脱质子化1,3环加成石墨烯外在固定位上的贵金属纳米线
蔡 潜 蔡秋霞 庄桂林 钟 兴 王新德 李小年 王建国
(浙江工业大学化学工程与材料学院,绿色化学合成技术国家重点实验室培育基地,杭州310032)
1 Introduction
Noble metal nanoparticles/nanowires supported on graphene have attracted much research attention because of their unique optical,electrical,magnetic,and mechanical properties.1-7However,the weak interaction between metal nanoparticles and graphene has become a vital problem.Different methods are adopted to modify the inert properties of pristine graphene.
The modification methods can be divided into two types,which are internal modification and external modification.The internal modification mainly includes the creation of carbon vacancies8-14and the substitution of the lattice carbon with foreign elements.15-18Density functional theory(DFT)calculations studied by Wilcox et al.12showed that the graphene vacancies act as anchoring points for pure metal nanoparticles,the strong binding of Fe13and Al13nanoparticles on defective graphene is due to the strong hybridization of metal nanoparticles with the sp2dangling bonds of neighboring carbons near the vacancy.Ramaprabhu et al.18prepared the N-doped graphene as a catalyst support material for Pt particles in oxygen reduction reaction(ORR)by plasma treatment.It indicates that the improved performance of Pt/N-G(Pt adsorbed on graphene doped with nitrogen)compared to Pt/G is due to the improved carbon-catalyst binding and increased electrical conductivity induced by nitrogen doping.Our previous study20also showed that point defects can enhance the bonding of noble metal atoms on the graphene with the sequence of vacancy defect>>B-doping>N-doping.The external modification method is to introduce surface functional groups on the graphene.21-26Among the various surface functional groups,1,3-dipolar cycloaddition becomes a hot topic because it has been successfully applied in the organic functionalization of carbon nanotubes27-29and fullerenes30,31for many applications,like biotechnology,electrooptical devices,and solar energy conversion.32,33In addition,Trapalis et al.25confirmed that graphene can be effectively functionalized through 1,3-dipolar cycloaddition.Recently,Prato et al.34prepared 1,3-dipolar cycloaddition graphene experimentally,demonstrating that gold nanorods can distribute uniformly over the modified graphene surface.Among the noble metals,Pt is commonly used as catalyst material.However,the mechanism for the adsorption properties of Pt nanoparticles over external anchoring carbon carries is still unclear.
In this study,we identify the anchoring site for small Pt cluster and the formation of Pt nanowires on deprotonated 1,3-dipolar cycloaddition(DDC)graphene by means of DFT calculations.A series of electronic structure analysis(Bader charge,charge density,band structure,and density of states)were used to rationalize the mechanism of external anchoring for Pt clusters or nanowires on the modified graphene.
2 Computational section
All calculations were performed using the Vienna ab initio Simulation Package(VASP)code35,36based on density functional theory.The projector augmented wave(PAW)potentials were used to describe the interaction between ions and electrons.37,38The nonlocal exchange correlation energy was evaluated using the Perdew-Burke-Ernzehof(PBE)functional.The electron wave function was expanded using plane waves with an energy cutoff of 400 eV.A 4×4×1 Monkhorst-Pack k-point mesh was used.In this study,every 32-atom graphene slab was modified by one 1,3-dipolar cycloaddition,which derived from experimental literature reported.34The supercell was chosen to be 0.9880 nm×0.8556 nm×2 nm.The adsorption energy(Ead)of noble metal clusters or nanowires on DDC graphene is calculated as follows:

where EMn+G,EMn,and EGrepresent the energies of the combined systems of Mn(n denotes the number of metal atoms)and modified graphene,the most stable gas phase of metal cluster or nanowire,and the modified graphene,respectively.
3 Results and discussion
3.1 Deprotonation of 1,3-dipolar cycloaddition graphene
We firstly investigated the adsorption of Pt monomer and dimer on 1,3-dipolar cycloaddition(DC)graphene.It is found that the most stable site for Pt monomer is ortho-carbon of nitrogen in 1,3-dipolar cycloaddition graphene,20with the hydrogen atom transfer from ortho-carbon to the platinum atom(Fig.1).Several different initial configurations had been optimized,we always found that hydrogen transfers from carbon to Pt once Pt adsorbed.The only stable geometry in which hydrogen does not transfer is Pt bonding with nitrogen.The adsorption energies of Pt monomer on ortho-carbon and nitrogen are-2.16 and-1.21 eV,respectively.Similarly,the most stable structure of Pt dimer is both of Pt located at atop sites of the two ortho-carbons of nitrogen,leading to the hydrogen atoms transfering from carbon to platinum.The adsorption energy of Pt dimer on the ortho-carbons is-1.68 eV,which is much more stable than that on the nitrogen atoms(-0.68 eV).It indicates that the hydrogen transfer is easily occurred after one or two Pt atoms adsorption.Therefore,in the following section,the DDC graphene is served as the model to investigate the adsorption of small Pt clusters/nanowires.
3.2 Formation of small Pt clusters on deprotonated 1,3-dipolar cycloaddition graphene

Fig.1 Optimized geometries of Ptn(n=1-2),Ptn(n=3-6)on 1,3-dipolar cycloaddition graphene,deprotonated 1,3-dipolar cycloaddition graphene
The adsorption of small noble metal clusters(Ptn(n=3-6))on DDC graphene is investigated(Fig.1).For Pt3configurations,the triangle Pt structures are suspended via two Pt atoms adsorbing on the top sites of ortho-carbons or one Pt adsorbing on bridge site of the two neighboring ortho-carbons,the adsorption energies are-2.67 and-2.26 eV,respectively.For Ptn(n=4-6)clusters,the same rule is found,in which the orthocarbon acts as anchoring sites via bonding with one or two Pt atoms.Different from internal anchoring site of carbon vacancy,in which more than two or three Pt bond with vacancy even for Pt3or Pt4clusters,39while the ortho-carbon of nitrogen acts as external anchoring sites only bound with one or two Pt atoms for these small clusters Ptn(n=3-6).
3.3 Formation of Pt nanowires on deprotonated 1,3-dipolar cycloaddition graphene
From the above analysis,the anchoring site of DDC graphene for small Pt clusters is the ortho-carbon of nitrogen by bonding with one or two Pt atoms.It is very interesting to investigate the growth of noble metal nanowires between two neighboring deprotonated pyridine alkyne units.Inspired by one recent experimental study,it reported that gold binding to the graphene can easily form nanorods on 1,3-dipolar cycloaddition graphene34,we investigate Pt nanowires grown on DDC graphene,as shown in Fig.2.
Pt3is used as the"bridge"to connect two neighboring deprotonated pyridine alkyne(PY)units,which form the PY-Pt3-PY one-dimensional nanowire.Ptn(n=4-6)nanowires can form based on the Pt3nanowire adsorption configuration.Compared with Pt3nanowire,Pt4forms an integrated zigzag nanowire.For Pt5and Pt6nanowires,whole configuration appears flat growth trend.The adsorption energies for Ptn(n=4-6)nanowire are-4.11,-4.02,and-4.83 eV,respectively.The adsorption strengths for them are much stronger than the corresponding most stable Ptn(n=4-6)clusters by more than 1.5 eV,while the adsorption strength of Pt3nanowire is just a little stronger than Pt3cluster.The Pt―C bond distances vary from 0.204 to 0.215 nm,and Pt―Pt bond distances vary from 0.245 to 0.265 nm depending on the nanowire size and shape.It is seen that the orthocarbon of nitrogen in DDC graphene again acts as the anchoring site for nanowires via bonding with one or two metal atoms,which provides another explanation why gold nanorods are easily formed on 1,3-dipolar cycloaddition graphene.34
3.4 Electronic properties modification of deprotonated 1,3-dipolar cycloaddition graphene by clusters and nanowires
In order to rationalize the above mentioned results,the average bader charge of carbon,nitrogen,and Pt in different systems were analyzed,as summarized in Table 1.We can find that in DDCG,the charges of nitrogen and ortho-carbon are-2.674e and 1.000e,respectively.While in C-DCG,the charge of carbon that replaces nitrogen is 0.277e,and the“ortho-carbon”is-0.155e.It indicates that nitrogen induces huge charge changes in ortho-carbon and makes these ortho-carbons act as adsorption sites for noble metal clusters and nanowires.
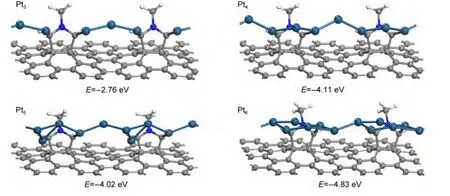
Fig.2 Pt nanowires on deprotonated 1,3-dipolar cycloaddition graphene
Charge density difference is another important electronic property for analyzing local bonding process or charge transfer.Generally,we calculate the required charge density difference according to the following equation:

where Δρrepresents charge density difference,ρMn+G,ρMn,and ρGare the charge densities of noble metal on DDC graphene,pure noble metal,and pure DDC graphene,respectively.For DDC graphene,the more electronegative N atom(electronegativityof 3.04)leads the ortho-carbons of nitrogen to be obviously electropositive(Fig.3(b)).According to the studies of N-doped graphene,the active site locates at the atoms with high charge density.40Therefore,ortho-carbons of nitrogen act as active sites,which is in agreement with the configurations mentioned above.As comparison,the charge density of DC graphene distributes rather average(Fig.3(a)).From Fig.3(c,d),we can easily find that the metal atom bonding to the modified graphene mainly shows charge depletion,while other metal atoms show slight charge accumulation.The bonding property of metal on DDC graphene coincides with average Bader charge analysis and work function.The work functions of Pt6and DDC graphene are 5.55 and 3.96 eV,respectively.It is seen that the adsorbed metal clusters or nanowires induce charge redistribution again(Table 1 and Fig.3).For Pt cluster,the electrons of nitrogen transfer to carbon and the adsorbed metal,which leads to the charge reduction of nitrogen and carbon atoms and negative charge of whole metal clusters(Table 1).While for Pt nanowire,in addition to the electron of nitrogen transfering to carbon and the adsorbed metal,the electrons also transfer from bonding Pt to carbon,which explains the positive charge of bonding metal.For Pt clusters adsorbed on carbon vacancy graphene20or CNTs,39the positive charge is also identified.The unique electronic properties of DDC graphene lead to the negative charge of metal clusters or nanowires.

Table 1 Average Bader charge(e)anaysis of 1,3-dipolar cycloaddition graphene with or without Pt6adsorption

Fig.3 Calculated charge density difference of(a)DC graphene,(b)DDC graphene,(c)Pt6cluster/DDC graphene,and(d)Pt6nanowire/DDC graphene
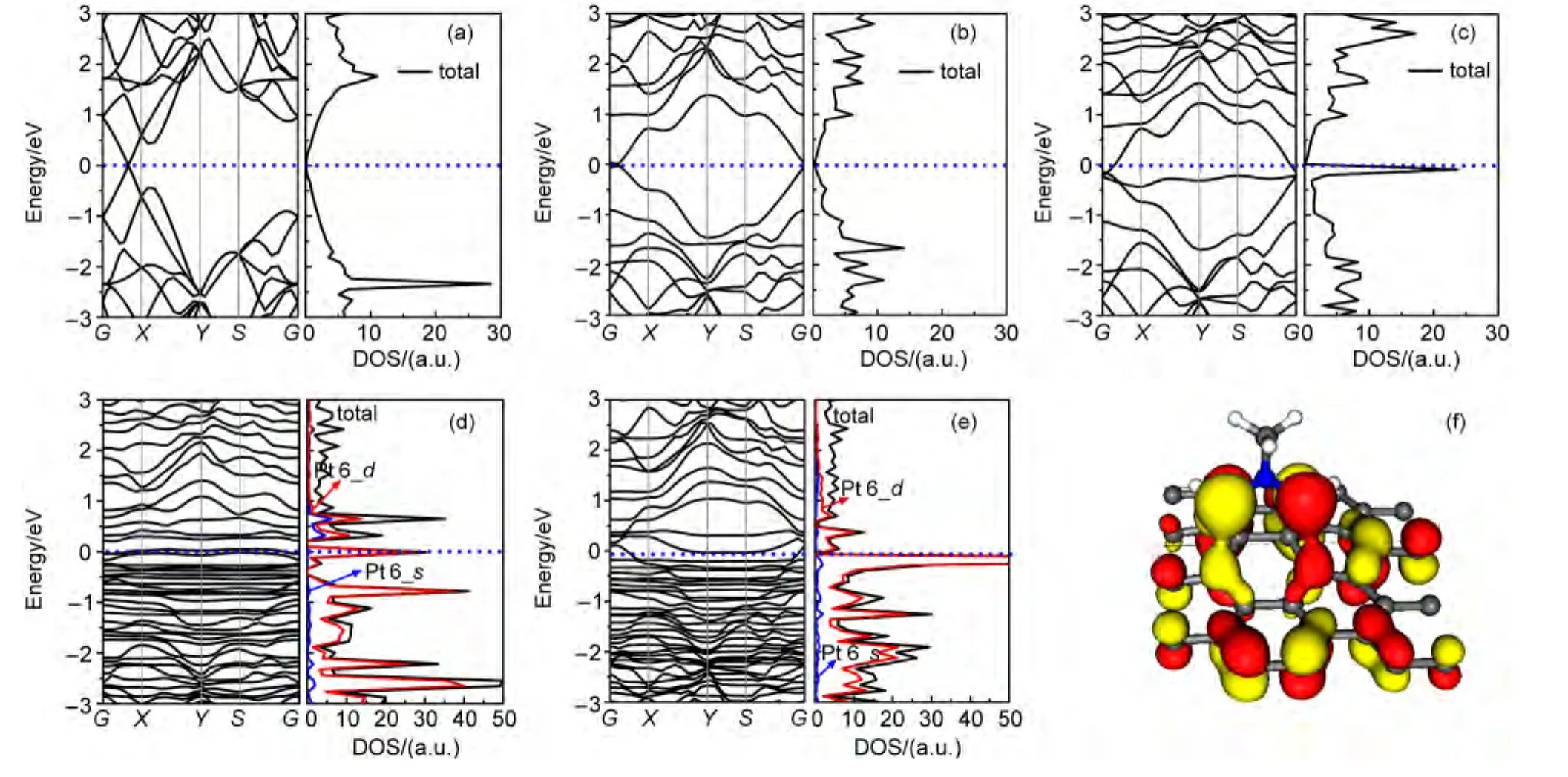
Fig.4 Band structures and DOS of(a)graphene,(b)DC graphene,(c)DDC graphene,(d)Pt6cluster/DDC graphene,(e)Pt6nanowire/DDC graphene,and(f)the highest occupied molecular orbit(HOMO)of DDC graphene
To further investigate the electronic structure of the materials,density of states(DOS)and band structure calculations are performed.The result of DFT calculation reveals that pristine graphene and DDC graphene are semimetallic(Fig.4(a-c)).Detailed difference between them can be concluded:(a)disappearance of dirac point and one strong DOS peak at about 2.4 eV below Fermi level in DDC graphene;(b)emergence of new remarkable DOS peak of DDC graphene at the vicinity of Fermi level;(c)reducing of the dispersion degrees of bands both in valance band(VB)and conduction band(CB)in DDC graphene.Generally,the phenomenon may be due to the slightly broken conjugated system of graphene induced by deprotonated 1,3-dipolar cycloaddition.In this regard,particular attention must be drawn on the new DOS peak of DDC graphene at the vicinity of Fermi level,which plays an important role in anchoring effect of DDC graphene.To further understand it,an orbit analysis at the Gamma point was performed for deprotonated 1,3-dipolar cycloaddition graphene(Fig.4(f)).Noticeably,the highest occupied molecular orbital(HOMO)mainly involves the localized bond of the two ortho-carbons of nitrogen in the deprotonated 1,3-dipolar cycloaddition and the bonding orbits of graphene.Therefore,it is observed that the two orthocarbons of nitrogen in the DDC graphene serve as anchoring sites.As displayed in Fig.4(d,e),the band structures show that Pt metal adsorptions never essentially change the electronic characteristic of DDC graphene substrate and only mix with some impurity bands of doped metals,consisting of d and s bands.Furthermore,the doped states differently influence the electronic properties of Pt/DDC graphene.Pt6cluster makes the composite be metallic,while Pt6nanowire adsorption composite keeps semimetallic,which might have new applications in the electronic or photovoltaic devices of such composite materials.
4 Conclusions
From our study,several important issues concerning the adsorption of noble metal Pt on deprotonated 1,3-dipolar cycloaddition graphene are clear:(1)Pt atom adsorption leads to deprotonation of 1,3-dipolar cycloaddition graphene;(2)ortho-carbon of nitrogen acts as anchoring site of the DDC graphene for noble metals,with the average Bader charge as high as 1.0e.(3)Ptn(n=3-6)nanowire adsorption configurations are more stable than the corresponding Ptn(n=3-6)clusters on the DDC graphene;(4)electronic structure analysis shows that the electronic characteristics of DDC graphene is not essentially changed by the Pt metal adsorptions,the doped states introduced by Pt metal differently influence the electronic properties of Pt/DDC graphene,Pt6nanowire adsorption composites keep semimetallic,while Pt6cluster adsorption composite is metallic.
(1) Chan,K.T.;Neaton,J.B.;Cohen,M.L.Phys.Rev.B 2008,77,235430.doi:10.1103/PhysRevB.77.235430
(2) Chang,S.W.;Nair,A.K.;Buehler,M.J.J.Phys.-Condes.Matter 2012,24,245301.doi:10.1088/0953-8984/24/24/245301
(3) Wang,S.Y.;Jiang,S.P.;Wang,X.Electrochim.Acta 2011,56,3338.doi:10.1016/j.electacta.2011.01.016
(4)Xu,C.;Wang,X.;Zhu,J.W.J.Phys.Chem.C 2008,112,19841.doi:10.1021/jp807989b
(5) Muszynski,R.;Seger,B.;Kamat,P.V.J.Phys.Chem.C 2008,112,5263.doi:10.1021/jp800977b
(6) Entani,S.;Sakai,S.;Matsumoto,Y.;Naramoto,H.;Hao,T.;Maeda,Y.J.Phys.Chem.C 2010,114,20042.doi:10.1021/jp106188w
(7)Wang,W.L.;Ma,Z.F.Acta Phys.-Chim.Sin.2012,28,2879.[王万丽,马紫峰.物理化学学报,2012,28,2879.]doi:10.3866/PKU.WHXB201209252
(8) Palacios,J.J.;Fernandez-Rossier,J.;Brey,L.Phys.Rev.B 2008,77,195428.10.1103/PhysRevB.77.195428
(9) Boukhvalov,D.W.;Katsnelson,M.I.Nano Lett.2008,8,4373.doi:10.1021/nl802234n
(10) Cretu,O.;Krasheninnikov,A.V.;Rodriguez-Manzo,J.A.;Sun,L.T.;Nieminen,R.M.;Banhart,F.Phys.Rev.Lett.2010,105,196102.doi:10.1103/PhysRevLett.105.196102
(11) Lahiri,J.;Lin,Y.;Bozkurt,P.;Oleynik,I.I.;Batzill,M.Nat.Nanotechnol.2010,5,326.
(12) Lim,D.H.;Negreira,A.S.;Wilcox,J.J.Phys.Chem.C 2011,115,8961.doi:10.1038/nnano.2010.53
(13)Srivastava,M.K.;Wang,Y.;Kemper,A.F.;Cheng,H.P.Phys.Rev.B 2012,85,165444.doi:10.1103/PhysRevB.85.165444
(14)Dai,X.Q.;Li,Y.H.;Zhao,J.H.;Tang,Y.N.Acta Phys.-Chim.Sin.2011,27,369.[戴宪起,李艳慧,赵建华,唐亚楠.物理化学学报,2011,27,369.]doi:10.3866/PKU.WHXB20110224
(15) Liu,H.T.;Liu,Y.Q.;Zhu,D.B.J.Mater.Chem.2011,21,3335.doi:10.1039/c0jm02922j
(16)Muhich,C.L.;Westcott,J.Y.;Morris,T.C.;Weimer,A.W.;Musgrave,C.B.J.Phys.Chem.C 2013,117,10523.
(17)Wei,D.C.;Liu,Y.Q.;Wang,Y.;Zhang,H.L.;Huang,L.P.;Yu,G.Nano Lett.2009,9,1752.
(18) Jafri,R.I.;Rajalakshmi,N.;Ramaprabhu,S.J.Mater.Chem.2010,20,7114.doi:10.1021/nl803279t
(19)Wu,X.Q.;Zong,R.L.;Mu,H.J.;Zhu,Y.F.Acta Phys.-Chim.Sin.2010,26,3002.[吴小琴,宗瑞隆,牟豪杰,朱永法.物理化学学报,2010,26,3002.]doi:10.3866/PKU.WHXB20101010
(20) Xie,P.Y.;Zhuang,G.L.;Lü,Y.A.;Wang,J.G.;Li,X.N.Acta Phys.-Chim.Sin.2012,28,331.[解鹏洋,庄桂林,吕永安,王建国,李小年.物理化学学报,2012,28,331.]doi:10.3866/PKU.WHXB201111021
(21)Wehling,T.O.;Novoselov,K.S.;Morozov,S.V.;Vdovin,E.E.;Katsnelson,M.I.;Geim,A.K.;Lichtenstein,A.I.Nano Lett.2008,8,173.doi:10.1021/nl072364w
(22) Boukhvalov,D.W.;Katsnelson,M.I.Phys.Rev.B 2008,78,085413.doi:10.1103/PhysRevB.78.085413
(23) Medeiros,P.V.C.;Mascarenhas,A.J.S.;Mota,F.D.;de Castilho,C.M.C.Nanotechnology 2010,21,485701.doi:10.1088/0957-4484/21/48/485701
(24) Xu,Y.F.;Liu,Z.B.;Zhang,X.L.;Wang,Y.;Tian,J.G.;Huang,Y.;Ma,Y.F.;Zhang,X.Y.;Chen,Y.S.Adv.Mater.2009,21,1275.doi:10.1002/adma.v21:12
(25) Georgakilas,V.;Bourlinos,A.B.;Zboril,R.;Steriotis,T.A.;Dallas,P.;Stubos,A.K.;Trapalis,C.Chem.Commun.2010,46,1766.doi:10.1039/b922081j
(26) Bosch-Navarro,C.;Coronado,E.;Marti-Gastaldo,C.Carbon 2013,54,201.doi:10.1016/j.carbon.2012.11.027
(27) Georgakilas,V.;Kordatos,K.;Prato,M.;Guldi,D.M.;Holzinger,M.;Hirsch,A.J.Am.Chem.Soc.2002,124,760.doi:10.1021/ja016954m
(28)Tasis,D.;Tagmatarchis,N.;Bianco,A.;Prato,M.Chem.Rev.2006,106,1105.doi:10.1021/cr050569o
(29) Singh,P.;Campidelli,S.;Giordani,S.;Bonifazi,D.;Bianco,A.;Prato,M.Chem.Soc.Rev.2009,38,2214.doi:10.1039/b518111a
(30)Maggini,M.;Scorrano,G.;Prato,M.J.Am.Chem.Soc.1993,115,9798.doi:10.1021/ja00074a056
(31) Tagmatarchis,N.;Prato,M.Synlett 2003,6,768.
(32) Tagmatarchis,N.;Prato,M.J.Mater.Chem.2004,14,437.doi:10.1039/b314039c
(33) Prato,M.J.Mater.Chem.1997,7,1097.doi:10.1039/a700080d
(34)Quintana,M.;Spyrou,K.;Grzelczak,M.;Browne,W.R.;Rudolf,P.;Prato,M.ACS Nano 2010,4,3527.doi:10.1021/nn100883p
(35) Kresse,G.;Furthmüller,J.Comput.Mater.Sci.1996,6,15.doi:10.1016/0927-0256(96)00008-0
(36) Kresse,G.;Furthmüller,J.Phys.Rev.B 1996,54,11169.doi:10.1103/PhysRevB.54.11169
(37) BlöCHL,P.E.Phys.Rev.B 1994,50,17953.doi:10.1103/PhysRevB.50.17953
(38) Kresse,G.;Joubert,D.Phys.Rev.B 1999,59,1758.
(39)Wang,J.G.;Lv,Y.A.;Li,X.N.;Dong,M.D.J.Phys.Chem.C 2009,113,890.doi:10.1021/jp810277b
(40) Zhang,L.P.;Xia,Z.H.J.Phys.Chem.C 2011,115,11170.

