MMP-2 and MT1-MMP contribute to vasculogenic mimicry and poor prognosis in human primary gallbladder carcinomas
SUN Wei,FAN Yue-Zu,ZHANG Wen-Zhong,GE Chun-Yan
Department of Surgery,Tongji Hospital,Tongji University School of Medicine,Shanghai 200065,China
Introduction
Gallbladder carcinoma(GBC)isthemost common malignancy of the biliary tract,the fifth or sixth common malignant neoplasm of the digestive tract and the leading cause of cancer-related deaths in western countries,China and in Shanghai[1,2].This carcinomaisan aggressive tumorwith a poor prognosis because of its inherent biology and often advanced stage at diagnosis.Despite advances in imaging technologies enabling earlier diagnosis of GBC,surgery and response rates of radiotherapy and chemotherapy,the clinical outcome of GBC patients remains unsatisfactory[3].Thus,improvement of GBC treatment is a major public health goal.However,to date,the molecular mechanism of its development and progression,and special biological behavior remains poorly understood.
Vasculogenic mimicry(VM),which is different from angiogenesis and mosaic vessel,was introduced to describe the unique ability of highly aggressive tumor cells to form a capillary-like structure and matrix-rich patterned network in three-dimensionalculture that mimic the embryonic vasculogenic network[4].After the initial observation in melanoma[4-6],evidence for VM has been reported in other tumors,such as cell renal cell carcinoma[7], ovarian carcinoma[8,9], hepatocellular carcinoma[10],breast cancer[11-13],laryngeal squamous cell carcinoma[14], glioblastomas[15], gastric adenocarcinoma[16],colorectal cancer[17],esophageal[18]and gastrointestinal[19]stromal carcinoma.
Matrix metalloproteinases(MMPs)are a broad family of zinc-biding endopepeidases that participate in the extracellular matrix(ECM)degradation that accompanies cancercellinvasion,metastasis,and angiogenesis[20-22].There are 23 MMPs in humans,many of which have been characterized.MMPs can be divided into two subgroups based on whether the enzymes are secreted or expressed on the cell surface in a membrane-tethered form:soluble MMPs and membrane type MMPs(MT-MMP)[23].Interestingly,recent multiple studies have indicated that matrix metalloproteinases-2(MMP-2)and membrane type 1-matrix metalloproteinases(MT1-MMP;MMP-14)expression were significantly related to VM formation in melanoma and ovarian carcinoma cells in threedimensional culture[9,24].Microarray analysis revealed that MMPs(-1,2,9 and 14)were all more highly expressed in aggressive melanoma with VM channels compared with poorly aggressivemelanomawith absence of VM[25].Several recently published papers have indicated that phosphoinositide 3-kinase(PI3-K)is an important adjustor of directly affecting the cooperative interactions of MT1-MMP and MMP-2 activity.PI3-K regulates MT1-MMP activity,which promotes the conversion of pro-MMP into its active conformation through an interaction with tissue inhibitor ofmetalloproteinase-2 (TIMP-2).Both enzymatically active MT1-MMP and MMP-2 may then promote the cleavage of Ln-5γ2chain into promigratoryγ2and γ2xfragments.The deposition of these fragments into tumor extracellular milieu may result in increased migration,invasion and VM formation[26,27].Antisense oligonucleotides to the Ln-5γ2chain and antibodies to MMP-2 or MMP-14 could inhibit VM formation.Special inhibitors of PI3-K could also impair VM formation and decrease MTI-MMP and MMP-2 activity[24].Moreover,anotherstudy has demonstrated that MMP-2 and MMP-9 expression were associated to VM channels in mouse ischemic limbs inoculated by B16 melanoma cells[28].Furthermore,numerous groups have investigated that MMP-2 and MMP-9 expression were correlated to VM in human gastric adenocarcinoma[16],gastrointestinal stromal carcinoma[19]and hepatocellular carcinoma tissue samples[10].VM,an angiogenesis-independent novel pathway,was also reported to be resistant to angiogenesis inhibitor such as endostain and TPN-470 in melanoma tumor cells and the B16F10 murine melanoma model[29].
We have previously reported VM existed in human primary GBC tissue samples and its correlation with the patient's poor prognosis[30].However,the fact that whether MMPs expression was associated with VM in GBC tissue samples remains unclear.Thus,it is necessary to explore MMPs(-1,2,9 and 14)expression and their correlation with VM-positive ornegative GBC,clinicopathologic parameters,and prognosis of the GBC patients with VM-positive and VM-negative.In the present study,our results suggest that over-expression of MMP-2 and MMP-14 was significantly correlated with VM in GBC.Elevated MMP-2 and MMP-14 expressionscould exhibit different associations with clinicopathologic parameters in human primary GBC with VM and non-VM,respectively.MMP-2 and MMP-14 were independent factors for the overall survival(OS)rate of GBC patients.In addition,low expressions of both MMP-2 and MMP-14 were associated with better 5-year survival rate compared to patients with high expressions of MMP-2 and MMP-14 either in VM negative or positive group.
Materials and methods
Patientsand tissue specimens
For this study,we retrospectively selected 94 cases of patients withGBC who underwent curative surgical resection from January 1994 to August 2005 at Shanghai Tongji Hospital of Tongji University.To reduce effects directly related to surgery,patients who died within one month after surgical resection were not included.No patients had history of chemotherapy or radiotherapy before surgery.According to the patients’ clinical record,routine visit record,telephone number,and address,clinical outcome was followed from the date of surgery to the date of death or until the end of August 31,2005.Cases lost during follow-up were regarded as censored data for the survival analysis.Finally,89 resection specimens with complete clinical and prognostic data were collected for final analysis.The diagnosis of these GBC samples(n=89)was verified by two different pathologists who were blinded to the patients,clinical status.According to WHO criteria and Nevin stage system,detailed pathological and clinical data were collected by reviewing medical charts and pathological records for all of the samples,including age,gender,tumor location,tumor size,histological grade,Nevin stage,invasion depth,live and lymph node metastasis.The group comprised 31 men and 58 women with a mean age of 63±15 years(mean ± SD,range 31 to 92 years).The median follow-up period for all patients was 20.16(range,1.5-60)months.R0,1 and R2 forGBC patientswere observed in 62 of89(69.66%)and 27 of 89(30.34%),respectively.The present study was carried out with approval from Research Ethical Review Broad in Tongji University(Shanghai,China).Clinical and histopathologic data were summarized in Table1.

Tab.1 Demographic and clinicopathologic parameters of GBC patients included in this study n(%)表1 本研究所涉及的胆囊癌患者的人口统计学和临床病理参数
MMP-1,-2,-9 and-14 immunohistochemical staining
The immunohistochemical method was performed as described previously[16]. Briefly, after deparaffinizing,inactivating endogenous peroxide activity,Thesamples werewashed in phosphate buffered saline(PBS,pH7.4),thenpretreated 0.01 M citrate buffer(pH 6.0)for 30 min at 100 ℃in a boiler,then the slides were allowed to cool at room temperature and washed in PBS again.Slides were then incubated respectively with the primary antibodies to MMP-1(rabbit monoclonal,EP1247Y,dilution 1:100,Abcam,USA),MMP-2(rabbit polyclone,Z2089,dilution 1:200,ZETA,USA),MMP-9(rabbit polyclone,Z2090,dilution 1:100,ZETA,USA)and MMP-14(rabbit monoclonal,EP1264Y,dilution 1:100,Abcam,USA)at 4℃overnight.Then by a brief rinse in PBS,the sections were incubated with goat anti-rabbit Envision Kit(GK400305,Genetech,USA)for 30 min at 37 ℃,3,3-diaminobenzidine(DAB)for 5 min and counterstained by hematoxylin.Negative controls were established by replacing the primary antibody with PBS in all samples,known immunoassaying-positive sections were used as positive controls.
Computer-Assisted Image Analysis
The positive expression of MMP-1,-2 and-9 was in the cytoplasm.MMP-14 expression in the tumor cells was mainly detected on the cell membrane and in the cytoplasm.To evaluate precisely the expression level of MMP-1,-2,-9 and -14 protein, all immunohistochemistry stained sections were examined in aZesissphotomicroscope(CarlZesiss,Inc,Thornwood,NY)equipped with a three-chip chargecoupled device color camera(model DXC-960 MD;Sony Corp,Tokyo,Japan),High-resolution(1 024 ×1 024 pixels)images were obtained from each histospot at×40 magnification and stored digitally on a computer.The image analysis to quantify intensity of color reaction was analyzed using Image-Pro Plus Software(IPP version 4.5;Media Cybernetics,CA,USA)[31,32].The staining intensity levels of MMPs were measured using arbitrary unit(AU)on a linear scale ranging from 0 (non-detectable)to 255(highest intensity).This procedure can be divided into seven different steps:①creating and measuring the area of interest(AOI);②calibrating the optical density(OD);③acquiring,converting and saving images;④performingthebackgroundand background staining correction;⑤setting of the AOI in the acquired image to measure the optical density(OD,AU),integrated optical density(IOD,AU),tumor tissue area and immunohistochemistry(IHC)-positive area;⑥measuring mean density(IOD sum/tumor tissue area);⑦creating macros.Mean density of five different fields in each zone were quantified by a reader who was kept blinded from the clinical outcome.Therefore,the OD(mean density)results of protein expression were analyzed for statistical significance.
Selection of cut-off scores
Cut-off scores forMMPs(-1,-2,-9 and-14)expression were selected based on receiver operating characteristic(ROC)curve analysis[33,34].ROC curve was plotted for the outcome of GBC patients under study by calculating the sensitivity and specificity on its points.The score closest to the points(0.0,1.0)on the curve with a maximum sensitivity and specificity was selected as the cut-off score leading to the greatest number of tumors classified with or without clinical outcome.The area under the ROC curve was calculated respectively to estimate the discriminatory power of MMPs protein over the entire range of scores for overall survival(OS)rate of GBC patients.The ROC curve was generated and analyzed using the MedCalc statistical software package 11.0.1(MedCalc Software bvba,Belgium).
Statistical analysis
All data were expressed as mean± SD and performed using SAS 9.0 software(SAS Institute Inc.,Cary,NC,USA).The comparison and association
between VM and categorical variables were analyzed by t/F testand Spearmen correlation analysis,respectively.Survival curves were calculated with the Kaplan-Meier method and were compared using the log-rank test.Multivariate analysis of prognostic factors was performed using the Cox’s regression model.P <0.05 was considered statistically significant.
Results
MMP-2 and-14 expression levels are significantly correlated with VM in GBC patients
According to the evidence of VM in human primary GBC by using H&E and CD31-PAS double staining(Fig.1)[30],we further evaluate the MMP-1,-2,-9 and -14 expressions in both VM-positive and VM-negative group.Representative pictures of immunohistochemical staining of MMP-1,-2,-9 and-14 are shown in Fig.2.The mean density(OD)of MMP-2 was significantly greater in the VM-positive than in the VM-negative group(0.083 ±0.032 vs.0.059 ± 0.024,P=0.0007;Fig.2b3),as was expression of MMP-14(0.010 ±0.003 vs.0.007 ±0.002,P=0.0013;Fig.2d3).However,the mean density of MMP-1(P=0.0763;Fig.2a3)and MMP-9(P=0.3553;Fig.2c3)showed no significant difference between the VM-positive and VM-negative groups,respectively.
ROC curve analysisfor MMPs(-1,-2,-9 and-14)was used to select the cut-off score for high or low IHC reactivity.Based on the above results,as to MMP-2 protein expression(Fig.3c),threshold value of 0.0622 was the closest to the point with both maximum sensitivity and specificity,and thereby selected as the cut-off score.The AUC of our ROC curve analysis was 0.736(95%CI:0.632-0.824).In addition,for MMP-14 protein expression(Fig.3d),the threshold value was 0.0083.The AUC of our ROC curve analysis was 0.783(95%CI:0.683-0.864).Therefore,the 89 GBC specimens were categorized into high and low MMP-2 and MMP-14 expression groups,respectively.The expression level ofMMP-2 and MMP-14 was up-regulated in 27(38.6%)and 13(18.6%)cases and down-regulated in 43(61.4%)and 57(81.4%)cases in VM-negative group,respectively.Similarly,the expression level of MMP-2 and MMP-14 was up-regulated in 16(84.2%)and 13(68.4%)cases and down-regulated in 3(15.8%)and 6(31.6%)cases in VM-positive group,respectively.Correlation analysis revealed a positive correlation between MMP-2(r=0.374,P=0.0003)and MMP-14 r=0.449,P=0.0001)expression and VM,respectively.
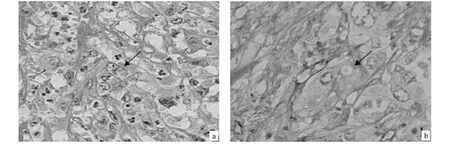
Fig.1 Evidence of vasculogenic mimicry(VM)in human gallbladder carcinoma(GBC).Morphologic appearance of VM(Envision,original magnification ×400)with HE staining(A)and CD31-Periodic acid-Schiff(PAS)double staining(B).A:The VM channel(black arrow)was lined only by tumor cells,several red blood cells(RBCs)therein.B:the presence of CD31-PAS double staining of VM(black arrowhead);the wall of VM channel was positive for PAS staining,while tumor cells lining the external wall were negative for CD31 staining;CD31-negative tumor cell-lined vascular structures were seen to contain RBCs(black arrowhead).CD31-positive vascular endothelial cells(yellow arrowhead).图1 人胆囊癌存在血管生成拟态的证据
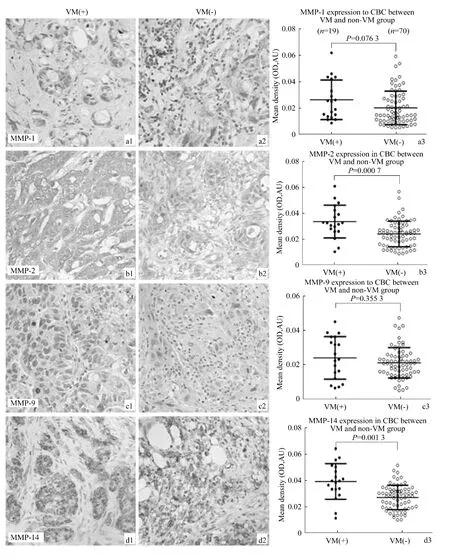
Fig.2 Expression of MMPs(-1,-2,-9 and-14)and quantitative analysis in GBC samples with VM and non-VM by immunohistochemistry(Envision,original magnification ×400).MMPs(-1,-2 and-9)expression showed cytoplasmic immunostaining(A,B and C);of them,representative staining of MMPs(-1,-2 and-9)in GBC samples with VM(A1,B1and C1)and non-VM(A2,B2and C2).MMP-14 expression showed mixed membraneous and cytoplasmic staining(D1-2);of them,representative staining of MMP-14 in GBC samples with VM(D1)and non-VM(D2).The mean density(OD)of MMP-2(B3)and MMP-14(D3)was significantly higher in the VM-positive than in the VM-negative group(P=0.0007;P=0.0013).The mean density of MMP-1 and MMP-9 showed no significant difference between the VM-positive and VM-negative groups(P=0.0763;P=0.3553),respectively.图2 有、无血管生成拟态胆囊癌中MMPs(-1,-2,-9 and-14)表达(免疫组化Envision法)

Fig.3 Receiver operating characteristic(ROC)analysis of MMPs(-1,-2,-9 and-14)protein expression and selection of cut-off score.Eighty-nine GBC specimens were categorized into low and high MMPs(-1,-2,-9 and-14)expression groups,respectively.图3 MMPs(-1,-2,-9 and-14)蛋白表达的ROC分析和截点得分选择
Associations between expression of MMP-2,-14 and clinicopathologic data in GBC with VM and non-VM
We next explore the relationships between mean intensity of MMP-2 and MMP-14 and clinicopathologic data in GBC with VM or without VM.No significant correlations were observed between the staining intensity of MMP-2 and MMP-14 and clinicopathologic variable such as age,gender,tumor location and tumor size.The representative results were shown in Fig.4,increased MMP-2 expression in GBC specimens with non-VM correlated with Nevin stage(0.049 ±0.023 vs.0.064 ±0.025,P=0.027),liver metastasis(0.074 ± 0.026 vs.0.055 ± 0.023,P=0.005),anddegreeof differentiation(G1 0.040 ± 0.013,G2 0.055±0.019,G3 0.071 ± 0.027;PG1/G2=0.025,PG1/G3=0.001,PG2/G3=0.012),respectively.The similar correlation was also observed in GBC samples with VM in terms of liver metastasis(0.094 ± 0.028 vs.0.061 ± 0.029,P=0.029)and serosal invasion(0.083 ± 0.026 vs.0.054 ± 0.023,P=0.004).Furthermore,when comparing the VM-positive with the VM-negative group,the mean density of MMP-2 was higher significantly in GBC patients with VM than those patients without VM in the following conditions,including Nevin stage(S3 ~ S5;0.088 ±0.028 vs.0.064 ± 0.025,P=0.019),positive liver metastasis(P =0.046),positivelymphnode metastasis(0.073 ± 0.031 vs.0.056 ± 0.021;P=0.009),positive serosal invasion(0.083 ±0.026 vs.0.062 ± 0.026,P=0.0001),andmoderate differentiation degree(G2)(0.079 ± 0.015 vs.0.055 ±0.019,P=0.003).

Fig.4 Relationship between MMP-2 expression and clinicopathological parameters in GBC with VM and non-VM group图4 MMP-2表达与有、无血管生成拟态胆囊癌临床病理参数相关性
As shown in Fig.5,in VM-negative group,significant relationship was observed between elevated MMP-14 expression and liver metastasis(0.008 ±0.003 vs.0.006 ± 0.002,P=0.003),Nevin stage(0.006 ± 0.002 vs.0.007 ± 0.002,P=0.044)and degree of differentiation(G1:0.004 ± 0.001,G2:0.007 ±0.002,G3:0.007 ±0.002;PG2/G2=0.0001,PG1/G3=0.0001),respectively.However,we failed to find significant correlation between MMP-14 expression and clinicopathologic features in VM-positive group.In addition,the expression of MMP-14 in GBC patients with VM was higher significantly than those patients without VM in the following conditions,including Nevin stage(S1 ~ S2;0.008 ±0.001 vs.0.006 ± 0.002,P=0.032),Nevin stage(S3 ~ S5;0.01 ± 0.004 vs.0.007 ± 0.002,P=0.01),negative(0.01 ± 0.004 vs.0.007 ± 0.002,P=0.016)and positive(0.01 ± 0.001 vs.0.007 ±0.002,P=0.021)serosal invasion,positive lymph node metastasis(0.012 ± 0.002 vs.0.007 ± 0.002,P=0.0001),negative liver metastasis(0.008 ±0.003 vs.0.006 ± 0.002,P=0.027),moderate differentiation degree(G2)(0.009 ± 0.002 vs.0.007 ±0.002,P=0.002)and poor differentiation degree(G3)(0.011 ±0.004 vs.0.008 ±0.002,P=0.033),respectively.
Univariate and multivariate survival analysis
Factors involved in overall survival(OS)rate of GBC patients were identified using the Cox proportionalhazardsmodel(Table2).Univariate analysis showed that liver metastasis(P=0.013),VM(P=0.026),curability(P=0.001),Nevin stage(P=0.03),MMP-2 expression(P=0.000)and MMP-14 expression(P =0.013)were found to be the significant prognostic indicators for the OS rate of GBC patients,and thereby selected as the parameters to be included in the same Cox regression model.Further multivariate analysis confirmed Nevin stage(hazard risk=2.85,95%CI:1.645-4.939,P=0.000),curability(hazard risk=2.183,95%CI:1.306-3.650,P=0.003),MMP-2 expression(hazard risk=2.487,95%CI:1.393-4.441,P=0.002)and MMP-14 expression(hazard risk=5.418,95%CI:2.806-10.463,P=0.000)were the independent prognostic factors for the OS rate of GBC patients.
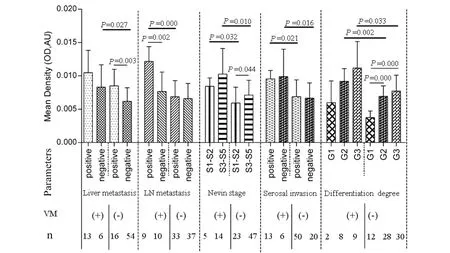
Fig.5 Relationship between MMP-14 expression and clinicopathological parameters in GBC with VM and non-VM group图5 MMP-14表达与有、无血管生成拟态胆囊癌临床病理参数相关性
Kaplan-Meier analysis and the log-rank test were used to further evaluate theeffects of VM,MMP-2 and-14 expressions on survival of GBC patients.The means and medians for survival time of the VM group were 11.13 months and 9 months,compared with 22.62 months and 22 months for non-VM group.The cumulative 1- ,3-and 5-year OS rate were 36.84%,0%and 0%in the VM group and 71.43%,21.43% and 4.29% in the non-VM group,respectively.The survival time of the VM group were significantly shorter than that of the non-VM group(Fig.6a,P=0.0005).In addition,patients with low MMP-2 expression had a better survival than those with high MMP-2 expression(Fig.6b1,P=0.003)in the VM group,and an improved survival than those with high MMP-2 expression(Fig.6b2,P=0.000)in the non-VM group.The OS rate was significantly higher in patients with low MMP-14 expression than those with high MMP-14 expression in either VM(Fig.6c1,P=0.018)or non-VM group(Fig.6c2,P=0.000).We also analyzed the prognostic value of VM in different status ofMMP-2 and MMP-14 expression.No significant difference was observed betweenVM-positiveand VM-negativein GBC samples with high or low MMP-2 and MMP-14 expression(P >0.05,figures not shown).
Discussion
In the currentstudy,we found thatoverexpression of MMP-2 and MMP-14 was significantly associated with VM in GBC;and with Nevin stage,degree of differentiation and liver metastasis in non-VM GBC group.Also,MMP-2 expressionwas associated with serosal invasion and liver metastasis in VM group.More importantly,we showed that overexpression of MMP-2 in GBC patients was associated with VM in the same subset of clinicopathologic parameters, including moderate degree of differentiation,Nevin stage(S3-S5),positive liver metastasis,serosal invasion and lymph node metastasis;over-expressionofMMP-14inGBC patients was correlated with VM in the same subset of clinicopathologic parameters,including degree of differentiation(G2 and G3),Nevin stage,positive lymph node metastasis and serosal invasion,negative liver metastasis and serosal invasion.Multivariate analysis confirmed that MMP-2 and MMP-14 were independent factors for the overall survival(OS)rate of GBC patients and associated with poor prognosis either in VM negative or positive group.

Tab.2 Univariate and multivariate analysis of overall survival rate of GBC patients with Cox proportional hazards model表2 应用Cox风险模型的胆囊癌患者生存的单因素和多因素分析
The evolution and progression of carcinoma is a multi-step process,which requires the degradation or remodeling of ECM and basement membrane(BM)by proteolytic enzymes.Among these proteinases,MMPs are particularly implicated due to their specific spectrum of substrates and central mediators of tumor metastasis[20]. In fact, the research about the relationship between GBC and MMPs expression was sparse.Karadag et al[35]reported that MMP-2,MMP-9,and MMP-14 were expressed in GBC epithelium but also the expression in the stromal component,the latter may be essential for the malignant potential of GBC.In previous study,we observed that MMP-2/TIMP-2 ratio might be a new significant marker in the judgment of invasion or metastasis and the estimate of prognosis in patients with GBC[36].On the other hand,ECM remodeling,one of prerequisite steps governing VM formation,could provide the space needed for VM and was correlated with MMP secretion by tumor cells[9,37].Recent observations have indicated that MMP-2 and MMP-9 degrade the ECM components and facilitate tumor angiogenesis,invasion,metastasis[38,39]and also promote VM channels formation induced byhypoxia-induced factor-1α (HIF-1α)[28].Unlike the other members of the MMPs family,MT-MMP are notsecreted,butinstead remain attached to cell surface[40].MMP-14 activates pro-MMP-2,and the zymogen interacts with MMP-14 by a mutualTIMP-2 binding partner,which formsa receptor complex on the cell surface[41].Numerous studies have revealed that MMP-14 is important for endothelial tubulogenesis in fibrin gels[42], in endothelial cell migration on three-dimensional collagen gels[43],and both MMP-14 and MMP-2 are up-regulated when endothelial cells[44]or tumor cells[9,24]are cultured on a three-dimensional matrix.
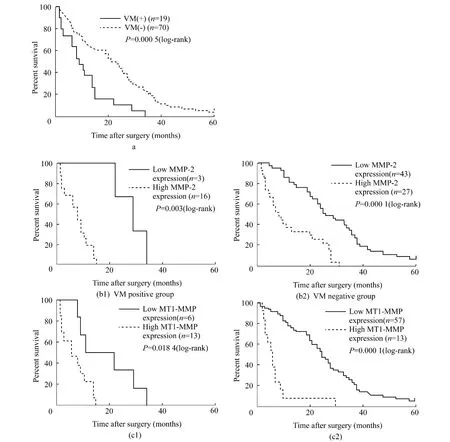
Fig.6 Kaplan-Meier survival curves for patients with gallbladder carcinoma according to VM(A),MMP-2 expression(B1-2)and MMP-14 expression(C1-2).These Kaplan-Meier curves demonstrate significantly worse outcomes for patients with(A)vasculogenic mimicry,high expression of MMP-2(B1-2)and MMP-14(C1-2)either in VM negative or positive group.图6 根据VM和MMP-2、MMP-14表达的胆囊癌患者的Kaplan-Meier生存曲线
Severalstudies have examined relationships between MMPs(-2and-9)expression and VM formation in several formalin-fixed paraffin-embedded tumor tissue samples[10,16,19]. Elevated MMP-14 expression was found to associate with VM only in ovarian carcinoma tissue samples by using immunohistochemistry(IHC)[9].In addition,our study used computer-assisted image analysis system to quantitatively analyse the MMPs expression in VM-positive and VM-negative GBC patients,and the cutoff score was selected based on ROC curve analysis,so that the trade-off between sensitivity and specificity was the smallest,leading to the greatest overall number of correctly classified tumors with and without clinical outcome.WeobservedthatMMP-2and MMP-14 expression correlated significantly with VM in human GBC,which is in line with the above research results,suggesting that MMP-2 and MMP-14 could degrade and remodel the ECM to facilitate VM formation and were key mediators of VM formation in GBC.However,no significant association was found between MMP-1 and MMP-9 expression and VM,which is variance with previous studies indicating that the significant correlation between MMP-9 expression and VM.It is plausible that different tissue origin,different methods of evaluation applied(semiquantitative evaluation based on the percentage of positive cells vs.quantitative digital analysis)and technical factors could explain why our results differ from other studies.
Our data suggested that elevated MMPs(-2 and-14)expressions were correlated with Nevin stage,degree of differentiation and liver metastasis in non-VM group.Moreover,MMP-2 expression was also associated with serosal invasion and liver metastasis in VM group.However,no significant correlation between MMP-14 expression and clinicpathological features was found in VM-positive group.Hence,we initially speculated that high expression of MMP-2 and MMP-14 is compatible with more aggressive histological behaviors and metastasis of GBC patients with non-VM.In addition,high MMP-2 expression only might reflect more invasion and metastasis of GBC patients with VM.Taken together,these findings indicate that MMP-2 and MMP-14 could exhibit different clinical significance under the influence of VM channel in human primary GBC.It is necessary to evaluate whether VM exists in GBC before exploring the relationship between MMP-2 and MMP-14 expression and clinicpathological parameters in human primary GBC.
As far as we know,we reported here the firstthe relation between MMP-2 and MMP-14 expression and VM in the same subgroup ofclinicopathologic parameters in GBC by using IHC quantitative analysis.Ourcurrentanalysisrevealed thathigh MMP-2 expression in GBC patients was associated with VM in the same subset of clinicopathologic parameters, including moderate degree of differentiation,Nevin stage(S3-S5),positive liver metastasis,serosal invasion and lymph node metastasis.We also demonstrated that high MMP-14 expression in GBC patients was correlated with VM in the same subsetofclinicopathologic parameters,including degree of differentiation(G2 and G3),Nevin stage,positive lymph node metastasis and serosal invasion,negative liver metastasis and serosal invasion.Therefore,based upon our collective data it appears that the association between MMP-2 expression and VM wasnotan enough early phenomenon in GBC development.Additionally,MMP-14 might play an important role in early and advanced stage of VM formation in GBC.
Our multivariate analysis showed that MMP-2 and MMP-14 expression levels in primary GBC could be highly significant independent risk factors.In contrast,VM had no statistically significant association with prognosis.This different finding about VM in univariate and multivariate analysis was opposite of the expected.Therefore,furtherstudy with large sample size (especially for VM-positive GBC samples)isneeded to obtain a clearpicture.According to the ROC curves for MMPs(-1,-2,-9 and-14),89 GBC patients were divided into high and low expression group,respectively.The data showed VM was related to decreased 5-year survival.High MMP-2 and MMP-14 expression levels were associated with poor prognosis either in VM negative or positive group.The present study suggested that MMP-2 and MMP-14might serveas prognostic factors in human primary GBC patients either with VM or non-VM channels.
In conclusion,MMP-2 and MMP-14 might play a functionally significant rolein VM formation in human primaryGBC.Moreover,elevated MMP-2 expression contributed to progression of GBC by promoting liver metastasis in either VM or non-VM group.MMP-14 only contributed to progression of GBC by promoting serosalinvasion and liver metastasis in non-VM group.High expression MMP-2 and MMP-14 was significantly associated with VM in the severalsame subgroups of clinicopathologic parameters.In addition,MMP-2 and MMP-14 were independent factors for GBC patients and associated with poor prognosis.However,more cases need to be studied to verify our findings,especially in view of the contradictory results.Further investigations are warranted to elucidate the role of MMP-2 and MMP-14 in the pathogenesis of VM in GBC in vivo and in vitro for the development of effective treatment.
[1] Miller G,Jarnagin WR.Gallbladder carcinoma[J].EJSO,2008,34(3):306-312.
[2] Hsing AW,Gao YT,Devesa SS,et al.Rising incidence of biliary tract cancers in Shanghai,China[J].Int J Cancer,1998,75(3):368-370.
[3] Zhu AX,Hong TS,Hezel AF,et al.Current management of gallbladder carcinoma[J].Oncologist,2010,15(2):168-181.
[4] Maniotis AJ,Folberg R,HessA,etal.Vascularchannel formation by human melanoma cells in vivo and in vitro:vasculogenic mimicry[J].Am J Pathol,1999,155(3):739-752.
[5] Hendrix MJ,Seftor EA,Hess AR,et al.Vasculogenic mimicry and tumor-cell plasticity:lessons from melanoma[J].Nat Rev Cancer,2003,3(6):411-421.
[6] Lissitzky JC,ParriauxD,Ristorcelli E,et al.Cyclic AMP signaling as a mediator of vasculogenic mimicry in aggressive human melanoma cells in vitro[J].Cancer Res,2009(3),69:802-809.
[7] Vartanian AA,Stepanova EV,Gutorov SL,et al.Prognostic significance of periodic acid-Schiff-positive patterns in clear cell renal cell carcinoma[J].Can J Urol,2009,16(4):4726-4732.
[8] Sood AK,Fletcher MS,Zahn CM,et al.The clinical significance of tumor cell-lined vasculature in ovarian carcinoma:implications for anti-vasculogenic therapy[J].Cancer Biol Ther,2002,1(6):661-664.
[9] Sood AK,Fletcher MS,Coffin JE,et al.Functional role of matrix metalloproteinases in ovarian tumor cell plasticity[J].Am J Obstet Gynecol,2004,190(4):899-909.
[10] Sun B,Zhang S,Zhang D,et al.Vasculogenic mimicry is associated with high tumor grade,invasion and metastasis,and short survival in patients with hepatocellular carcinoma[J].Oncol Rep,2006,16(4):693-698.
[11] Shirakawa K,Wakasugi H,Heike Y,et al.Vasculogenic mimicry and pseudo-comedo formation in breast cancer[J].Int J Cancer,2002,99(6):821-828.
[12] Basu GD,Liang WS,Stephan DA,et al.A novel role for cyclooxygenase-2 in regulating vascular channel formation by human breast cancer cells[J].Breast Cancer Res,2006,8(6):R69.
[13] Robertson FM,SimeoneAM,LucciA,etal.Differential regulation of the aggressive phenotype of inflammatory breast cancer cells by prostanoid receptors EP3 and EP4[J].Cancer,2010,116(11):2806-2814.
[14] Wang W,Lin P,Han C,et al.Vasculogenic mimicry contributes to lymph node metastasis of laryngeal squamous cell carcinoma[J].J Exp Clin Cancer Res,2010,Jun 2;29:60.
[15] El Hallani S,Boisselier B,Peglion F,et al.A new alternative mechanism in glioblastoma vascularization:tubular vasculogenic mimicry[J].Brain,2010,133(Pt 4):973-982.
[16] Li M,Gu Y,Zhang Z,et al.Vasculogenic mimicry:a new prognostic sign of gastric adenocarcinoma[J].Pathol Oncol Res,2010,16(2):259-266.
[17] Baeten CI,Hillen F,Pauwels P,et al.Prognostic role of vasculogenic mimicryin colorectal cancer[J].Dis Colon Rectum,2009,52(12):2028-2035.
[18] Zhao H,Gu XM.Study on vasculogenic mimicry in malignant esophageal stromal tumors[J].World J Gastroenterol,2008,14(15):2430-2433.
[19] Sun B,QieS,ZhangS,etal.Roleandmechanism of vasculogenic mimicry in gastrointestinal stromal tumors[J].Hum Pathol,2008,39(3):444-451.
[20] McCawley LJ, Matrisian LM. Matrix metalloproteinases:multifunctional contributors to tumor progression[J].Mol Med Today,2000,6(4):149-156.
[21] Noel A,Gilles C,Bajou K,et al.Emerging roles for proteinases in cancer[J].Invasion & Metastasis,1997,17(5):221-239.
[22] Stetler-Stevenson WG. Matrix metalloproteinases in angiogenesis:a moving target for therapeutic intervention[J].J Clin Invest,1999,103(9):1237-1241.
[23] Seiki M.Membrane-type matrix metalloproteinases[J].Apmis,1999,107(1):137-143.
[24] Hess AR,Seftor EA,Seftor RE,et al.Phosphoinositide 3-kinase regulates membrane Type 1-matrix metalloproteinase(MMP)and MMP-2 activity during melanoma cell vasculogenic mimicry[J].Cancer Res,2003,63(16):4757-4762.
[25] Hendrix MJ,Seftor EA,Kirschmann DA,et al.Remodeling of the microenvironment by aggressive melanoma tumor cells[J].Ann N Y Acad Sci,2003,May,995:151-161.
[26] Seftor RE,Seftor EA,Kirschmann DA,et al.Targeting the tumor microenvironmentwith chemically modified tetracyclines:inhibition of laminin 5 gamma 2 chain promigratory fragments and vasculogenic mimicry[J].Mol Cancer Ther,2002,1:1173-1179.
[27] Seftor RE,SeftorEA,Koshikawa N,etal.Cooperative interactions of laminin 5 gamma2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required formimicry ofembryonic vasculogenesis by aggressive melanoma[J].Cancer Res,2001,61(17):6322-6327.
[28] Sun B,Zhang D,Zhang S,etal.Hypoxia influences vasculogenic mimicry channel formation and tumor invasionrelated protein expression in melanoma[J].Cancer Lett,2007,249(2):188-197.
[29] van der Schaft DW,Seftor RE,Seftor EA,et al.Effects of angiogenesis inhibitors on vascular network formation by human endothelial and melanoma cells[J].J Natl Cancer Inst,2004,96(19):1473-1477.
[30] Fan YZ,Sun W,Zhang WZ,et al.Vasculogenic mimicry in human primary gallbladder carcinoma and clinical significance thereof[J].Zhonghua Yi Xue Za Zhi,2007,87(3):145-149.
[31] Wang CJ,Zhou ZG,Holmqvist A,et al.Survivin Expression Quantified by Image Pro-plus Compared With Visual Assessment[J].Appl Immunohistochem Mol Morphol,2009,17(6):530-535.
[32] Xavier LL,Viola GG,Ferraz AC,et al.A simple and fast densitometric method for the analysis of tyrosine hydroxylase immunoreactivity in the substantia nigra pars compacta and in the ventral tegmental area[J].Brain Res Protoc,2005,16(1-3):58-64.
[33] Situ DR,Hu Y,Zhu ZH,et al.Prognostic relevance of betacatenin expression in T2-3N0M0 esophageal squamous cell carcinoma[J].World J Gastroenterol,2010,16(41):5195-5202.
[34] Zlobec I, Steele R, Terracciano L, et al. Selecting immunohistochemical cut-off scores for novel biomarkers of progression and survival in colorectal cancer[J].J Clin Pathol,2007,60(10):1112-1116.
[35] Karadag N,Kirimlioglu H,Isik B,et al.Expression of matrix metalloproteinases in gallbladder carcinoma and their significance in carcinogenesis[J].Appl Immunohistochem Mol Morphol,2008,16(2):148-152.
[36] Fan YZ,Zhang JT,Yang HC,et al.Expression of MMP-2,TIMP-2 protein and the ratio of MMP-2/TIMP-2 in gallbladder carcinoma and their significance[J].World J Gastroenterol,2002,8(6):1138-1143.
[37] Zhang S,Zhang D,Sun B.Vasculogenic mimicry:current status and future prospects[J].Cancer Lett,2007,254(2):157-164.
[38] Williams TM,Medina F,Badano I,et al.Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo.Role ofCav-1 in cell invasiveness and matrix metalloproteinase(MMP-2/9)secretion[J].J Biol Chem,2004,279(49):51630-51646.
[39] Miyamori H,Takino T,Kobayashi Y,et al.Claudin promotes activation of pro-matrix metalloproteinase-2 mediated by membrane-type matrix metalloproteinases[J].J Biol Chem,2001,276(30):28204-28211.
[40] Nelson AR,Fingleton B,Rothenberg ML,etal.Matrix metalloproteinases:Biologic activity and clinical implications[J].J Clin Oncol,2000,18(5):1135-1149.
[41] Zucker S,Drews M,Conner C,et al.Tissue inhibitor of metalloproteinase-2(TIMP-2)binds to the catalytic domain of the cell surface receptor,membrane type 1 matrix metalloproteinase 1(MT1-MMP)[J].J Biol Chem,1998,273(2):1216-1222.
[42] Lafleur MA,HandsleyMM,Knauper V,et al.Endothelial tubulogenesis within fibrin gels specifically requires the activity of membrane-type-matrix metalloproteinases(MT-MMPs)[J].J Cell Sci,2002,115(17):3427-3438.
[43] Koike T,Vernon RB,Hamner MA,et al.MT1-MMP,but not secreted MMPs,influences the migration of human microvascular endothelial cells in 3-dimensional collagen gels[J].J Cell Biochem,2002,86(4):748-758.
[44] Haas TL,Davis SJ,Madri JA.Three-dimensional type I collagen lattices induce coordinate expression of matrix metalloproteinases MT1-MMP and MMP-2 in microvascular endothelial cells[J].J Biol Chem,1998,273:3604-3610.

