新型马来松香基双官能化合物的合成及其抑菌活性
董淑求, 段文贵, 岑 波, 许雪棠, 韦有杰, 李龙生
(广西大学 化学化工学院,广西 南宁 530004)
松香是我国的一项优势资源,广泛应用于油墨、合成原料、油漆、肥皂等行业中。其改性产品也越来越受到人们的重视[1~4],马来松香是重要的改性产品之一。以其为原料可以制备性能良好的抗菌剂、表面活性剂、防锈剂、功能材料、涂料等[5~10]。噁二唑杂环化合物近年受到人们的广泛关注[11,12],具有杀虫、抗菌等生物活性[13,14]。有关酰胺类化合物的研究报道也较多,其具有较好的除草和抑菌等生物活性[15~17]。
本文以松香为原料合成了马来松香(1); 1经酰氯化、肼解和酰化反应制得马来松香双酰肼(4a~4g);以三氯氧磷为脱水剂,4a~4g关环合成了7个新型的马来松香基双官能化合物(5a~5g, Scheme 1),其结构经1H NMR,13C NMR, IR和MS 表征。初步抑菌活性测试结果表明5a~5g对黄瓜枯萎病菌、苹果轮纹病菌、番茄早疫病菌、花生褐斑病菌和小麦赤霉病菌均有一定杀菌活性(尤其是对苹果轮纹病菌)。

CompabcdefgArC6H5-4-CH3C6H4-4-O2NC6H4-4-MeOC6H4-2-ClC6H4-4-ClC6H4-3-FC6H4-
Scheme1
1 实验部分
1.1 仪器与试剂
X-4型数字显示显微熔点仪(温度未校正);Bruker AV 600 MHz型核磁共振仪(DMSO-d6为溶剂,TMS为内标);Nicolet Nexus 470 FT-IR型红外光谱仪(KBr压片);Agilent 1100 LC/MSD Trap SL型高效液相色谱-质谱联用仪。
1[18]和马来松香酸酰氯(2)[9]按文献方法合成;特级松香,广西梧州松脂股份有限公司;其余所用试剂均为分析纯。
1.2 合成
(1) 马来松香酰肼(3)的合成
在反应瓶中加入80%水合肼10 mL和CH2Cl250 mL,搅拌下于室温缓慢加入2 10 mmol的CH2Cl2(20 mL)溶液,反应5 h(TLC监测)。加入饱和氯化钠溶液50 mL,分液,有机相旋蒸脱溶,残余物用乙醇重结晶得白色固体马来松香酰肼(3),收率70%, m.p.255 ℃~258 ℃;1H NMRδ: 8.81(s, 1H, NH), 5.33(s, 1H, 14-H), 4.83(d,J=9.3 Hz, 2H, NH2), 4.21(s, 2H, NH2), 2.86(d,J=1.5 Hz, 1H, 21-H), 2.81(dd,J=7.8, 2.8 Hz, 1H, 22-H), 2.45(d,J=7.9 Hz, 1H, 12-H), 2.34(dd,J=10.6, 3.1 Hz, 1H, 5-H), 2.11(m, 1H, CH ini-Pr), 1.80(m, 1H, 9-H), 1.73~1.24(m, 10H, CH2), 1.09(s, 2H, CH2), 1.04(s, 3H, 18-H), 0.89(m, 6H, CH3ini-Pr), 0.53(s, 3H, 20-H);13C NMRδ: 177.27, 175.24, 174.55, 146.13, 124.29, 53.33, 53.21, 50.24, 48.96, 45.16, 42.78, 37.63, 37.20, 36.44, 35.05, 34.96, 32.22, 27.16, 20.72, 20.66, 20.16, 16.81, 16.28, 15.53; IRν: 3 345, 3 283, 3 245, 1 777, 1 698, 1 623, 1 515 cm-1; ESI-MSm/z: 429{[M+H]+}。
(2)4a~4g的合成(以4a为例)
在反应瓶中加入35 mmol, THF 25 mL,搅拌下于回流状态缓慢滴加苯甲酰氯(Ⅰa)10 mmol的THF(20 mL)溶液,滴毕,反应2 h(TLC监测)。旋蒸脱溶,残余物经硅胶柱层析[洗脱剂:A=V(乙酸乙酯) ∶V(石油醚)=1 ∶2]纯化得白色固体4a。
用类似方法合成白色固体4b~4g,其表征数据分别见表1和表2。
(3)5a~5g的合成(以5a为例)
在反应瓶中加入45 mmol,乙腈25 mL,搅拌下缓慢滴加三氯氧磷3.07 g(20 mmol)的乙腈(20 mL)溶液,滴毕,回流反应3 h(TLC监测)。倾入冰水中(析出沉淀),静置,过滤,滤饼经硅胶柱层析(洗脱剂:A=1 ∶3)纯化得白色固体5a。
用类似方法合成白色固体5b~5g,其表征数据分别见表1和表2。
1.3 5的抑菌活性测试
将供试药剂溶解在丙酮中,用2×10-4mg·L-1sorporl-144乳化剂稀释成5×10-4mg·L-1药液。取药液1 mL,注入培养皿内,加入PSA培养基9 mL,制成最终浓度为5×10-5mg·L-1含药平板。将培养好的供试菌用打孔器打取直径5 mm菌饼,置于含药平板内,每皿三块呈等边三角形摆放。以不加药剂做空白对照。将各处理于(24±1) ℃培养箱内培养48 h,计量各处理菌丝扩展直径,并与对照相比较,计算相对抑菌率,结果见表3。
2 结果与讨论
2.1 合成
(1)4a~4g的合成
3与Ⅰa~Ⅰg的反应分两步进行。 TLC监测发现滴加Ⅰ时,开始有一个新产物生成,继续滴加该新点消失,生成另一个新点,可能是由于酰肼的活性大于环酰亚胺的活性。另外,实验发现该反应易于进行,不需要添加缚酸剂或催化剂。
(2)5a~5g的合成
4可以用多聚磷酸、三氯氧磷等脱水关环,实验发现,用三氯氧磷效果较好、杂质较少。另外,温度对反应影响较大,于乙腈回流温度反应较快。
2.2 表征
在4的IR谱图中, 3 000 cm-1以上为N-H吸收峰, 1 720 cm-1和1 795 cm-1处为酰亚胺五元环上两个羰基C=O的伸缩振动特征吸收峰, 1 630 cm-1为酰胺羰基振动吸收峰, 1 500 cm-1为苯环骨架伸缩振动吸收峰;1H NMR和13C NMR分析表明, 9.5~11.0的三个吸收峰为N-H, ArH吸收峰位于7.0~8.2,苯环上碳吸收峰在123~150。 由ESI-MS结果可见4的分子离子峰或者准分子离子峰。
在5的IR谱图中, 1 550 cm-1为C=N的吸收峰。1H NMR分析结果表明N-H在11附近只剩一个吸收峰,说明有两个质子氢脱去,发生了环合反应。 ESI-MS结果可见5的分子离子峰或者准分子离子峰。
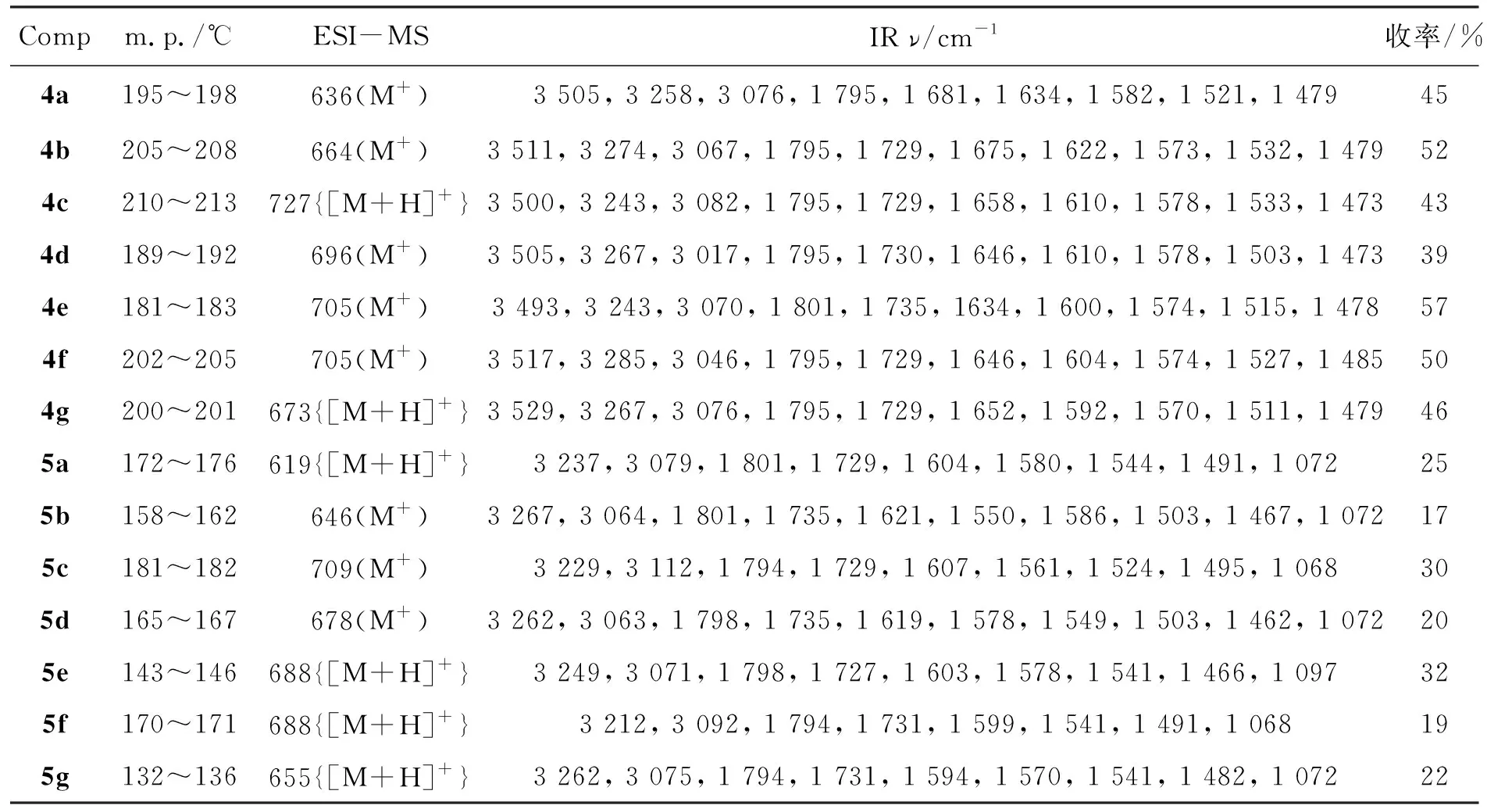
表1 4和5的实验结果,MS和IR数据Table 1 Experimental results, MS and IR data of 4 and 5

表 2 4和5的NMR数据Table 2 NMR data of 4 and 5
续表2

Comp1H NMR δ(J/Hz)13C NMR δ4f11.09(s, 1H, NH), 10.28(s, 1H, NH), 9.62(s, 1H, NH), 7.91(d, J=8.6, 4H, ArH), 7.61(d, J=8.2, 2H, ArH), 7.58(d, J=8.5, 2H, ArH), 5.46(s, 1H, 14-H), 3.10(m, 1H, 21-H), 2.96(s, 1H, 22-H), 2.69(m, 1H, 12-H), 2.32(t, J=15.8, 1H, 5-H), 2.17(m, 1H, CH in i-Pr), 1.81(t, J=11.4, 1H, 9-H), 1.78~1.68(m, 2H, CH2), 1.67~1.30(m, 8H, CH2), 1.16(m, 2H, CH2), 1.14(d, J=7.8, 3H, 18-H), 0.96(m, 6H, CH3 in i-Pr), 0.59(s, 3H, 20-H)177.32, 174.72, 173.93, 164.74, 163.58, 146.26, 137.50, 136.56, 131.53, 129.73, 129.36, 128.82, 128.59, 124.46, 124.22, 53.26, 50.34, 50.24, 49.03, 45.75, 42.88, 37.49, 37.43, 36.33, 34.96, 34.86, 32.31, 27.05, 20.49, 20.02, 18.59, 16.82, 16.58, 15.534g11.11(s, 1H, NH), 10.26(d, 1H, NH), 9.63(d, 1H, NH), 7.77~7.70(m, 2H, ArH), 7.67(dt, J=10.9, 5.8, 2H, ArH), 7.62~7.54(m, 2H, ArH), 7.50(t, J=7.6, 1H, ArH), 7.43(td, J=8.4, 2.2, 1H, ArH), 5.46(s, 1H, 14-H), 3.11(m, 1H, 21-H), 2.96(s, 1H, 22-H), 2.70(m, 1H, 12-H), 2.32(t, J=15.4, 1H, 5-H), 2.17(m, 1H, CH in i-Pr), 1.82(dd, J=13.3, 9.3, 1H, 9-H), 1.76(d, J=12.2, 2H), 1.68~1.47(m, 4H, CH2), 1.47~1.33(m, 4H, CH2), 1.16(dd, J=13.2, 6.0, 2H, CH2), 1.14(d, J=7.1, 3H, 18-H), 0.99~0.92(m, 6H, CH3 in i-Pr), 0.59(s, 3H, 20-H)177.50, 174.76, 173.78, 164.28, 163.31, 146.43, 135.09, 130.98, 130.75, 124.50, 124.04, 123.87, 123.60, 119.70, 119.55, 118.74, 118.60, 114.30, 114.14(C-D), 53.55, 53.27, 50.25, 49.03, 45.76, 42.94, 37.57, 37.43, 36.33, 34.95, 34.87, 32.30, 27.06, 20.49, 20.03, 19.43, 16.82, 16.58, 15.535a10.97(s, 1H, NH), 8.06~7.96(m, 2H, ArH), 7.84(m, 2H, ArH), 7.61(d, J=7.4 Hz, 4H), 7.51(t, J=7.3 Hz, 2H), 5.47(s, 1H, 14-H), 3.10(d, J=8.3 Hz, 1H, 21-H), 2.98(s, 1H, 22-H), 2.77(m, 1H, 12-H), 2.28(d, J=11.7 Hz, 1H, 5-H), 2.23~2.12(m, 1H, CH in i-Pr), 1.89(m, 1H, 9-H), 1.82(m, 2H, CH2), 1.79~1.44(m, 8H, CH2), 1.43(s, 2H, CH2), 1.07~1.00(m, 3H, 18-H), 0.96(m, 6H, CH3 in i-Pr), 0.67(s, 3H, 20-H)174.68, 173.83, 173.43, 164.54, 163.75, 146.60, 132.53, 131.79, 131.01, 129.38, 128.58, 127.77, 127.62, 126.46, 123.66, 53.24, 52.94, 50.51, 49.86, 45.17, 42.84, 37.74, 37.52, 34.93, 34.78, 34.59, 32.29, 27.11, 20.68, 20.49, 20.00, 17.30, 16.56, 15.485b10.87(s, 1H, NH), 7.90(d, J=8.2 Hz, 2H, ArH), 7.77(d, J=8.0 Hz, 1H, ArH), 7.73(d, J=7.6 Hz, 1H, ArH), 7.41(d, J=8.2 Hz, 2H, ArH), 7.31(d, J=7.7 Hz, 2H, ArH), 5.46(s, 1H, 14-H), 3.07(t, J=10.8 Hz, 1H, 21-H), 2.97(s, 1H, 22-H), 2.75(m, 1H, 12-H), 2.38(m, 6H, CH3-Ar), 2.32~2.23(m, 1H, 5-H), 2.17(dd, J=13.3, 6.4 Hz, 1H, CH in i-Pr), 1.91~1.85(m, 1H, 9-H), 1.48(m, 12H, CH2), 1.02(s, 3H, 18-H), 0.96(m, 6H, CH3 in i-Pr), 0.66(s, 3H, 20-H)174.64, 173.89, 173.07, 164.38, 163.83, 146.57, 129.92, 129.32, 129.13, 129.09, 128.21, 127.81, 127.65, 126.42, 124.27, 52.94, 50.69, 50.52 49.84, 45.43, 42.83, 37.74, 37.53, 36.67, 34.94, 34.62, 32.29, 27.11, 21.12, 21.04, 20.68, 20.49, 20.00, 17.31, 16.57, 15.485c11.36(s, 1H, NH), 8.46~8.39(m, 2H, ArH), 8.36(d, J=8.2, 2H, ArH), 8.30~8.24(m, 2H, ArH), 8.08(d, J=8.6, 2H, ArH), 5.47(s, 1H, 14-H), 3.17~3.06(m, 1H, 21-H), 2.99(s, 1H, 22-H), 2.77(m, 1H, 12-H), 2.27(d, J=12.8, 1H, 5-H), 2.18(d, J=6.4, 1H, CH in i-Pr), 1.89(dt, J=13.4, 6.8, 1H, 9-H), 1.86~1.40(m, 12H, CH2), 1.09~0.99(m, 3H, 18-H), 0.96(m, 6H, CH3 in i-Pr), 0.67(s, 3H, 20-H)174.44, 173.58, 173.46, 163.14, 162.57, 149.78, 149.08, 146.66, 136.45, 130.71, 129.36, 129.29, 127.89, 124.57, 123.85, 52.95, 50.64, 50.53, 49.96, 42.87, 37.77, 37.70, 37.48, 34.95, 34.58, 32.29, 27.10, 20.63, 20.50, 20.01, 17.28, 16.53, 15.48
续表2

Comp1H NMR δ(J/Hz)13C NMR δ5d10.79(s, 1H, NH), 7.97~7.92(m, 2H, ArH), 7.85(d, J=8.7, 2H, ArH), 7.17~7.11(m, 2H, ArH), 7.04(d, J=8.6, 2H, ArH), 5.46(s, 1H, 14-H), 3.83(m, 6H, CH3O), 3.07(t, J=10.8, 1H, 21-H), 2.97(s, 1H, 22-H), 2.74(m, 1H, 12-H), 2.35~2.24(m, 1H, 5-H), 2.21~2.08(m, 1H, CH in i-Pr), 1.89~1.84(m, 1H, 9-H), 1.84~1.62(m, 4H, CH2), 1.47(d, J=82.0, 8H, CH2), 1.02(d, J=5.6, 3H, 18-H), 0.96(m, 6H, CH3 in i-Pr), 0.66(s, 3H, 20-H) 174.82, 173.98, 172.87, 163.62, 161.89, 129.81, 129.62, 129.48, 128.27, 124.27, 123.11, 116.07, 114.83, 113.81, 55.51, 55.45, 53.26, 52.97, 50.46, 49.85, 42.83, 37.75, 37.54, 34.94, 34.80, 34.63, 32.30, 27.01, 20.66, 20.50, 20.00, 17.33, 16.58, 15.485e11.01(s, 1H, NH), 7.98(dd, J=7.8, 1.5, 1H, ArH), 7.72(d, J=8.1, 1H, ArH), 7.66(td, J=7.8, 1.7, 1H, ArH), 7.58(td, J=7.6, 1.1, 1H), 7.53(m, 2H, ArH), 7.48(dd, J=7.6, 1.6, 1H, Ar), 7.46~7.41(m, 1H, ArH), 5.40(s, 1H, 14-H), 3.08(m, 1H, 21-H), 2.96(s, 1H, 22-H), 2.75(m, 1H, 12-H), 2.34(s, 1H, 5-H), 2.18~2.09(m, 1H, CH in i-Pr), 1.85(dd, J=13.3, 3.6, 1H, 9-H), 1.43(s, 12H, CH2), 1.02(m, 3H, 18-H), 0.92(m, 6H, CH3 in i-Pr), 0.66(s, 3H, 20-H)174.36, 173.99, 173.31, 162.21, 162.09, 147.08, 133.22, 132.02, 131.84, 131.40, 130.99, 130.48, 129.50, 127.93, 127.22, 124.26, 123.04, 52.87, 50.70, 50.03, 49.60, 45.94, 42.81, 37.77, 37.56, 36.79, 35.15, 34.62, 32.27, 27.14, 20.72, 20.52, 19.96, 17.29, 16.56, 15.475f11.09(s, 1H, NH), 8.03(d, J=8.5, 2H, ArH), 7.89(d, J=8.3, 2H, ArH), 7.72~7.65(m, 2H, ArH), 7.61(d, J=8.1, 2H, ArH), 5.47(s, 1H, 14-H), 3.10(m, 1H, 21-H), 2.98(s, 1H, 22-H), 2.76(m, 1H, 12-H), 2.28(d, J=11.4, 1H, 5-H), 2.21~2.11(m, 1H, CH in i-Pr), 1.88(m, 1H, 9-H), 1.84~1.62(m, 4H, CH2), 1.60~1.41(m, 8H, CH2), 1.02(d, J=6.3, 3H, 18-H), 0.96(m, 6H, CH3 in i-Pr), 0.67(s, 3H, 20-H)174.62, 173.77, 173.65, 163.60, 163.07, 146.64, 137.47, 136.52, 131.16, 129.73, 129.57, 128.80, 128.76, 128.32, 124.29, 52.94, 50.49, 49.99, 49.89, 42.85, 37.75, 37.50, 34.96, 34.84, 34.59, 32.32, 27.12, 20.66, 20.52, 20.03, 17.30, 16.56, 15.495g11.10(d, J=24.1, 1H, NH), 7.87(dd, J=7.8, 1.0, 1H, ArH), 7.83~7.77(m, 1H, Ar),7.74~7.54(m, 4H, Ar), 7.52~7.44(m, 2H, ArH), 5.47(s, 1H, 14-H), 3.11(d, J=8.4, 1H, 21-H), 2.98(s, 1H, 22-H), 2.77(m, 1H, 12-H), 2.27(d, J=12.2, 1H, 5-H), 2.21~2.11(m, 1H, CH in i-Pr), 1.88(dd, J=18.2, 14.4, 1H, 9-H), 1.42~1.83(m, 12H, CH2), 1.05(s, 3H, 18-H), 0.96(m, 6H, CH3 in i-Pr), 0.66(s, 3H, 20-H)174.56, 173.85, 173.10, 162.90, 161.47, 146.61, 133.16, 131.84, 131.01, 125.75, 125.45, 124.35, 124.04, 122.77, 119.55, 118.88, 115.66, 114.58, 113.13, 53.19, 52.89, 50.41, 49.87, 42.86, 37.75, 37.68, 37.48, 34.92, 34.53, 32.30, 27.11, 20.51, 20.03, 19.41, 17.28, 16.56, 15.48
2.3 抑菌活性
4和5在用药量为50 μg·mL-1时对黄瓜枯萎病菌、苹果轮纹病菌、番茄早疫病菌、花生褐斑病菌和小麦赤霉病菌具有不同程度的抑制作用,结果列于表3。从表3可知,4和5对测试病菌均有一定的抑制作用,尤其是对苹果轮纹病菌抑制效果较好。

表 3 4和5的抑菌活性*Table 3 Fungicidal activity of 4 and 5
*A:Fusariumoxysporumf.sp.cucumerinum, B:Physalosporapiricola, C:Alternariasolani, D: Cercospora rachidicola,E: Fusarium graminearum
[1] 朱岩, 任莹. 改性松香水分散液的制备及其粘合性能研究[J].精细化工,2010,27(4) :405-408.
[2] Duan W G, Shen C M, Fang H X,etal. Synthesis of dehydroabietic acid-modified chitosan and its drug release behavior[J].Carbohydrate Research,2009,344(1):9-13.
[3] 李双月. 几种抑木腐菌活性松香衍生物的合成与表征[D].黑龙江:东北林业大学硕士学位论文,2009.
[4] 田小艳, 杨达, 潘英明, 等. 含脱氢松香酸骨架的吖啶染料的合成及其理化性质研究[J].有机化学,2011,31(3):346-355.
[5] Hess S C, Farah M I, Eguchib S Y,etal. Synthetic studies with pinus elliottiis′rosin derivatives oxidation of maleopimaric anhydride methyl ester and trimethyl
fumaropimarate[J].J Braz Chem Soc,2000,11(1):59-63.
[6] Panda R, Panda H. Mold growth inhibitor from maleopimaric acid[J].Chemistry and Industry of Forest Products,1995,15(1):40-42.
[7] 崔锦峰, 康博, 杨保平, 等. 马来松香-聚氧乙烯醚双酯羧酸钠表面活性剂[J].兰州理工大学学报,2009,35(6):64-67.
[8] 王慧媛.新型防锈剂马来海松酸醇酰胺的制备及其应用研究[D]. 南京:南京理工大学硕士学位论文,2009.
[9] Jong S L, Sung H. Synthesis of acrylic rosin derivatives and application as negative photoresist[J].European Polymer Journal,2002,38:387-392.
[10] Atta A M, El-Saeed S M, Farag R K. New vinyl ester resins based on rosin for coating applications[J].Reactive and Functional Polymers,2006,66:1596-1608.
[11] Lv H S, Zhao B X, Li J K,etal. The synthesis, characterization and optical properties of novel, substituted, pyrazoly 1,3,4-oxadiazole derivatives[J].Dyes and pigments,2010,86:25-31.
[12] Gilani S, Khan S, Siddiqui N. Synthesis and pharmacological evaluation of condensed heterocyclic 6-substituted 1,2,4-triazolo-[3,4-b]-1,3,4-oxadiazole derivatives of isoniazid[J].Bioorganic and Medicinal Chemistry Letters,2010,20:4762-4765.
[13] 马献力, 陈丽, 段文贵, 等. 脂肪族二酸二烷基双去氢枞基双噁二唑的合成及除草活性[J].有机化学,2011,31(7):1069-1075.
[14] Chandrakantha B, Shetty P, Nambiyar V,etal. Synthesis, characterization and biological activity of some new 1,3,4-oxadiazole bearing 2-flouro-4-methoxy phenyl moiety[J].European Journal of Medical Chemistry,2010,45: 1206-1210.
[15] 李宇彬,段文贵,陈秋菊,等. 丙烯海松酸基双酰胺类化合物的合成及生物活性研究[J].化学试剂,2012,34(1):9-15,54.
[16] 邹荣霞, 岑波, 段文贵, 等. 蒎酸基双苯酰胺类化合物的合成及其除草活性[J].广西大学学报,2011,36(3):463-469.
[17] 李欢欢, 王振军, 王力钟, 等.α-取代苯乙酰胺类化合物的合成及生物活性[J].高等学校化学学报,2011,32(1):79-83.

