新型螺[氧化吲哚-3,4'-噁唑啉]-5'-磷酸酯类化合物的合成*
陈永正,赵建强,白玫,,袁伟成
(1.遵义医学院药学院,贵州遵义 563000; 2.中国科学院成都有机化学研究所,四川成都 610041;3.中国科学院大学,北京 100049)
新型螺[氧化吲哚-3,4'-噁唑啉]-5'-磷酸酯类化合物的合成*
陈永正1,赵建强2,3,白玫1,2,3,袁伟成2
(1.遵义医学院药学院,贵州遵义 563000; 2.中国科学院成都有机化学研究所,四川成都 610041;3.中国科学院大学,北京 100049)
以10 mol%三乙烯二胺为催化剂,3-异硫氰酸酯氧化吲哚与α-羰基磷酸酯经aldol/环化反应,合成了一系列新型的螺[氧化吲哚-3,4'-噁唑啉]-5'-磷酸酯类化合物,收率86%~92%,dr>99∶1,其结构经1H NMR 和13C NMR表征。
3-异硫氰酸酯氧化吲哚;α-羰基磷酸酯;aldol/环化;合成
螺环氧化吲哚结构单元广泛存在于许多天然分子中,已发现的含3,3'-螺环结构的氧化吲哚类化合物大多具有较高的生物活性,如抗病毒、抗细胞毒素、抗氧化等[1-2]。如Spirobrassinin及其类似物Methoxyspirobrassinin(Chart 1)是一类植物抗毒素氧化吲哚,具有杀菌、抗肿瘤活性[3]。而含磷化合物由于其独特的医药价值,受到化学家们越来越多的关注,如Estramustine phosphate(Chart 1)对前列腺癌具有明显的治疗作用[4]。鉴于含磷化合物与螺环氧化吲哚类化合物广泛的生理与药物价值,构建此类化合物具有重要的意义。
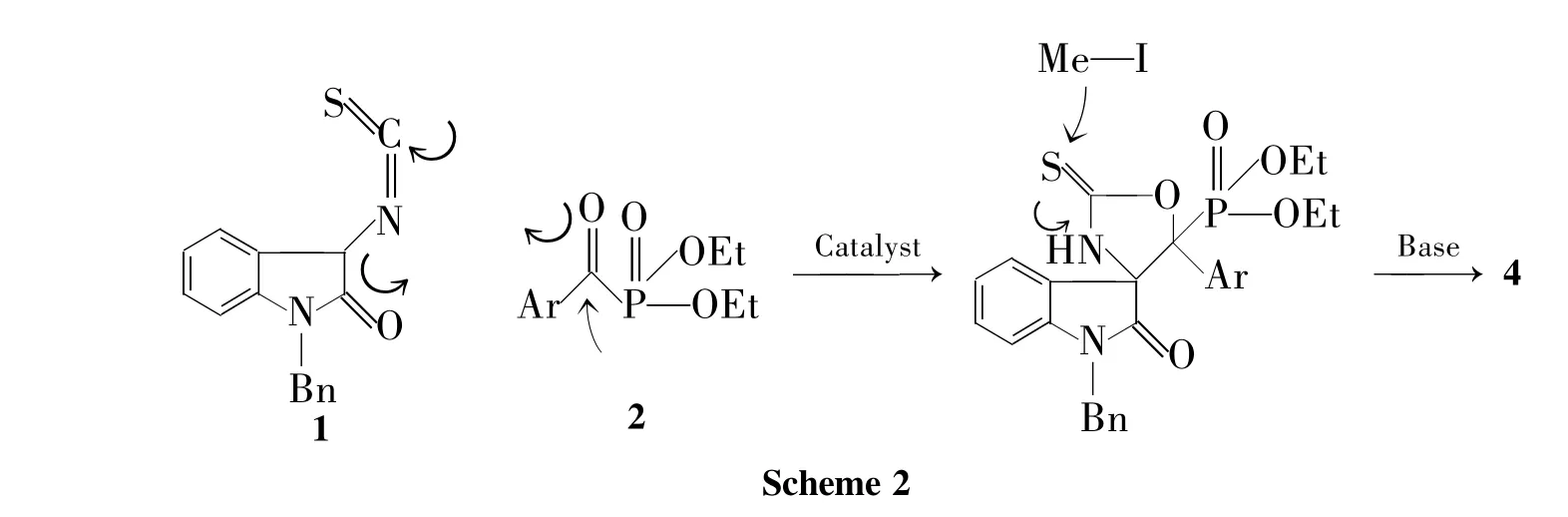
本课题组[5-7]曾报道了3-异硫氰酸酯氧化吲哚(1)的合成,并研究了1与醛、酮、亚胺及不饱和双键的加成环化反应,但是1与α-羰基磷酸酯的反应还未见报道。根据本课题组多年的研究基础预测1与α-羰基磷酸酯可发生aldol/环化反应,用于构建含磷酸酯的螺环氧化吲哚类化合物。
本文以10 mol%三乙烯二胺(DABCO)为催化剂,1和α-羰基磷酸酯(2a~2h)经aldol/环化一步构建含磷酸酯的螺环氧化吲哚类化合物(3a~3h);由于3不太稳定(可能发生逆aldol反应),通过一步简单的甲基化反应,将其转化为另一类稳定的新型螺[氧化吲哚-3,4'-噁唑啉]-5'-磷酸酯类化合物(4a~4h,Scheme 1),收率86%~92%,dr>99∶1,其结构经1H NMR和13C NMR表征。

1 实验部分
1.1 仪器与试剂
Bruker-300型核磁共振仪(CDCl3为溶剂,TMS为内标)。
所用试剂均为分析纯。
1.2 合成
(1)4的合成通法
在硬质反应管中加入DABCO 2.2 mg(0.01 mmol),2 0.2 mmol和THF 3 mL,冰浴冷却下搅拌3 min;加入1 28.0 mg(0.1 mmol),反应30 min (TLC监测)。减压旋蒸除去THF后经硅胶柱层析[洗脱剂:A=V(乙酸乙酯)∶V(石油醚)= 1∶2~1∶1.5]纯化得3。
将3溶于4 mL丙酮中,依次加入K2CO316.5 mg(0.12 mmol)和MeI 17 mg(0.12 mmol),搅拌下于室温反应过夜。减压蒸除丙酮后经硅胶柱层析(洗脱剂:A=1∶3~1∶2)纯化得无色油状液体4。
4 a:收率90%,dr>99∶1;1H NMR δ:0.94 (t,J=6.9 Hz,3H),1.43(t,J=6.9 Hz,3H),2.58(s,3H),3.25~3.39(m,1H),3.70~3.82 (m,1H),4.30~4.56(m,2H),4.71(d,J= 15.6 Hz,1H),5.23(d,J=15.6 Hz,1H),6.22 (d,J=7.5 Hz,1H),6.46~6.54(m,2H),6.79~6.81(m,1H),6.88~7.06(m,3H),7.24~7.30(m,2H),7.34~7.40(m,2H),7.52(d,J=7.2 Hz,3H);13C NMR δ:14.7,16.1(d,J=5.5 Hz,1C),16.4(d,J=6.1 Hz,1C),44.5,64.0(d,J=7.4 Hz,1C),64.1(d,J=7.7 Hz,1C),81.9,95.3(d,J=160.5 Hz,1C),108.7,121.9,124.4(d,J=5.6 Hz),126.0,126.6(d,J=2.3 Hz,1C),127.3(d,J= 2.4 Hz,1C),127.6,127.7,127.8(d,J=2.5 Hz,1C),127.9,128.7,129.2,134.6(d,J= 3.1 Hz,1C),135.7,142.2,169.5,173.7(d,J=6.0 Hz,1C)。
4 b:收率89%,dr>99∶1;1H NMR δ:0.96 (t,J=6.9 Hz,3H),1.41(t,J=6.9 Hz,3H),2.16(s,3H),2.57(s,3H),3.26~3.39(m,1H),3.69~3.82(m,1H),4.28~4.51(m,2H),4.69(d,J=15.6 Hz,1H),5.24(d,J=15.6 Hz,1H),6.23(d,J=7.5 Hz,1H),6.48~6.53(m,2H),6.61~6.72(m,2H),6.89~6.91(m,1H),7.05~7.07(m,1H),7.26~7.27(m,1H),7.30~7.41(m,3H),7.52(d,J=7.2 Hz,2H);13C NMR δ:14.1,16.1(d,J=5.4 Hz,1C),16.4 (d,J=6.2 Hz,1C),20.9,44.5,63.9(d,J=3.3Hz,1C),64.0(d,J=3.8 Hz,1C),81.8,95.4 (d,J=160.6 Hz,1C),108.6,121.9,124.4(d,J= 5.5 Hz,1C),126.1,126.5,127.4,127.5,127.7,128.1,128.4,128.7,129.2,131.4(d,J=2.9 Hz,1C),135.8,137.5(d,J=2.6 Hz,1C),169.5(d,J=2.6 Hz,1C),173.7(d,J=5.8 Hz,1C)。
4 c:收率91%,dr>99∶1;1H NMR δ:0.97 (t,J=6.9 Hz,3H),1.41(t,J=6.9 Hz,3H),2.57(s,3H),3.27~3.41(m,1H),3.66(s,3H),3.70~3.84(m,1H),4.30~4.50(m,2H),4.70(d,J=15.6 Hz,1H),5.21(d,J= 15.6 Hz,1H),6.22(d,J=7.5 Hz,1H),6.35~6.55(m,3H),6.75~6.79(m,2H),6.89~6.91(m,1H),7.26~7.29(m,1H),7.33~7.38(m,3H),7.50~7.52(m,2H);13C NMR δ:14.7,16.2(d,J=5.4 Hz,1C),16.4(d,J=6.1 Hz,1C),44.5,55.0,63.9(d,J=4.9 Hz,1C),64.0(d,J=5.4 Hz,1C),81.9,94.0(d,J=161.6 Hz,1C),108.6,112.9 (d,J=9.8 Hz,1C),122.0,125.8,125.9,126.2,127.3,127.5,127.7,127.9,128.7,129.2,135.8,142.2,159.0(d,J=2.3 Hz,1C),169.4(d,J=3.4 Hz,1C),173.8(d,J= 5.8 Hz,1C)。
4 d:收率87%,dr=59∶41;1H NMR δ: 1.00(t,J=6.9 Hz,3H),1.40(t,J=6.9 Hz,3H),2.57(s,3H),3.60~3.77(m,1H),3.80~4.00(m,1H),4.32~4.46(m,2H),4.77(d,J=15.6 Hz,1H),5.00(d,J=15.6 Hz,1H),6.24(d,J=7.5 Hz,1H),6.76~6.79 (m,2H),7.04~7.07(m,3H),7.10~7.33 (m,5H),7.33~7.48(m,1H),8.06(d,J= 7.5 Hz,1H);13C NMR δ:14.6,15.9(d,J=5.3 Hz,1C),16.3(d,J=6.5 Hz,1C),44.3,64.1 (d,J=7.2 Hz,1C),64.8(d,J=8.0 Hz,1C),83.3,94.1(d,J=161.2 Hz,1C),109.2,122.7,125.8(d,J=2.6 Hz,1C),126.4,127.0,127.5,127.8,128.0,128.4,128.8,129.9,130.7,132.5(d,J=1.6 Hz,1C),135.2,143.9,170.6,172.8。
4 e:收率86%,dr=50∶50;1H NMR δ:1.00 (t,J=6.9 Hz,3H),1.41(t,J=6.9 Hz,3H),2.58(s,3H),3.60~3.78(m,1H),3.85~3.93 (m,1H),4.30~4.51(m,2H),4.75(d,J= 15.6 Hz,1H),5.29(d,J=15.6 Hz,1H),6.53~6.60(m,1H),6.76~6.80(m,3H),7.04~7.13(m,2H),7.21~7.29(m,4H),7.35~7.52(m,2H),8.04(d,J=7.5 Hz,1H);13C NMR δ:14.6,16.0(d,J=5.3 Hz,1C),16.2(d,J=5.8 Hz,1C),44.2,63.9(d,J= 7.6 Hz,1C),64.8(d,J=8.0 Hz,1C),83.3,93.8(d,J=161.3 Hz,1C),109.2,120.0,122.7,125.1,126.3,126.8,127.5,127.8,128.2,128.5,128.9,129.5,130.3,134.1,135.2,136.2,143.9,170.3,172.8。
4 f:收率90%,dr=91∶9;1H NMR δ:1.04 (t,J=6.9 Hz,3H),1.40(t,J=6.9 Hz,3H),2.59(s,3H),3.46~3.54(m,1H),3.89~3.97 (m,1H),4.29~4.68(m,2H),4.66(d,J=15.6 Hz,1H),5.22(d,J=15.6 Hz,1H),6.40(d,J= 7.5 Hz,1H),6.52~6.71(m,4H),6.98~6.99 (m,1H),7.09~7.10(m,1H),7.26~7.27(m,1H),7.34~7.38(m,2H),7.52(d,J=7.2 Hz,2H);13C NMR δ:14.9,16.2(d,J=5.6 Hz,1C),16.4(d,J=6.0 Hz,1C),44.6,64.0(d,J=6.2 Hz,1C),64.4(d,J=5.6 Hz,1C),82.6,92.7 (d,J=164.5 Hz,1C),108.8,121.8,124.8(d,J=2.6 Hz,1C),125.8,126.3(d,J=4.4 Hz,1C),127.0(d,J=2.4 Hz,1C),127.5,127.4,128.7,129.2,135.7,137.5,142.4,169.6,173.5 (d,J=5.4 Hz,1C)。
4 g:收率89%,dr>99∶1;1H NMR δ:0.92 (t,J=6.9 Hz,3H),1.36(t,J=6.9 Hz,3H),2.62(s,3H),3.42~3.55(m,1H),3.62~3.74 (m,1H),4.11(d,J=15.6 Hz,1H),4.22~4.32 (m,2H),4.72(d,J=15.6 Hz,1H),6.72(d,J=7.2 Hz,1H),6.71~6.87(m,3H),6.94~6.96(m,3H),7.14~7.20(m,1H),7.26~7.46 (m,4H),7.48~7.90(m,2H),8.16~8.18(m,2H);13C NMR δ:14.8,15.9(d,J=5.6 Hz,1C),16.4(d,J=5.8 Hz,1C),44.4,63.8(d,J=7.6 Hz,1C),64.3(d,J=8.1 Hz,1C),81.3,94.1 (d,J=161.0 Hz,1C),109.1,122.6,122.7,123.6(d,J=4.4 Hz,1C),124.5,125.3(d,J= 5.8 Hz,1C),126.1,126.4,127.2(d,J=5.8 Hz,1C),127.4,127.7(d,J=8.3 Hz,1C),128.0,128.4,128.7,129.3,130.3(d,J=7.8 Hz,1C),131.0,132.7,132.9,135.0(d,J=9.1 Hz,1C),144.1,144.3,170.7,172.9。
4 h:收率92%,dr>99∶1;1H NMR δ:0.98 (t,J=6.9 Hz,3H),1.42(t,J=6.9 Hz,3H),2.57(s,3H),3.34~3.47(m,1H),3.72~3.85 (m,1H),4.32~4.56(m,2H),4.70(d,J=15.6 Hz,1H),5.22(d,J=15.6 Hz,1H),6.21~6.24 (m,1H),6.49~6.56(m,3H),6.90~6.96(m,3H),7.26~7.30(m,1H),7.34~7.39(m,2H),7.50~7.52(m,3H);13C NMR δ:14.8,16.1(d,J=5.5 Hz,1C),16.4(d,J=6.2 Hz,1C),44.5,64.0(d,J=7.4 Hz,1C),64.3(d,J=7.8 Hz,1C),81.8,93.9(d,J=161.2 Hz,1C),108.8,114.6(d,J=19.6 Hz,1C),122.1,126.0,126.5,127.2(d,J=10.2 Hz,1C),127.6,127.7,128.4 (d,J=8.1 Hz,1C),128.8,129.4,130.6,135.7,142.1,160.5(d,J=2.7 Hz,1C),163.8 (d,J=2.7 Hz,1C),169.5,173.6(d,J=5.8 Hz,1C)。
2 结果与讨论
2.1 合成
在4的合成中,2中的Ar无论是吸电子取代基(2d,2e和2h)还是给电子取代基(2b和2c),对反应活性基本无影响,均可高收率合成4;当Ar 的2-位为吸电子取代基(2d和2e)时,反应的非对应选择性(dr)大大降低;对于杂芳环和大位阻的萘环(2g),该反应体系也有很好的适应性,只是对于芳杂环,dr有所降低(2f),而Ar为萘环时收率89%,dr>99∶1。
2.2 反应机理
推测合成4的机理如Scheme 2所示。
3 结论
以10 mol%三乙烯二胺为催化剂,通过aldol/环化反应,高收率的合成了一系列螺[氧化吲哚-3,4'-噁唑啉]-5'-磷酸酯类化合物,非对应选择性>99∶1。该方法为合成含磷螺环氧化吲哚类化合物提供了很好的途径。
[1]Lin H,Danishefsky S J.Gelsemine.A thought-provoking target for total synthesis[J].Angew Chem,Int Ed,2003,42(1):36-51.
[2]Marti C,Carreira E M.Construction of spiro[pyrrolidine-3,3-oxindoles]-recent applications to the synthesis of oxindole alkaloids[J].Eur J Org Chem,2003,2003(12):2209-2219.
[3]Suchý M,Kutschy P,Monde K,et al.Synthesis,absolute configuration,and enantiomeric enrichment of a cruciferous oxindole phytoalexin,(S)-(-)-spirobrassinin,and its oxazoline analog[J].J Org Chem,2001,66:3940-3947.
[4]Giribone D,Fontana E.Synthesis of estromustine,estramustine and estramustine phosphate labelled with deuterium[J].J Labelled Compd Radiopharm,2004,47:161-165.
[5]Chen W B,Wu Z J,Hu J,et al.Organocatalytic direct asymmetric aldol reactions of 3-isothiocyanato oxindoles to ketones:Stereocontrolled synthesis of spirooxindoles bearing highly congested contiguous tetrasubstituted stereocenters[J].Org Lett,2011,13(9): 2472-2475.
[6]Han Y Y,Chen W B,Han W Y,et al.Highly efficient and stereoselective construction of dispiro[oxazolidine-2-thione]bisoxindoles and dispiro[imidazolidine-2-thione]bisoxindoles[J].Org Lett,2012,14 (2):490-493.
[7]Liu X L,Han W Y,Zhang X M,et al.Highly efficient and stereocontrolled construction of 3,3'-pyrrolidonyl spirooxindoles via organocatalytic domino michael/cyclization reaction[J].Org Lett,2013,15(6):1246-1249.
Synthesis of Novel Spiro[indoline-3,4'-oxazoline]-5'-ylphosphonates
CHEN Yong-zheng1,ZHAO Jian-qiang2,3,BAI Mei1,2,3,YUAN Wei-cheng2
(1.School of Pharmacy,Zunyi Medical University,Zunyi 563000,China; 2.Chengdu Institute of Organic Chemistry,Chinese Academy of Sciences,Chengdu 610041,China; 3.University of Chinese Academy of Science,Beijing 100049,China)
A series of novel spiro[indoline-3,4'-oxazoline]-5'-ylphosphonates were synthesized with yield of 86%~92%and excellent diastereoselectivities(dr>99∶1)by aldol/cyclization reaction of 3-isothiocyanato oxindole and α-carboxyl phosphates using triethylenediamine as the catalyst.The structures were characterized by1H NMR and13C NMR.
3-isothiocyanatooxindole;α-carboxyl phosphates;aldol/cyclization;synthesis
O626;O621.3
A
1005-1511(2014)03-0346-04
2014-01-15;
2014-04-03
国家自然科学基金资助项目(21372217);贵州省科学技术基金资助项目[黔科合J字LKZ(2013)27号];遵义医学院博士启动基金资助项目(F-563)
陈永正(1982-),男,汉族,贵州遵义人,博士,副教授,主要从事不对称合成的研究。E-mail:yzchen@zmc.edu.cn
袁伟成,研究员,博士生导师,E-mail:yuanwc@cioc.ac.cn

