Neuroprotective effects of Asiaticoside
Feng-yan Qi, Le Yang, Zhen Tian, Ming-gao Zhao, Shui-bing Liu, Jia-ze An
1 Department of Pharmacology, School of Pharmacy, Fourth Military Medical University of Chinese PLA, Xi’an, Shaanxi Province, China
2 Department of Hepatobiliary Surgery, Xijing Hospital, Fourth Military Medical University of Chinese PLA, Xi’an, Shaanxi Province, China
Neuroprotective effects of Asiaticoside
Feng-yan Qi1, Le Yang1, Zhen Tian1, Ming-gao Zhao1, Shui-bing Liu1, Jia-ze An2
1 Department of Pharmacology, School of Pharmacy, Fourth Military Medical University of Chinese PLA, Xi’an, Shaanxi Province, China
2 Department of Hepatobiliary Surgery, Xijing Hospital, Fourth Military Medical University of Chinese PLA, Xi’an, Shaanxi Province, China
Fengyan Qi and Le Yang contributed equally to this work.
Jiaze An, Department of Hepatobiliary
Surgery, Xijing Hospital, Fourth
Military Medical University of Chinese
PLA, Xi’an 710032, Shaanxi Province,
China, anchen@fmmu.edu.cn.
Shuibing Liu, Department of
Pharmacology, School of Pharmacy,
Fourth Military Medical University of
Chinese PLA, Xi’an 710032, Shaanxi
Province, China, liushb1974@aliyun.com.
In the central nervous system, Asiaticoside has been shown to attenuate in vitro neuronal damage caused by exposure to β-amyloid. In vivo studies demonstrated that Asiaticoside could attenuate neurobehavioral, neurochemical and histological changes in transient focal middle cerebral artery occlusion animals. In addition, Asiaticoside showed anxiolytic effects in acute and chronic stress animals. However, its potential neuroprotective properties in glutamate-induced excitotoxicity have not been fully studied. We investigated the neuroprotective effects of Asiaticoside in primary cultured mouse cortical neurons exposed to glutamate-induced excitotoxicity invoked by N-methyl-D-aspartate. Pretreatment with Asiaticoside decreased neuronal cell loss in a concentration-dependent manner and restored changes in expression of apoptotic-related proteins Bcl-2 and Bax. Asiaticoside pretreatment also attenuated the upregulation of NR2B expression, a subunit of N-methyl-D-aspartate receptors, but did not affect expression of NR2A subunits. Additionally, in cultured neurons, Asiaticoside significantly inhibited Ca2+influx induced by N-methyl-D-aspartate. These experimental fi ndings provide preliminary evidence that during excitotoxicity induced by N-methyl-D-aspartate exposure in cultured cortical neurons, the neuroprotective effects of Asiaticoside are mediated through inhibition of calcium in fl ux. Aside from its anti-oxidant activity, down-regulation of NR2B-containing N-methyl-D-aspartate receptors may be one of the underlying mechanisms in Asiaticoside neuroprotection.
nerve regeneration; brain injury; Asiaticoside; apoptosis; N-methyl-D-aspartate; glutamate; neurotransmitter; neurotoxicity; calcium imaging; Bcl-2; Bax; NSFC grant; neural regeneration
Funding: This study was supported by the National Natural Science Foundation of China, No. 31271126, 81372606.
Qi FY, Yang L, Tian Z, Zhao MG, Liu SB, An JZ. Neuroprotective effects of Asiaticoside. Neural Regen Res. 2014;9(13):1275-1282.
Introduction
Glutamate plays a critical role in synaptic transmission and plasticity of the central nervous system. Excessive glutamate, however, is the main cause of excitotoxicity and leads to neuronal damage as seen in stroke, spinal cord injury, traumatic brain injury, and neurodegenerative diseases (Mattson, 2003). The N-methyl-D-aspartate (NMDA) subtype of glutamate receptor is a calcium permeable ion channel which mediates glutamate excitotoxicity (Hardingham and Bading, 2003). The receptor is composed of different subunits that link to different intracellular cascades, which subsequently determine synaptic plasticity and cytotoxic potential. NR2B-containing NMDA receptors are reported to selectively trigger excitotoxicity (Krapivinsky et al., 2003; Liu et al., 2007) and our previous studies are consistent with these fi ndings (Liu et al., 2012; Zhang et al., 2013). Ca2+infl ux from NMDA receptors activates reactive oxygen species which are the cause of excitotoxic neuronal death (Carriedo et al., 2000; Stanciu et al., 2000; Zhang et al., 2013).
Asiaticoside (Figure 1) is a triterpenoid product derived from the plant Centella asiatica. It has been shown to have wound healing effects (Lee et al., 2012; Somboonwong et al., 2012), anti-in fl ammatory effects (Wan et al., 2013), and promotes liver protective activity (Zhang et al., 2010) in vivo. Previous studies have also shown Asiaticoside to ameliorate cognitive impairment and neurotoxicity in animal models (De fi llipo et al., 2012; Xu et al., 2012). However, the neuroprotective activity of Asiaticoside and the underlying pathways that inhibit neuronal apoptosis caused by glutamate are not clear. In this study, we used an NMDA-induced injury model to investigate the protective effects of Asiaticoside in cultured mouse cortical neurons with an attempt to uncover the underlying mechanisms involved.
Materials and Methods
Primary culture of neurons
All animal protocols used were approved by the Animal Care and Use Committee of the Fourth Military Medical University of Chinese PLA, Xi’an, Shaanxi Province, China. Primary cortical neuronal cultures were harvested from brains of E15-E16 C57BL/6 mouse embryos (the Animal Center of the Fourth Military Medical University of Chinese PLA). Briefly, disassociated cortex tissues were incubated with 0.125% trypsin (Sigma, St Louis, MO, USA) in Ca2+and Mg2+-free Hank’s balanced salt solution for 10 minutes at 37°C. Trypsin was inactivated by washing with DMEM (Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA). Cortices were then furtherdissociated by trituration. The single cell suspension was cultured on poly-D-lysine coated plates in Neurobasal media supplemented with 2% B27 (Invitrogen), 0.5 mmol/L glutamine (Sigma), 100 U/mL penicillin (Sigma), and 100 U/mL streptomycin (Sigma). Maturation of cortical neurons took 7 days and half of the medium was changed every 2 days. Anti-MAP2 antibody (Sigma) staining revealed that more than 95% cells were neurons obtained by this culture procedure (Yang et al., 2012). All plates were pre-coated with 50 μg/mL poly-D-lysine (Sigma) in water.

Figure 2 Effects of Asiaticoside (AS) on cell viability in cultured cortical neurons.

Figure 1 Chemical structure of Asiaticoside.
Treatment of the neurons
Asiaticoside was purchased from the Shanghai PureOne Biotechnology (Shanghai, China). Its purity was 98%, detected by high-performance liquid chromatography. Asiaticoside was dissolved in ethanol with the fi nal ethanol concentration less than 1%.
Cortical neurons cultured for 7 days in vitro were divided into three groups: control, NMDA treatment (200 μmol/L NMDA and 10 μmol/L glycine, NMDA receptor co-activator) and Asiaticoside pretreatment (Asiaticoside (less than 1%) pretreatment for 24 hours, then NMDA (200 μmol/L) and glycine (10 μmol/L) treatment for another 30 minutes). The NR2B receptor speci fi c antagonist, RO-25-6981 (Sigma), was used as a positive control. The cells were then returned to the original culture medium for further 24 hours.
Cell viability analysis
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma) assay was used to detect cell viability, as previously described (Yang et al., 2010). Neurons used for the experiments were cultured in vitro for 7 days (DIV 7). Cells were incubated with MTT (0.5 mg/mL) at 37°C for 4 hours on day 9. Cells were then washed and incubated in 150 μL dimethyl sulfoxide. The absorbance value was read on a Universal Microplate Reader (Elx800, Bio-TEK instruments Inc., Winooski, VT, USA) at 570 nm (taking 630 nm as a reference). Cell viability was expressed as a percentage of control value (%). All data were collected from three independent experiments.
Hoechst/propidium iodide double staining
Cortical neurons were cultured in 24-well plates at a density of 3 × 105cells per well. Propidium iodide (Sigma) and Hoechst 33258 (Sigma) double fluorescent staining was used to determine either cell death or apoptosis as described previously (Liu et al., 2012). Neurons were incubated with propidium iodide (10 μg/mL) and Hoechst 33258 (10 μg/mL) for 15 minutes, and then fi xed in 4% formaldehyde for 20 minutes. Imaging was detected under a fluorescence microscope (Olympus BX61, Tokyo, Japan) at 340 and 620 nm, respectively. Six visual fields were selected randomly from each well and data were collected from three independent experiments. The percentage of propidium iodide positive neurons compared with total Hoechst stained neuronswas used to indicate cell death or apoptosis.
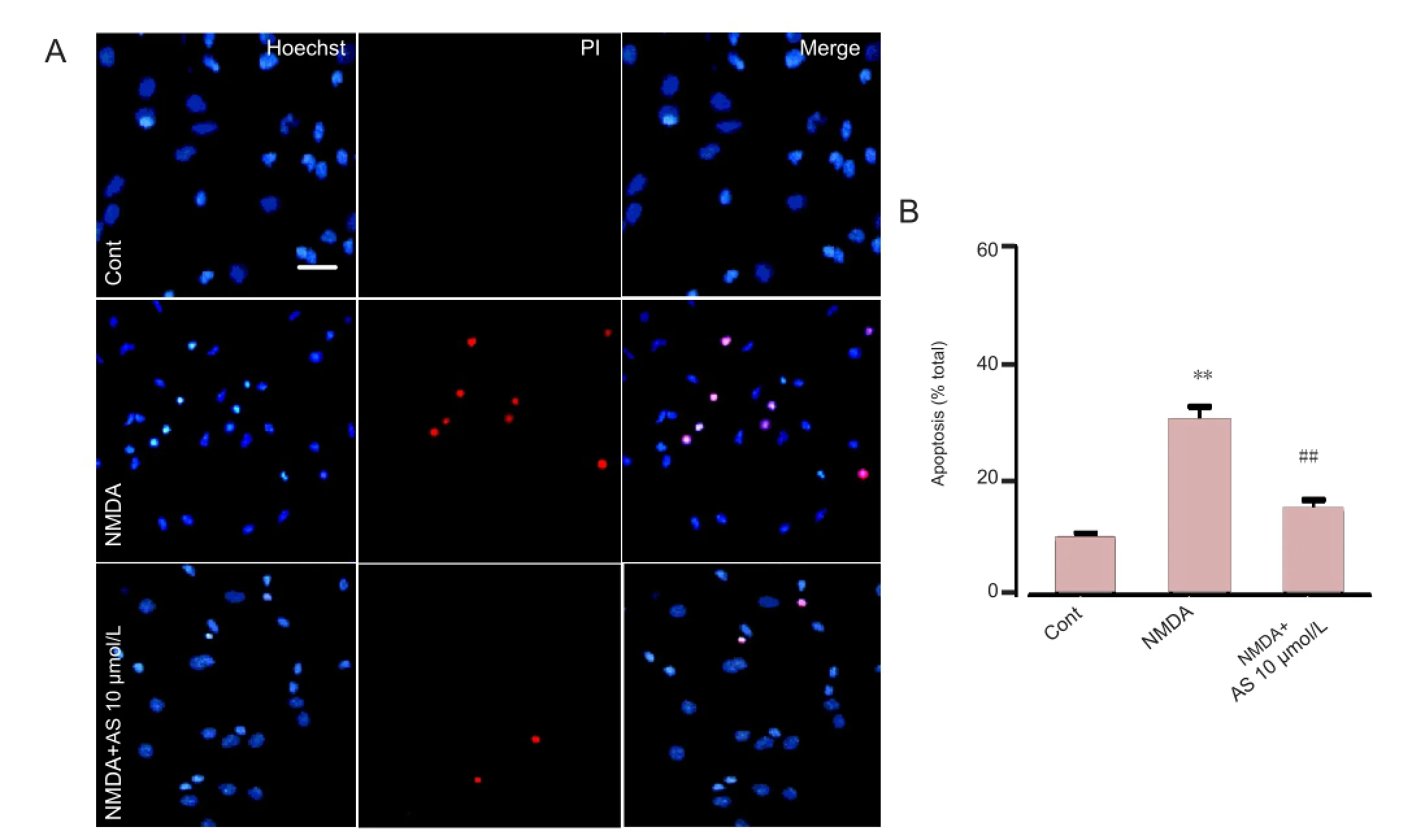
Figure 3 Neuroprotective effect of Asiaticoside (10 μmol/L) in cultured cortical neurons treated with N-methyl-D-aspartate (NMDA) (Hoechst 33258 and propidium iodide double staining).
Western blot analysis
For western blot analysis, neurons were cultured in 6-well plates at a density of 2 × 106cells/well. After pretreatment with Asiaticoside for 24 hours, cells were treated with NMDA (200 μmol/L) and glycine (10 μmol/L) for another 30 minutes. The next day, cells were rinsed twice with PBS and lysed using M-PER Protein Extraction Buffer containing 1 × protease inhibitor cocktail. Equal amounts of protein (50 μg) were separated on 10% polyacrylamide gel and then transferred onto an Immun-Blot polyvinylidene difluoride membrane. To block the membrane for 1 hour, 5% non-fat milk in Tris-Phosphate buffer containing 0.05% Tween 20 was used. Membranes were incubated with the appropriate antibody overnight at 4°C; either mouse anti-NR2A (1:200), anti-NR2B (1:1,000), anti-Bax (1:1,000), or anti-Bcl-2 (1:1,000), with β-actin (1:10,000) as a loading control. Bands were visualized using an ECL system (Bio-Rad, Hercules, CA, USA) after incubation with the appropriate secondary antibody (goat anti-mouse immunoglobulin; Boster, Wuhan, Hubei Province, China). Anti-NR2A was purchased from Millipore (Billerica, MA, USA). Anti-NR2B, anti-Bax, and anti-Bcl-2 antibodies were purchased from Chemicon (Temecula, CA, USA). β-Actin antibody was purchased from Sigma. Levels of protein were expressed as the percentage of control (β-actin).
Calcium imaging
Calcium imaging was performed as previously described (Yang et al., 2013). Neurons were cultured in 3.5 mm plates made especially for laser scanning microscope at a density of 3 × 105per plate. Neurons were washed twice using Mg2+-free extracellular solution. The extracellular solution contained NaCl (140 mmol/L), KCl (3 mmol/L), CaCl2(2 mmol/L), HEPES (10 mmol/L), and glucose (10 mmol/L). To the extracellular solution, 2.5 μmol/L fluo-3/AM was added and neurons were incubated for 30 minutes at 37°C to load the dye. The neurons were washed and incubated in the culture medium for another 30 minutes. Calcium fl uorescence curves were detected by a confocal laser scanning microscope (Olympus). Basal calcium levels were scanned for approximately 1 minute before NMDA (200 μmol/L) was added to the cultures. Calcium fl uorescence curves were compared between control neurons and Asiaticoside pretreated neurons. The results show the fl uorescence intensitywas expressed as a change relative to basal levels.

Figure 4 Effects of Asiaticoside (AS; 10 μmol/L) on the expression levels of Bcl-2 and Bax in cultured cortical neurons.
Statistical analysis
Data were presented as mean ± SEM. Data analysis was performed using SPSS 10.0 software (SPSS, Chicago, IL, USA). One-way analysis of variance was used for comparison among multiple groups followed by Tukey’s multiple comparison tests as a post hoc comparison. In all cases, P < 0.05 was considered statistically signi fi cant.
Results
Effects of Asiaticoside on cell viability in cultured cortical neurons
Exogenous NMDA was used to induce excitotoxicity in cultured mouse cortical neurons and cell viability was subsequently determined using an MTT assay. Neurons were cultured for 7 days in vitro and then pretreated with Asiaticoside for 24 hours. On day 8, NMDA (200 μmol/L) and glycine (10 μmol/L) were added to the medium containing Asiaticoside and the cells were incubated for 30 minutes. Cells were then washed and cultured in the original culture medium for further 24 hours. As shown inFigure 2A, high concentrations of NMDA led to decreased cell viability measured by MTT assay (63%, P < 0.01 compared with control group). However, pretreatment with Asiaticoside signi fi cantly increased the cell viability of neurons in a dose-dependent manner. Neuroprotection was most effective at 10 μmol/L Asiaticoside (84%, P < 0.01 compared with NMDA treatment group), and 100 μmol/L Asiaticoside did not improve the viability of the neurons. Therefore, 10 μmol/L Asiaticoside was used in subsequent experiments. Asiaticoside (10 μmol/L) itself did affect the cell viability but Ro 25-6981 (0.3 μmol/L, added with the NMDA), an NR2B-selective antagonist, completely blocked the injury induced by NMDA (98%, P < 0.01 compared with NMDA treatment group;Figure 2B).
Effects of Asiaticoside on NMDA-induced excitotoxicity in cultured cortical neurons

Figure 5 Effects of Asiaticoside (AS; 10 μmol/L) on the protein expression levels of N-methyl-D-aspartate (NMDA) receptor subunits, NR2A and NR2B.
Hoechst and propidium iodide double staining were used to determine the neuroprotective effects of Asiaticoside. Hoechst and propidium iodide are dyes that stain nucleic acids. Propidium iodide, however, cannot pass through living cells, and therefore only stains apoptotic and necrotic cells. In normal culture conditions, only 9.7 ± 1.5% of cells were positive for propidium iodide staining. NMDA treatment for 30 minutes induced signi fi cant cell death or apoptosis, as 29.58 ± 2.41% of the cells were propidium iodide positive (P< 0.01 compared with control group;Figure 3). Asiaticoside pretreatment (10 μmol/L) prevented cell damage caused by NMDA treatment as fewer cells were positive for propidium iodide staining (14.61 ± 2.14%; P < 0.01 compared with NMDA treatment group;Figure 3). These results demonstrate that Asiaticoside is protective against injury induced by NMDA.
Anti-apoptotic effects of Asiaticoside
Western blot analysis was used to detect the levels of apoptosis-related proteins, anti-apoptotic Bcl-2 and pro-apoptotic Bax. The ratio of Bax/Bcl-2 re fl ects the susceptibility of cells to harmful response (Liu et al., 2012). Treatment with 200 μmol/L NMDA markedly decreased levels of Bcl-2 (32% of control; P < 0.01 compared with control group;Figure 4A, B) and increased the levels of Bax (211% of control; P < 0.01, compared with control group;Figure 4A, C) and the ratio of Bax/Bcl-2 (652% of control; P < 0.01 compared with control group;Figure 4A, D). However, pretreatment of Asiaticoside (10 μmol/L) prior to administration of NMDA signi fi cantly changed the levels of Bcl-2 (76% of control; P < 0.01 compared with NMDA treatment group;Figure 4A, B) and Bax (137% of control; P < 0.05 compared with NMDA treatment;Figure 4A, C), and the ratio of Bax/Bcl-2 (180% of control; P< 0.01 compared with NMDA treatment group;Figure 4A, D).
Effects of Asiaticoside on the levels of NMDA receptors
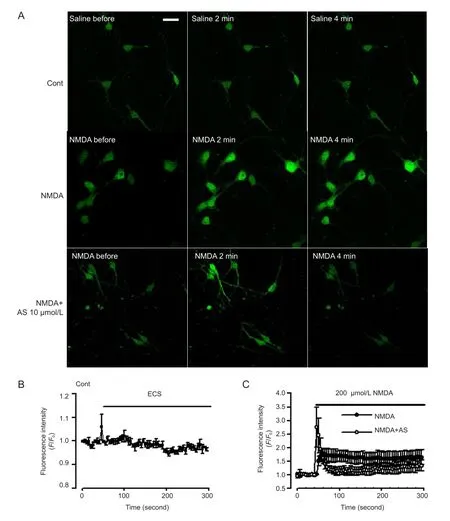
Figure 6 Effect of Asiaticoside (AS) on Ca2+levels mediated by N-methyl-D-aspartate (NMDA) receptors.
NMDA receptor subunit composition (NR2A or NR2B) and NMDA receptor localization (intrasynapse or extrasynapse) determine the intracellular cascades and functions of the receptor in neurons (Liu et al., 2007). NR2B-containing NMDA receptors, particularly those located extrasynaptically, contribute to neuronal damage (Liu et al., 2007; Shenet al., 2008; Stanika et al., 2009). Western blot analysis indicated that NMDA treatment caused an increase of NR2B expression (214% of control; P < 0.01 compared with control group;Figure 5A, C) but expression of NR2A subtype did not change (Figure 5A, B). Asiaticoside (10 μmol/L) pretreatment notably reduced the up-regulation of NR2B levels by NMDA exposure (95% of control; P < 0.01 compared with NMDA treatment group;Figure 5A, C). Therefore, down-regulation of NR2B levels may be the major mechanism underlying the neuroprotective effects of Asiaticoside.
Asiaticoside decreases NMDA-induced Ca2+overload
Following excessive activation of NMDA receptors, Ca2+influx from NMDA receptors and sustained intracellular Ca2+overload contribute to neuronal injury (Vander Jagt et al., 2009). Cytoplasmic Ca2+concentration was marked by fluorescence intensity under the laser confocal microscopy (Matsumoto et al., 2004). Under control conditions, a stable recording of Ca2+levels was observed in neurons (Figure 6A, B). NMDA (200 μmol/L) evoked a rapid increase in cytoplasmic Ca2+which stayed at a high concentration for the next 4 minutes (Figure 6C). In neurons pretreated with Asiaticoside (10 μmol/L) Ca2+levels were markedly attenuated in response to NMDA exposure (Figure 6A, C).
Discussion
Glutamate plays a key role in neural transmission, development and synaptic plasticity. Glutamate binds to ionotropic receptors including NMDA, kainate, and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Watkins and Jane, 2006). In pathological conditions such as stroke, traumatic damage and inflammation, excessive accumulation of cytoplasmic Ca2+influx through NMDA receptors triggers excitotoxicity and neuronal damage (Jayanarayanan et al., 2013). In this study, elevation of Ca2+stimulated by NMDA was inhibited by Asiaticoside, suggesting inhibition of calcium influx through NMDA receptors contributes, at least partly, to neuroprotection by Asiaticoside.
Asiaticoside exhibits antipyretic, anti-inflammatory and antioxidant activity (Tabassum et al., 2013; Wan et al., 2013). In the central nervous system, Asiaticoside could attenuate neurobehavioral, neurochemical and histological changes in transient focal middle cerebral artery occlusion rats (Tabassum et al., 2013). Another study demonstrated anxiolytic effects of Asiaticoside in both acutely and chronically stressed animals (Wanasuntronwong et al., 2012). In this study, we found that Asiaticoside pretreatment dose-dependently attenuated reduction in cell viability by exposure to high concentration of NMDA. Hoechst and propidium iodide double staining further verified the neuroprotective effects of Asiaticoside, although the staining could not discriminate between apoptosis and necrosis. Western blot analysis suggested Asiaticoside has anti-apoptotic activities as it affected the expression of apoptotic proteins, Bcl-2 and Bax. Further experiments showed that Asiaticoside attenuated expression of NR2B-containing NMDA receptors, but not NR2A-containing NMDA receptors. This is consistent with previous fi ndings that suggest NR2B is responsible for mediating excitotoxicity (Liu et al., 2007). NMDA receptors that have more NR2B than NR2A subunits (a low NR2A:NR2B ratio) open for a longer length of time. Thus, neurons with a low NR2A:NR2B ratio are more sensitive to glutamate (Yamakura and Shimoji, 1999). Pretreatment with Asiaticoside decreased the level of NR2B receptors. It is possible that lowered NR2B expression resulted in a shorter opening time of NMDA receptors and subsequently less calcium in fl ux into neurons, and consequently a neuroprotective effect.
In conclusion, our results provide a new insight into the neuroprotective effects of Asiaticoside. Aside from its anti-oxidant activity, down-regulation of NR2B-containing NMDA receptors may be one of the underlying mechanisms in Asiaticoside neuroprotection. Our results suggest that Asiaticoside protects neurons from excitotoxicity induced by NMDA exposure by inhibiting cell apoptosis and calcium overload.
Acknowledgments:We would like to thank Dr. Wu YM from the Fourth Military Medical University of Chinese PLA for her helpful comments for the discussion.
Author contributions:An JZ and Zhao MG designed the study. Qi FY, Yang L and Tian Z performed the experiments. Liu SB analyzed data. An JZ wrote the paper. All authors approved the final version of the manuscript.
Con fl icts of interest:None declared.
Carriedo SG, Sensi SL, Yin HZ, Weiss JH (2000) AMPA exposures induce mitochondrial Ca(2+) overload and ROS generation in spinal motor neurons in vitro. J Neurosci 20:240-250.
De fi llipo PP, Raposo AH, Fedoce AG, Ferreira AS, Polonini HC, Gattaz WF, Raposo NR (2012) Inhibition of cPLA2 and sPLA2 activities in primary cultures of rat cortical neurons by Centella asiatica water extract. Nat Prod Commun 7:841-843.
Hardingham GE, Bading H (2003) The Yin and Yang of NMDA receptor signalling. Trends Neurosci 26:81-89.
Jayanarayanan S, Smijin S, Peeyush KT, Anju TR, Paulose CS (2013) NMDA and AMPA receptor mediated excitotoxicity in cerebral cortex of streptozotocin induced diabetic rat: ameliorating effects of curcumin. Chem Biol Interact 201:39-48.
Krapivinsky G, Krapivinsky L, Manasian Y, Ivanov A, Tyzio R, Pellegrino C, Ben-Ari Y, Clapham DE, Medina I (2003) The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron 40:775-784.
Lee JH, Kim HL, Lee MH, You KE, Kwon BJ, Seo HJ, Park JC (2012) Asiaticoside enhances normal human skin cell migration, attachment and growth in vitro wound healing model. Phytomedicine 19:1223-1227.
Liu SB, Zhang N, Guo YY, Zhao R, Shi TY, Feng SF, Wang SQ, Yang Q, Li XQ, Wu YM, Ma L, Hou Y, Xiong LZ, Zhang W, Zhao MG (2012) G-protein-coupled receptor 30 mediates rapid neuroprotective effects of estrogen via depression of NR2B-containing NMDA receptors. J Neurosci 32:4887-4900.
Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT (2007) NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci 27:2846-2857.
Matsumoto Y, Yamamoto S, Suzuki Y, Tsuboi T, Terakawa S, Ohashi N, Umemura K (2004) Na+/H+ exchanger inhibitor, SM-20220, is protective against excitotoxicity in cultured cortical neurons. Stroke 35:185-190.
Mattson MP (2003) Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Med 3:65-94.
Shen H, Yuan Y, Ding F, Liu J, Gu X (2008) The protective effects of Achyranthes bidentata polypeptides against NMDA-induced cell apoptosis in cultured hippocampal neurons through differential modulation of NR2A- and NR2B-containing NMDA receptors. Brain Res Bull 77:274-281.
Somboonwong J, Kankaisre M, Tantisira B, Tantisira MH (2012) Wound healing activities of different extracts of Centella asiatica in incision and burn wound models: an experimental animal study. BMC Complement Altern Med 12:103.
Stanciu M, Wang Y, Kentor R, Burke N, Watkins S, Kress G, Reynolds I, Klann E, Angiolieri MR, Johnson JW, DeFranco DB (2000) Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J Biol Chem 275:12200-12206.
Stanika RI, Pivovarova NB, Brantner CA, Watts CA, Winters CA, Andrews SB (2009) Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity. Proc Natl Acad Sci U S A 106:9854-9859.
Tabassum R, Vaibhav K, Shrivastava P, Khan A, Ejaz Ahmed M, Javed H, Islam F, Ahmad S, Saeed Siddiqui M, Safhi MM (2013) Centella asiatica attenuates the neurobehavioral, neurochemical and histological changes in transient focal middle cerebral artery occlusion rats. Neurol Sci 34:925-933.
Vander Jagt TA, Connor JA, Weiss JH, Shuttleworth CW (2009) Intracellular Zn2+ increases contribute to the progression of excitotoxic Ca2+ increases in apical dendrites of CA1 pyramidal neurons. Neuroscience 159:104-114.
Wan J, Gong X, Jiang R, Zhang Z, Zhang L (2013) Antipyretic and anti-in fl ammatory effects of asiaticoside in lipopolysaccharide-treated rat through up-regulation of heme oxygenase-1. Phytother Res 27:1136-1142.
Wanasuntronwong A, Tantisira MH, Tantisira B, Watanabe H (2012) Anxiolytic effects of standardized extract of Centella asiatica (ECa 233) after chronic immobilization stress in mice. J Ethnopharmacol 143:579-585.
Watkins JC, Jane DE (2006) The glutamate story. Br J Pharmacol 147 Suppl 1:S100-108.
Xu CL, Wang QZ, Sun LM, Li XM, Deng JM, Li LF, Zhang J, Xu R, Ma SP (2012) Asiaticoside: attenuation of neurotoxicity induced by MPTP in a rat model of Parkinsonism via maintaining redox balance and up-regulating the ratio of Bcl-2/Bax. Pharmacol Biochem Behav 100:413-418.
Yamakura T, Shimoji K (1999) Subunit- and site-speci fi c pharmacology of the NMDA receptor channel. Prog Neurobiol 59:279-298.
Yang L, Li XB, Yang Q, Zhang K, Zhang N, Guo YY, Feng B, Zhao MG, Wu YM (2013) The neuroprotective effect of praeruptorin C against NMDA-induced apoptosis through down-regulating of GluN2B-containing NMDA receptors. Toxicol In Vitro 27:908-914.
Yang Q, Feng B, Zhang K, Guo YY, Liu SB, Wu YM, Li XQ, Zhao MG (2012) Excessive astrocyte-derived neurotrophin-3 contributes to the abnormal neuronal dendritic development in a mouse model of fragile X syndrome. PLoS Genet 8:e1003172.
Yang Q, Yang ZF, Liu SB, Zhang XN, Hou Y, Li XQ, Wu YM, Wen AD, Zhao MG (2010) Neuroprotective effects of hydroxysaf fl or yellow A against excitotoxic neuronal death partially through down-regulation of NR2B-containing NMDA receptors. Neurochem Res 35:1353-1360.
Zhang K, Li YJ, Yang Q, Gerile O, Yang L, Li XB, Guo YY, Zhang N, Feng B, Liu SB, Zhao MG (2013) Neuroprotective effects of oxymatrine against excitotoxicity partially through down-regulation of NR2B-containing NMDA receptors. Phytomedicine 20:343-350.
Zhang L, Li HZ, Gong X, Luo FL, Wang B, Hu N, Wang CD, Zhang Z, Wan JY (2010) Protective effects of Asiaticoside on acute liver injury induced by lipopolysaccharide/D-galactosamine in mice. Phytomedicine 17:811-819.
Copyedited by Paul P, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.137574
http://www.nrronline.org/
Accepted: 2014-05-17
- 中国神经再生研究(英文版)的其它文章
- Stem cell therapy for central nerve system injuries: glial cells hold the key
- Beyond taxol: microtubule-based strategies for promoting nerve regeneration after injury
- Neuroprotective effect of the traditional Chinese herbal formula Tongxinluo: a PET imaging study in rats
- Treating Alzheimer’s disease with Yizhijiannao granules by regulating expression of multiple proteins in temporal lobe
- Autophagy activation aggravates neuronal injury in the hippocampus of vascular dementia rats
- Role of Notch-1 signaling pathway in PC12 cell apoptosis induced by amyloid beta-peptide (25-35)

