calcd. 20.10%). The sharp weight loss above 300 ℃ corresponds to the decomposition of framework structure.
2.4 Powder X-ray diffraction analysis
As shown in Fig.5, the peak positions of the experimental patterns are in a good agreement with the simulated patterns, which clearly indicates the good purity of the complex.
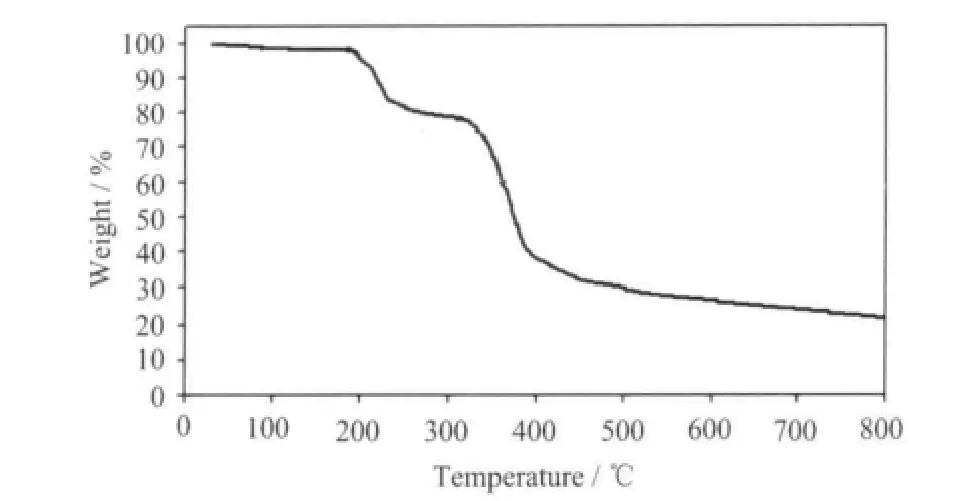
Fig.4 TG curve for complex 1

Fig.5 PXRD patterns of complex 1
2.5 UV-Vis absorption spectra
Fig.6 illustrates the UV-Vis absorption spectra of 1 in solid state. In the studied wavelength domain from 200~450 nm, the B band of 1-npac and dmpy ligands attributed to the π-π*transition is observed with the most intense of absorption around 281 nm.The second-most intense absorption at 362 corresponds to the K band of the L →M (charge transfer) transition involving 1-npac, dmpy ligands and Nd3+ions[20].
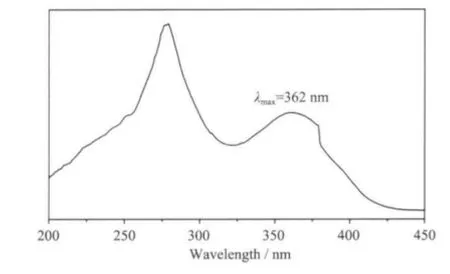
Fig.6 UV-Vis absorption spectroscopy of 1 in solid state
2.6 Luminescent properties
The excitation and emission spectra of complex 1 are shown in Fig.7. The excitation spectra of 1 show a broad band covering the 200 ~250 nm regions. The broad excitation band is assigned to the π-π electron transition of the ligands[21]. In the emission spectrum of 1, there are three characteristic fluorescence emission bands assigned with Sm3+ions, which are the characteristic peaks of the4G5/2→6HJtransitions (J=5/2,7/2, and 9/2)[22]. The peak at 562 nm corresponds to the4G5/2→6H5/2transition of Sm3+ions, the peak at 598 nm corresponds to the4G5/2→6H7/2transition of Sm3+ions, and the strongest peak at 642 nm corresponds to the4G5/2→6H9/2hypersensitive transition of Sm3+ions.The luminescent lifetime of solid complex 1 using an Edinburgh FLS920 phosphorimeter with 450 W xenon lamp as excitation source indicates a lifetime of 0.87 μs at 598 nm (Fig.8).
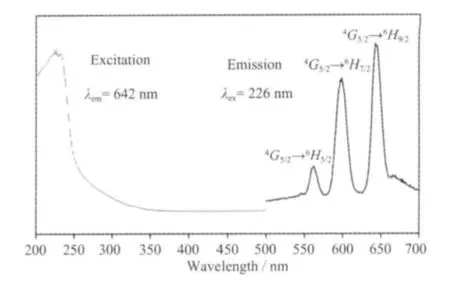
Fig.7 Solid-state excitation and emission spectra of complex 1 at room temperature
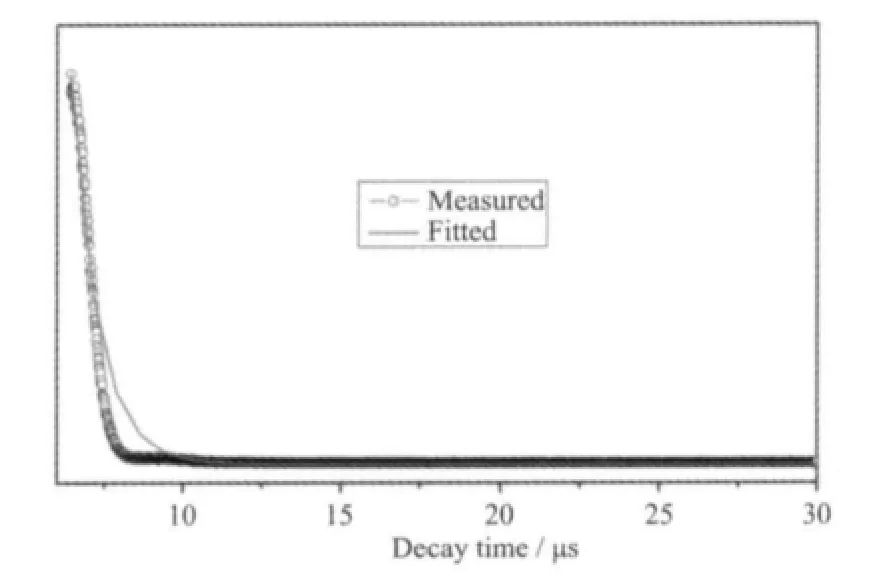
Fig.8 Luminescent lifetime for complex 1 in solid state at room temperaturef
3 Conclusions
In summary, a dinuclear samarium (Ⅲ)complex based on 1-npac and dmpy ligands has been synthesized and structurally characterized. The Sm3+ions are bridged by two bidentate and two terdentate carboxylato groups to give centrosymmetric dimers.Complex 1 emits intensive orange luminescence of Sm3+ion with fluorescence lifetime of 0.87 μs (598 nm) in the solid state at room temperature.
[1] Ma D, Wang W, Li Y, et al. CrystEngComm., 2010,12:4372-4377
[2] Tsukube H, Juanes S. Chem. Rev., 2002,102:2389-2403
[3] Bünzli J C G. Chem. Rev., 2010,110:2729-2755
[4] Smith P H, Brainard J R, Morris D E, et al. J. Am. Chem.Soc., 1989,111:7437-7443
[5] Galdwell J P, Henderson W, Kim N D. J. Forensic Sci.,2001,46:1332-1341
[6] Kido J, Okamoto Y. Chem. Rev., 2002,102:2357-2368
[7] Liu J Q. J. Coord. Chem., 2011,64:1503-1512
[8] Liang Y C, Cao R, Su W P, et al. Angew. Chem. Int. Ed.,2000,39:3304-3307
[9] Mortl K P, Sutter J P, Golhen S, et al. Inorg. Chem., 2000,39:1626-1627
[10]Li X, Sun H L, Wu X S, et al. Inorg. Chem., 2010,49:1865-1871
[11]Seward C, Hu N X, Wang S. Dalton Trans., 2001:134-137
[12]Liu Y F, Rong D F, Xia H T, et al. J. Coord. Chem., 2009,62:1835-1845
[13]Chen L F, Zhang J, Song L J, et al. Acta Cryst., 2004,E60:m1032-m1034
[14]Bruker. APEXII Software, Version 6.3.1, Bruker AXS Inc,Madison, Wisconsin, USA(2004).
[15]Sheldrick G M. Acta Cryst., 2008,A64:112-122
[16]Jongen L, Bromant C, Hinz-Hubner D, et al. Z. Anorg. Allg.Chem., 2003,629:975-980
[17]Sun S J, Zhang D H, Zhang J J, et al. J. Mol. Struct.,2010,977:17-25
[18]Lu Y Q, Lu W M, Wu B, et al. J. Coord. Chem., 2001,53:15-23
[19]Xia H T, Liu Y F, Chen L, et al. Acta Cryst., 2008,E64:m1419-m1420
[20]Yang Y, Zhang L, Liu L, et al. Inorg. Chim. Acta, 2007,360:2638-2646
[21]Wen S, Zhang X, Hu S, et al. Polymer, 2009,50:3269-3274
[22]Chen X Y, Jensen M P, Liu G K, et al. J. Phys. Chem. B,2005,109:13991-13999

