Surface Functionalization of Microporous Polypropylene Membrane with Polyols for Removal of Boron Acid from Aqueous Solution*
ZHOU Rong (周蓉), DI Ling (狄玲), WANG Cang (王苍), FANG Yan (方艳), WU Jian (吴健)and XU Zhikang (徐志康),**
1MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou 310027, China
2Department of Chemistry, Zhejiang University, Hangzhou 310027, China
Surface Functionalization of Microporous Polypropylene Membrane with Polyols for Removal of Boron Acid from Aqueous Solution*
ZHOU Rong (周蓉)1, DI Ling (狄玲)2, WANG Cang (王苍)2, FANG Yan (方艳)1, WU Jian (吴健)2and XU Zhikang (徐志康)1,**
1MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou 310027, China
2Department of Chemistry, Zhejiang University, Hangzhou 310027, China
Affinity membranes are fabricated for boric acid removal by the surface functionalization of microporous polypropylene membrane (MPPM) with lactose-based polyols. The affinity is based on specific complexation between boric acid and saccharide polyols. A photoinduced grafting-chemical reaction sequence was used to prepare these affinity membranes. Poly(2-aminoethyl methacrylate hydrochloride) [poly(AEMA)] was grafted on the surfaces of MPPM by UV-induced graft polymerization. Grafting in the membrane pores was visualized by dying the cross-section of poly(AEMA)-grafted MPPM with fluorescein disodium and imaging with confocal laser scanning microscopy. It is concluded that lactose ligands can be covalently immobilized on the external surface and in the pores by the subsequent coupling of poly(AEMA) with lactobionic acid (LA). Physical and chemical properties of the affinity membranes were characterized by field emission scanning electron microscopy and Fourier Transform Infrared/Attenuated Total Refraction spectroscopy (FT-IR/ATR). 3-Aminophenyl boric acid (3-APBA) was removed from aqueous solution by a single piece of lactose-functionalized MPPM in a dynamic filtration system. The results show that the 3-APBA removal reaches an optimal efficiency (39.5%) under the alkaline condition (pH 9.1), which can be improved by increasing the immobilization density of LA. Regeneration of these affinity membranes can be easily realized through acid-base washing because the complexation of boric acid and saccharide polyol is reversible.
surface functionalization, affinity membrane, microporous polypropylene membrane, boron removal, polyols
1 INTRODUCTION
Boron contamination of water is recognized as a serious environmental problem in recent years [1-3]. In aqueous solutions, dissolved boron is usually presentfound in seawater and wastewater at a concentration level of 5-100 mg·L−1or higher, far beyond the upper limit of 2.4 mg·L−1suggested by the World Health Organization (WHO) for drinking water and 0.5 mg·L−1for irrigation water [4]. Although a minute amount of boron plays an important role in human diet, an excessive level of boron will be toxic to human beings. Overdose of boron intake may cause symptoms such as nausea, vomiting, diarrhoea, dematitis, lethargy, poor appetite, mass loss, and decreased sexual activity [5]. “Boron poisoning” will result from boron concentration only a slightly higher than 0.5 mg·L−1for plants [6]. Therefore, it is a pressing challenge to develop ecological-economical technologies for removing boron compounds from water. Current methods include adsorption with various adsorbents [7-11], coagulation/precipitation [12], anion-exchange with boron selective resin (BSR) [13-23], and membrane processes (nanofiltration/reverse osmosis [24-31], adsorption membrane filtration [3, 32-34], electrodialysis [35], and direct contact membrane distillation [36]). The advantages and disadvantages of these methods have been commented in detail [1-3]. With the increasing demand for good quality water, more attention is paid to effective ways for boron removal at low cost with less energy consumption [19, 24, 26-29].
Boric acids/borates are complex compounds containing multiple hydroxyl groups (polyols) [37-42]. It was reported 50 years ago saccharides-based polyols form complexes with phenylboric acids [37]. This pioneering work led to a series of fundamental studies and potential applications [38-42], one of which was to remove boron with commercial BSR, synthesized from macroporous polystyrene matrix and modified with a polyol, N-methyl-D-glucamine [13-18]. Membrane processes were also used to integrate with specific complexation of polyols [33, 34]. For example, poly(vinyl alcohol) was used as boron complexant and separation of boron/polymer complexes was achieved with an ultrafiltration process [33]. More recently, Kabay et al. demonstrated an effective hybrid system to remove boron by economical treatment of reverse osmosis permeates [3, 32], in which BSR was combined with microfiltration polypropylene membrane, named as adsorption-membrane filtration (AMF). Further work is needed for developing and optimizing the membrane processes on membrane materials, boron removal efficiency, fouling phenomena, cleaning protocols, and long term operation.
It has been recognized that affinity membraneswith polyols can be used for water treatment [43, 44], such as a type of chitosan nanofibrous mats used as an efficient adsorbent for heavy metal ions in aqueous solutions [44]. The target pollutants are selectively captured by polyol ligands immobilized on the membrane surface. The affinity membranes with polyols as ligands can be utilized for boron removal [45]. We propose that the AMF system can be simplified to some extent by the surface functionalization of microporous polypropylene membrane (MPPM) with polyols. To confirm this idea, we explore the possibility of MPPM functionalized with saccharides as polyol ligands for boron removal from aqueous solutions. In this study, lactose is used because it has eight hydroxyl groups to give high binding capacity for boron compounds. Since the binding process of 3-aminophenyl boric acid (3-APBA) with polyols is analogous to that of boric acid, it is selected as a model compound of boric acid/borate due to its solubility in water for preparing aqueous solution and absorption at 281 nm for easy determination. We describe the fabrication, characterization and affinity filtration of these membranes for 3-APBA removal from aqueous solution. The affinity membranes based on saccharide functionalization may be promising for boron removal.
2 EXPERIMENTAL
2.1 Materials
MPPM was purchased from Membrana GmbH (Germany). The average pore size is 0.20 μm and the porosity is 75%-80%. Each membrane sample was cut into round shape with a diameter of 2.50 cm (area=4.91 cm2). Benzophenone (BP), acetone, and heptane with analytical grade were used as received. 2-Aminoethyl methacrylate hydrochloride (AEMA) was synthesized as described in our previous work [46]. 3-APBA was bought from Ningbo Yingfa Pengna Co., Ltd (China) and recrystallized before use. 1-Ethyl-3-(dimethyl aminopropyl)-carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) were purchased from Shanghai Medpep and Sigma (China), respectively. Lactobionic acid (LA) and fluorescein disodium are commercial products of Sigma-Aldrich Ltd (China) and were used as received.
2.2 Graft polymerization of AEMA on MPPM
AEMA was grafted onto MPPM by UV-irradiation with a 500 W high-pressure mercury lamp. First, a preweighted MPPM sample was soaked in 5 ml of photoinitiator solution (BP in heptane with a concentration of 3.64 g·L−1) for 60 min and dried in air for 30 min. Then the membrane was pre-wetted with acetone for 10 s to improve the accessibility of monomer aqueous solution onto the MPPM surface, and wiped with filter paper quickly. Each sample with pores wetted was immediately fixed between two filter papers and immersed into 1.0 ml of AEMA aqueous solution (pre-deaerated by bubbling with N2for 10 min) in a Petri dish. After 5 min of equilibration, the sample was put into an ultraviolet processor. UV irradiation was carried out for certain time under nitrogen gas environment. A constant-temperature water bath was connected to the system and maintained the grafting process at room temperature. Finally, the poly(AEMA)-grafted MPPM [designated as MPPM-g-poly(AEMA)] was thoroughly washed with deionized water for 24 h using a vibrator. After dried in a vacuum oven at 50 °C to constant mass, the grafting density (GD, μmol·cm−2) of the modified MPPM is calculated by the following equation

where m0is the mass of MPPM (mg), m1is the mass of MPPM-g-poly(AEMA) (mg), Amis the surface area of membrane (4.91 cm2), and 165.5 is the molar mass of AEMA. All the results are the average of three parallel experiments.
2.3 Lactose immobilization on MPPM-g-poly (AEMA)
Lactose ligands were immobilized onto the membrane surface by a reaction between amino groups of poly(AEMA) and carboxyl groups of LA via an EDC/NHS activation procedure as presented in Fig. 1. The amino groups of poly(AEMA) were first deprotonated by dipping MPPM-g-poly(AEMA) into a solution of triethylamine (TEA) (1.5%, by mass) for 2 h. Then the membrane sample was submerged into a LA solution [5-fold molar quantity of poly(AEMA) grafted on the membrane surfaces] at 4 °C in the presence of EDC/NHS (2 mg·L−1) with gentle vibration for 24 h. After washed with deionized water drastically with a vibrator for 24 h, the membrane was dried under vacuum at 50 °C. The immobilization density (ID, μmol·cm−2) of LA is defined as

where m2is the mass of membrane bound with lactose (designated as MPPM-g-LA) (mg), and 304.8 is the molar mass of LA. All the results are the average of three parallel experiments.
2.4 Characterization

Figure 1 Schematic diagram for UV-induced graft polymerization of AEMA and immobilization of lactose-based polyols on MPPM surface
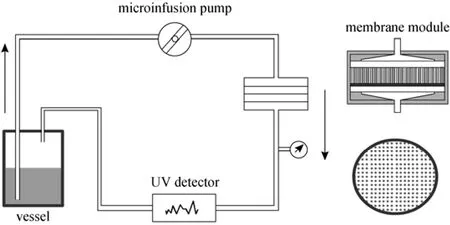
Figure 2 Set up of filtration system with the lactose-based polyol-functionalized affinity membrane
Fourier Transform Infrared/Attenuated Total Refraction spectra (FT-IR/ATR) were collected on Nicolet FT-IR/Nexus 470 spectrometer equipped with an ATR accessory (ZnSe crystal, 45°). Sixteen scans were taken for each spectrum at a resolution of 4 cm−1. Field emission scanning electron microscopy (FESEM, SIRION, FEI, USA) was used to observe the morphology of membrane surface after the sample was coated with a 20-nm-thick gold layer. Fluorescein disodium was used to stain the MPPM-g-poly(AEMA). Samples were incubated with 4 mL of aqueous solution of fluorescein disodium (0.5%, by mass) for 0.5 h at room temperature. After that, the membranes were washed with deionized water (6 times, 10 min each), dried at 50 °C under vacuum for 24 h, frozen in liquid nitrogen and fractured to obtain a tidy cross-section. Confocal laser scanning microscopy (CLSM) was used with a TCS SP5 confocal setup mounted on a Leica DMI 6000 CS inverted microscope (Leica Microsystems, Wetzlar, Germany) and operated under the Leica Application Suite Advanced Fluorescence (LAS AF) program. Confocal illumination was provided by an argon-ion laser at 488 nm for exciting. Fluorescence emission of fluorescein disodium on the membrane was recorded at 500-535 nm.
2.5 Filtration experiment
The filtration experiment with polyols-functionalized affinity membrane was performed with freshly prepared aqueous solution of 3-APBA in Tris-HCl buffer. As shown in Fig. 2, 20 ml 3-APBA solution was kept in a vessel and delivered in the loop at certain flow rate by a microinfusion pump. Before cycling, the filtration system was fed with 3-APBA solution (boron concentration of 7.89 mg·L−1) and then washed with Tris-HCl buffer to eliminate nonspecific adsorption of 3-APBA. One piece of MPPM-g-LA membrane was wetted with ethanol and exchanged with enough Tris-HCl buffer solution and put into the stainless steel module (Millipore, USA). The membrane holder was mounted in the filtration system. Finally, 3-APBA solution in Tris-HCl with boron concentration of 7.89mg·L−1was injected into the membrane holder with a constant flow rate (0.8 ml·min−1), and the 3-APBA concentration was determined from the UV absorbance at 281 nm with an online UV spectrometry detector. After the filtration, the system was washed with Tris-HCl buffer solution at a flow rate of 1.5 ml·min−1until 3-APBA was not detected in the solution. Then, the membrane was taken out from the membrane module, eluted with 0.3 mol·L−1HCl for 5 h and washed with deionized water for 24 h, dried, preserved, and used in the next circle.
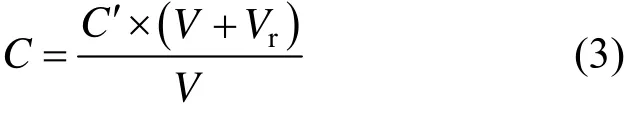
To eliminate the influence of residual buffer solution in the pipeline, the concentration of boron after filtration is corrected by the following equation where C′ is the concentration of boron recorded by online UV spectrometry (mg·L−1), V is the initial volume of 3-APBA solution (ml), and Vris the residual volume of Tris-HCl buffer (ml).

The 3-APBA removal is described by removal efficiency of boron (REB), defined as where C0and C are the concentrations of boron in aqueous solution before and after filtration, respectively (mg·L−1).
The binding capacity (BC, μmol·cm−2) of 3-APBA to the polyols-functionalized MPPM is calculated by
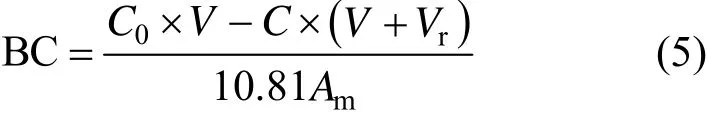
where 10.81 is the molar mass of boron.
3 RESULTS AND DISCUSSION
3.1 Surface functionalization of MPPM with lactosebased polyols
Ligands should be immobilized on both external surface and pore surface of membrane [43]. As shown in Fig. 1, lactose-based polyols are introduced on these surfaces of MPPM by a photoinduced graftingchemical reaction sequence. Amino-functionalized layer is resulted from the UV-induced grafting polymerization of AEMA. Since the grafting density of poly(AEMA) increases with AEMA concentration and UV irradiation time [46], the grafting density on the membrane surface is adjustable by controlling the monomer concentration and photoinduced grafting time in the experiment. Fluorescein disodium is used to visually verify the functionalization of the external and pore surfaces of MPPM with poly(AEMA). As the amino groups of poly(AEMA) are protected in a hydrochloride form, the poly(AEMA)-grafted membrane surfaces are positively charged. Therefore, these surfaces can be stained by fluorescein disodium, which is a negatively charged dye [47]. Fig. 3 displays typical photos taken from the external membrane surface and CLSM images taken from the membrane cross-section for MPPM-g-poly(AEMA) with various grafting densities treated with fluorescein disodium. The plain MPPM can not be stained by fluorescein disodium [Figs. 3 (a), 3 (e)]. Color intensity of the external surface increases with grafting density [Figs. 3 (b), 3 (c), 3 (d)]. The distribution of poly(AEMA) along the cross-sectionof membrane is characterized by CLSM [Figs. 3 (f), 3 (g), 3 (h)]. It indicates that the grafting of poly(AEMA) occurs on the external and pore surfaces of MPPM.
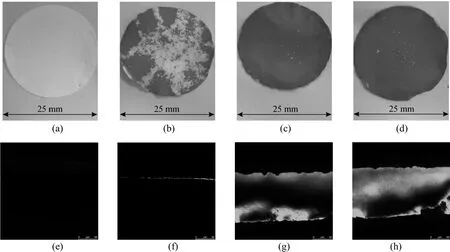
Figure 3 Photographs of external surface (a)-(d) and CLSM pictures of cross-section of pores (e)-(h) for the membranes stained by fluorescein disodium(a) MPPM; (b), (c), (d) MPPM-g-poly(AEMA) with GD = 0.56, 1.55, 2.45 µmol·cm−2;(e) MPPM; (f), (g), (h) MPPM-g-poly(AEMA) with GD = 0.53, 1.42, 2.56 µmol·cm−2
It is expected that lactose-based polyols can be immobilized on the surface with following steps. Lactose ligands are covalently bound with grafted poly(AEMA) chains through a reaction between the amino group of AEMA and the carboxyl group of LA. Before the reaction with LA, MPPM-g-poly(AEMA) sample is dipped in a TEA solution to neutralize hydrochloride to protect the amino group. When the membrane is transferred in a LA solution in the presence of EDC/NHS, the MPPM-g-poly(AEMA) sample reacts with LA under the same reaction condition (time, temperature, and concentration of TEA). Fig. 4 shows that the immobilization degree of LA increases almost linearly with the increase of GD. Therefore, more lactose ligands can be bound to poly(AEMA) chains when the GD of poly(AEMA) increases.

Figure 4 Dependence of lactose ID on GD of poly (AEMA)
3.2 Chemical and morphological changes of membrane surface
Chemical and physical properties of membrane surfaces are very important to separation characteristics. Fig. 5 shows the FT-IR/ATR spectra for MPPM, MPPM-g-poly(AEMA), and MPPM-g-LA to confirm the functionalization of membranes. After grafting, a new peak appears at 1720 cm−1in both MPPM-g-poly (AEMA) and MPPM-g-LA, attributed to the ester CO stretching vibration in COO−. The spectra of MPPM-g-poly(AEMA) and MPPM-g-LA show that two characteristic peaks at 1646 and 1543 cm−1for amide and 3360 cm−1for OH stretching vibration appear after functionalization with lactobionic acid. All these data indicate successful immobilization of lactose ligands. Fig. 6 shows the morphological change of membrane surfaces characterized by FESEM. These typical images demonstrate that the functional layer almost uniformly and conformably adheres to external membrane surfaces and covers part of membrane pores.

Figure 5 FT-IR/ATR spectra of MPPMs(a) MPPM; (b) MPPM-g-poly(AEMA) with GD of 0.31 µmol·cm−2; (c) MPPM-g-LA with GD of 0.85 µmol·cm−2and ID of 0.38 µmol·cm−2; (d) MPPM-g-LA with GD of 2.13 µmol·cm−2and ID of 0.95 µmol·cm−2

Figure 6 FESEM images of MPPMs(a) MPPM; (b) MPPM-g-poly(AEMA) with GD of 0.81 µmol·cm−2; (c) MPPM-g-LA with GD of 0.76 µmol·cm−2and ID of 0.31 µmol·cm−2; (d) MPPM-g-LA with GD of 4.03 µmol·cm−2and ID of 1.98 µmol·cm−2

Figure 7 Mechanism for complexation of boric acid and 3-APBA with polyols1, 5—boric acid; 2, 6—borate; 3, 4, 8—borate esters in tetrahedral; 7—borate ester in trigonal

Figure 8 Effect of pH value (a) and ID of lactose ligand (b) on the breakthrough curve for 3-APBA removal by the MPPM-g-LA
3.3 Removal of 3-APBA from aqueous solution by MPPM-g-LA
As shown in Fig. 7, boric acid and its derivatives (such as 3-APBA) react with compounds containing diol moieties with high affinity through reversible ester formation. Fig. 8 (a) shows the breakthrough curves of MPPM-g-LA with ID of 0.75 µmol·cm−2and Table 1 summarizes the removal efficiency of 3-APBA at different pH values (from 5.5 to 9.1). The effluent concentration increases rapidly to the maximum in the early stage. The maximal concentration is usually lower than the concentration of the original solution, which is diluted by residual Tris-HCl in the system. Then, affinity adsorption reduces the effluent concentration to a constant value. The pH value plays a significant role in the 3-APBA removal. Generally, the removal efficiency and binding capacity for 3-APBA increase with the pH value of aqueous solution. This isattributed to the equilibrium of boric acid and boronate ester in the solution. It is suggested that the complexation of boric acid with diol is related to the equilibrium between trigonal and tetrahedral borate esters formed [38]. The formation of tetrahedral ester brings about hydronium ion as a byproduct. Lowering the solution pH tends to reverse the complexation. It is reported that the optimum pH for the complexation isabove the pKaof boric acids [41, 42]. However, under strong basic condition, direct competition between hydroxyl ions and borate ions will result in low removal efficiency for boron [7, 45, 48]. The pKavalue of 3-APBA is 8.9, so pH of 9.1 is suitable for its removal in the present study. Further experiments were conducted under this pH value.

Table 1 Effect of pH value on the removal efficiency and binding capacity of 3-APBA by MPPM-g-LA membrane with an ID of 0.75 μmol·cm−2
The ID of lactose ligands on/in MPPM-g-LA has a great influence on 3-APBA removal, because the target binding depends on the number of ligands on the external surface and in the pores in affinity membrane chromatography [43, 44, 49]. Results calculated from the curves are listed in Table 2, showing that as the ID increases, the binding capacity is enhanced as expected. Fig. 8 (b) exhibits kinetic performance of MPPM-g-LA with different IDs. The curves are similar for ID of 0.30, 0.61 and 1.71 μmol·cm−2, but for ID of 2.01 μmol·cm−2, the effluent concentration decreases continuously in the whole experimental cycle. It implies unsaturation adsorption of 3-APBA by the affinity membrane. Most of lactose ligands locate on the outer layer of grafted poly(AEMA) chains and are easy to bind with 3-APBA when the ID is relatively low. However, when the ID of lactose is as high as 2.01 μmol·cm−2, the ligands may locate on the whole chains of poly(AEMA). 3-APBA adsorbed on the outer layer of flexible polyols chains can be transferred into the inner layer through the reversible binding between 3-APBA and lactose ligand [49]. Therefore, the effluent concentration decreases with adsorption time and takes longer time to reach adsorption equilibrium.

Table 2 Binding capacity and removal efficiency of 3-APBA by MPPM-g-LA with different IDs of lactose at pH 9.1
Durability and reusability of the affinity membrane can be evaluated from adsorption/elution cycles. The stability of boric acid-polyols complex is pH-dependent and under the acid condition the complex is easy to be hydrolyzed, which makes the regeneration of the affinity membrane possible. With extensive acid washing (300 mmol·L−1HCl for 3 h) to remove 3-APBA bound on the MPPM-g-LA, the membrane can be regenerated for further use. Fig. 9 displays the breakthrough curves for 3 continuous cycles, in which the breakthrough curves are similar, showing the durability and reusability of MPPM-g-LA.
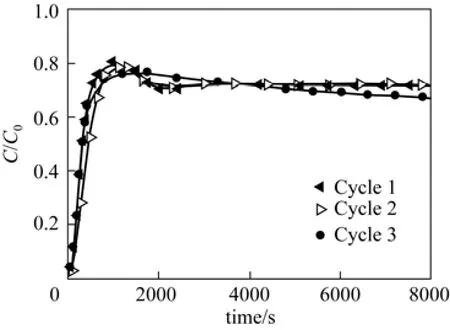
Figure 9 Breakthrough curves for 3-APBA removal by MPPM-g-LA with ID of lactose ligand 0.61 μmol·cm−2for three cycles
4 CONCLUSIONS
Microporous polypropylene membrane can be functionalized with poly(AEMA) grafting followed with immobilization of lactose-based polyols. These lactose-based polyols-functionalized membranes exhibit reasonable efficiency in the affinity adsorption of boric acid from aqueous solution. The removal efficiency can be improved by increasing the immobilization degree of lactose ligands on the external membrane surface and in the membrane pores. To further reduce boron concentration, subsequent work, such as filtration through multi-membranes, is ongoing in our laboratory and the results will be further reported.
REFERENCES
1 Hilal, N., Kim, G.J., Somerfield, C., “Boron removal from saline water: a comprehensive review”, Desalination, 273, 23-35 (2011).
2 Tu, K.L., Nghiem, L.D., Chivas, A.R., “Boron removal by reverse osmosis membranes in seawater desalination applications”, Sep. Purif. Technol., 75, 87-101 (2010).
3 Kabay, N., Bryjak, M., Schlosser, S., Kitis, M., Avlonitis, S., Matejka, Z., Al-Mutaz, I., Yuksel, M., “Adsorption-membrane filtration (AMF) hybrid process for boron removal from seawater: an overview”, Desalination, 223, 38-48 (2008).
4 World Health Organization, “Boron in drinking water—background document for development of WHO guidelines for drinking water quality”, WHO (2009).
5 Hunt, C.D., Benjamin, C., Boron, in Encyclopedia of Food Science and Nutrition, Academic Press, Oxford, 566-574 (2003).
6 Boncukcuoglu, R., Yilmaz, A.E., Kocakerim, M.M., Copur, M., “An emprical model for kinetics of boron removal from boron containing wastewaters by ion exchange in a batch reactor”, Desalination, 160, 159-166 (2004).
7 Karahan, S., Yurdakoc, M., Seki, Y., Yurdakoc, K., “Removal of boron from aqueous solution by clays and modified clays”, J. Colloid Interface Sci., 293, 36-42 (2006).
8 Ozturk, N., Kavak, D., “Boron removal from aqueous solutions by adsorption using full factorial design”, Fresenius Environ. Bull., 12, 1450-1456 (2003).
9 Garcia-Soto, M.M.F., Camachob, E.M., “Boron removal by means of adsorption with magnesium oxide”, Sep. Purif. Technol., 48, 36-44 (2006).
10 Ozturk, N., Kavak, D., “Boron removal from aqueous solutions by batch adsorption onto cerium oxide using full factorial design”, Desalination, 223, 106-112 (2008).
11 Chong, M.F., Lee, K.P., Chieng, H.J., Ramli, I., “Removal of boron from ceramic industry wastewater by adsorption-flocculationmechanism using palm oil mill boiler (POMB) bottom ash and polymer”, Water Res., 43, 3326-3334 (2009).
12 Yilmaz, A.E., Boncukcuoğlu, R., Kocakerim, M.M., “A quantitative comparison between electrocoagulation and chemical coagulation for boron removal from boron-containing solution”, J. Hazard. Mater., 149, 475-481 (2007).
13 Simonnot, M.O., Castel, C., Nicolai, M., Rosin, C., Sardin, M., Jauffret, H., “Boron removal from drinking water with a boron selective resin: is the treatment really selective?”, Water Res., 34, 109-116 (2000).
14 Kabay, N., Yılmaz, I., Yamac, S., Samatya, S., Yuksel, M., Yuksel, U., Arda, M., Saglam, M., Iwanaga, T., Hirowatari, K., “Removal and recovery of boron from geothermal wastewater by selective ion exchange resins. I. Laboratory tests”, React. Funct. Polym., 60, 163-170 (2004).
15 Kabay, N., Yılmaz, I., Yamac, S., Yuksel, M., Yuksel, U., Yildirim, N., Aydogdu, O., Iwanaga, T., Hirowatari, K., “Removal and recovery of boron from geothermal wastewater by selective ion-exchange resins. II. Field tests”, Desalination, 167, 427-438 (2004).
16 Kabay, N., Sarp, S., Yuksel, M., Kitis, M., Koseoğlu, H., Arar, O., Bryjak, M., Semiat, R., “Removal of boron from SWRO permeate by boron selective ion exchange resins containing N-methyl glucamine groups”, Desalination, 223, 49-56 (2008).
17 Kabay, N., Sarp, S., Yuksel, M., Arar, O., Bryjak, M., “Removal of boron from seawater by selective ion exchange resins”, React. Funct. Polym., 67, 1643-1650 (2007).
18 Jacob, C., “Seawater desalination: boron removal by ion exchange technology”, Desalination, 205, 47-52 (2007).
19 Samatya, S., Orhan, E., Kabay, N., Tuncel, A., “Comparative boron removal performance of monodisperse-porous particles with molecular brushes via ‘click chemistry’ and direct coupling”, Colloids Surf A: Physicochem. Eng. Aspects, 372, 102-106 (2010).
20 Bicak, N., Gazi, M., Senkal, B.F., “Polymer supported amino bis-(cis-propan 2,3-diol) functions for removal of trace boron from water”, React. & Funct. Polym., 65, 143-148 (2005).
21 Senkal, B.F., Bicak, N., “Polymer supported iminodipropylene glycol functions for removal of boron”, React. & Funct. Polym., 55, 27-33 (2003).
22 Wang, L., Qi, T., Gao, Z., Zhang, Y., Chu, J., “Synthesis of N-methylglucamine modified macroporous poly(GMA-co-TRIM) and its performance as a boron sorbent”, React. & Funct. Polym., 67, 202-209 (2007).
23 Liu, H.N., Ye, X.S., Li, Q., Kim, T., Qing, B.J., Guo, M., Ge, F., Wu, Z.J., Lee, K., “Boron adsorption using a new boron-selective hybrid gel and the commercial resin D564”, Colloid Surf., A, 341, 118-126 (2009).
24 Oo, M.H., Ong, S.L., “Implication of zeta potential at different salinities on boron removal by RO membranes”, J. Membr. Sci., 352, 1-6 (2010).
25 Oo, M.H., Song, L.F., “Effect of pH and ionic strength on boron removal by RO membranes”, Desalination, 246, 605-612 (2009).
26 Richards, L.A., Vuachère, M., Schäfer, A.I., “Impact of pH on the removal of fluoride, nitrate and boron by nanofiltration/reverse osmosis”, Desalination, 261, 331-337 (2010).
27 Kir, E., Gurler, B., Gulec, A., “Boron removal from aqueous solution by using plasma-modified and unmodified anion-exchange membranes”, Desalination, 267, 114-117 (2011).
28 Dominguez-Tagle, C., Romero-Ternero, V.J., Delgado-Torres, A.M.,“Boron removal efficiency in small seawater reverse osmosis systems”, Desalination, 265, 43-48 (2011).
29 Koseoglu, H., Harman, B.I., Yigit, N.O., Guler, E., Kabay, N., Kitis, M., “The effects of operating conditions on boron removal from geothermal waters by membrane processes”, Desalination, 258, 72-78 (2010).
30 Mane, P.P., Park, P.K., Hyung, H., Brown, J.C., Kim, J.H., “Modeling boron rejection in pilot- and full-scale reverse osmosis desalination processes”, J. Membr. Sci., 338, 119-127 (2009).
31 Cengeloglu, Y., Arslan, G., Tor, A., Kocak, I., Dursun, N., “Removal of boron from water by using reverse osmosis”, Sep. Purif. Technol., 64, 141-146 (2008).
32 Kabay, N., Yilmaz, I., Bryjak, M., Kabay, N., Yilmaz, I., Bryjak, M., Yuksel, M., “Removal of boron from aqueous solutions by a hybrid ion exchange-membrane process”, Desalination, 198, 158-165 (2006).
33 Dilek, C., Ozbelge, H.O., Bicak, N., Yilmaz, L., “Removal of boron from aqueous solutions by continuous polymer-enhanced ultrafiltration with polyvinyl alcohol”, Sep. Sci. Technol., 37, 1257-1271 (2002).
34 Melnyk, L., Goncharuk, V., Butnyk, I., Tsapiuk, E., “Boron removal from natural and wastewaters using combined sorption/membrane process”, Desalination, 185, 147-157 (2005).
35 Yazicigil, Z., Oztekin, Y., “Boron removal by electrodialysis with anion-exchange membranes”, Desalination, 190, 71-78 (2006).
36 Hou, D.Y., Wang, J., Sun, X.C., Luan, Z.K., Zhao, C.W., Ren, X.J.,“Boron removal from aqueous solution by direct contact membrane distillation”, J. Hazard. Mater., 177, 613-619 (2010).
37 Lorand, J.P., Edwards, J.O., “Polyol complexes and structure of the benzeneboronate ion”, J. Org. Chem., 24, 769-774 (1959).
38 Edwards, N.Y., Sager, T.W., McDevitt, J.T., Anslyn, E.V., “Boric acid based peptidic receptors for pattern-based saccharide sensing in neutral aqueous media, an application in real-life samples”, J. Am. Chem. Soc., 129, 13575-13583 (2007).
39 Kim, K.T., Cornelissen, J., Nolte, R.J.M., van Hest, J.C.M., “Polymeric monosaccharide receptors responsive at neutral pH”, J. Am. Chem. Soc., 131, 13908-13909 (2009).
40 Lu, C., Kostanski, L., Ketelson, H., Meadows, D., Pelton, R., “Hydroxypropyl guar-borate interactions with tear film mucin and lysozyme”, Langmuir, 21, 10032-10037 (2005).
41 Geffen, N., Semiat, R., Eisen, M.S., Balazs, Y., Katz, I., Dosoretz, C.G., “Boron removal from water by complexation to polyol compounds”, J. Membr. Sci., 286, 45-51 (2006).
42 Ivanov, A.E., Galaev, I.Y., Mattiasson, B., “Interaction of sugars, polysaccharides and cells with borate-containing copolymers: from solution to polymer brushes”, J. Mol. Recognit., 19, 322-331 (2006). 43 Klein, E., “Affinity membranes: a 10-year review”, J. Membr. Sci., 179, 1-27 (2000).
44 Haider, S., Park, S.Y., “Preparation of the electrospun chitosan nanofibers and their applications to the adsorption of Cu(II) and Pb(II) ions from an aqueous solution”, J. Membr. Sci., 328, 90-96 (2009).
45 Meng, J.Q., Yuan, J., Kang, Y.L., Zhang, Y.F., Du, Q.Y., “Surface glycosylation of polysulfone membrane towards a novel complexing membrane for boron removal”, J. Colloid Interface Sci., 368, 197-207 (2012).
46 Yang, Q., Xu, Z.K., Hu, M.X., Li, J.J., Wu, J., “Novel sequence for generating glycopolymer tethered on a membrane surface”, Langmuir, 21, 10717-10723 (2005).
47 Huang, J.Y., Murata, H., Koepsel, R.R., Russell, A.J., Matyjaszewski, K., “Antibacterial polypropylene via surface-initiated atom transfer radical polymerization”, Biomacromolecules, 8, 1396-1399 (2007).
48 Wei, Y.T., Zheng, Y.M., Chen, J.P., “Functionalization of regenerated cellulose membrane via surface initiated atom transfer radical polymerization for boron removal from aqueous solution”, Langmuir, 27, 6018-6025 (2011).
49 Hu, M.X., Wan, L.S., Xu, Z.K., “Multilayer adsorption of lectins on glycosylated microporous polypropylene membranes”, J. Membr. Sci., 335, 111-117 (2009).
10.1016/S1004-9541(14)60012-4
2012-11-21, accepted 2013-06-16.
* Supported by the National Natural Science Foundation of China (50933006), and the National Basic Research Program of China (2009CB623401).
** To whom correspondence should be addressed. E-mail: xuzk@zju.edu.cn
 Chinese Journal of Chemical Engineering2014年1期
Chinese Journal of Chemical Engineering2014年1期
- Chinese Journal of Chemical Engineering的其它文章
- Influence of Design Margin on Operation Optimization and Control Performance of Chemical Processes*
- Measurement and Modeling for the Solubility of Hydrogen Sulfide in Primene JM-T*
- Enhancing Structural Stability and Pervaporation Performance of Composite Membranes by Coating Gelatin onto Hydrophilically Modified Support Layer*
- Photocatalytical Inactivation of Enterococcus faecalis from Water Using Functional Materials Based on Natural Zeolite and Titanium Dioxide*
- A Group Contribution Method for the Correlation of Static Dielectric Constant of Ionic Liquids*
- Interaction Analysis and Decomposition Principle for Control Structure Design of Large-scale Systems*
