炭化米糠经臭氧活化制备活性炭及其去除Cr(VI)离子
Sivaraju Sugashini,Kadhar Mohamed Meera Sheriffa Begum
(Department of Chemical Engineering,National Institute of Technology,Tiruchirappalli 620015,Tamilnadu,India)
1 Introduction
The increase in industrial,domestic and agricultural activities leads to the generation of waste water containing harmful pollutants,which contaminate the fresh water.Among various harmful pollutants,heavy metals are of great concern and hence must be removed from water.One of the most harmful heavy metal is chromium.Chromium exists in two forms viz Cr (III)ions and Cr (VI)ions,which are generated from various industrial processes such as electropla ting,leather tanning,mining,dyes and pigments[1,2].Cr (III)ions are non toxic and play an essential role in the metabolism of plant and animals.Cr (VI)ions are highly toxic.Inhalation of Cr (VI)ions leads to the carcinogenetic problem.Other health effects of Cr(VI)ions are the skin allergy,liver and stomach problems.
Due to its severe toxicity,the World Health Organization set the tolerance limits of Cr (VI)ions in surface and potable water are 0.1 and 0.05 mg/L,respectively.Thus the removal of Cr (VI)ions becomes mandatory.Various methodologies have been used for the removal of Cr (VI)ions like electro chemical,ion exchange,membrane filtration,reverse osmosis and chemical coagulation,et al[3].However,these methods have their own shortcomings and limitations such as non economical,high reagent and energy requirements,generation of huge quantity of hazardous sludge that require a further disposal or treatment,and fouling of resin and membrane.
One of the most highly efficient methods is adsorption,owing to its simplicity,sludge free operation,easiness in handling,availability of various adsorbents and more efficient in removal of heavy metals down to a much low level[4].
Several investigators used different adsorbents for the removal of Cr (VI)ions such as activated carbons[5],chitosan[6],biosorbents[7],polymeric compounds[8].Activated carbon is a common adsorbent because of its high surface area and easy availability.However,commercially available activated carbon is highly expensive.This led to the search of activated carbons at low production cost using biomass.Rice husk is one of the cheapest biomass,in which the constituents of rice husk like cellulose (55%-60%),hemicellulose (20%-25%)and lignin (20%-25%)are precursors for the preparation of activated carbon[9,10].
The carbonized raw sources are further activated by treating them with chemicals such as acids,bases and oxidizing agents to increase the metal adsorption capacity and selectivity.Mostly,oxidizing agents are used to enhance the oxygen functional groups on the surface of the carbonized raw material,which are responsible for increasing the adsorption capacity[11].Recently,a powerful activating agent ozone was used to treat the commercial activated carbon[12]to modify its surface properties for removal of mercury[13]and volatile organic carbons[14].
In this paper,ozone was used to activate carbonized rice husk to prepare activated carbons for the removal of Cr (VI)ions from aqueous solutions.Adsorption isotherms were established to explain the solute and solvent interaction mechanism.Various kinetic models were used to determine the rate and adsorption kinetics.The adsorption thermodynamic was also investigated to evaluate the spontaneous nature of the adsorption process.
2 Materials and method
2.1 Materials
Raw rice husk was purchased from the local rice mill.Potassium dichromate was used for the preparation of Cr (VI)stock solutions.The pH value of the solutions was maintained by hydrochloric acid -potassium chloride for the pH range of 1-3,acetic acid -sodium acetate for the pH range of 4-6 and boric acid-sodium hydroxide for the pH range of 8-10[15].Analytical grade reagents of 1,5-diphenyl carbazide and acetone were used for analyzing the chromium ions.
2.2 Preparation of ozone treated rice husk carbon
Rice husk (size 2 mm)was soaked in water overnight and then filtered to remove the excess water.25 g of wet rice husk was carbonized at 800oC for 160 min under the N2flow at a heating rate of 5oC/min.The carbonaceous yield was 23.4%.Ozone was generated in a homemade dielectric barrier discharge reactor operating at an atmospheric pressure.Ozone discharge was created by applying a high voltage between 12.5 to 25 kV at 50 Hz frequency.The reactor was operated at 200 J/L (20 kV and 50 Hz)to get the ozone concentration around 1 100 mg/L.The carbon samples were placed at the outlet of the reactor and allowed to react with ozone for 3 h.
2.3 Characterization
The chemical characterization of adsorbent was performed using EDAX analysis.The specific surface area of the carbonized material was measured by using GeminiV2.00 Micromeritics.The morphological structure was observed by a Hitachi S3000 H scanning electron microscope.The crystallographic nature was measured by a Rigeku Ultima3 X Ray Diffractometer.FT-IR spectra were recorded using a Perkin Elmer,spectrum RXI FT-IR spectrophotometer to analyze the functional groups present in the adsorbent.
2.4 Adsorption studies
Adsorption were carried out for determining the effect of time and agitation speed on equilibrium for a known concentration of feed solution.Batch adsorption experiments were conducted to study the effect of initial metal ion concentration,adsorption dosage,agitation speed and pH value of the solution on equilibrium time in a temperature controlled rotary shaker.The supernatant liquid samples were filtered and then analyzed using a Jasco UV spectrophotometer at 540 nm to calculate the adsorption capacity and removal percentage.Experiments were repeated in triplicates and the average deviation was found to be 3%-5%.The amount of adsorption at equilibrium,qe(mg/g)and removal percentage (%)were calculated using the equations (1)and (2),respectively.

Where C0and Ceare the initial and equilibrium concentrations (mg/L),V is the volume of solution(L),qeis the adsorption capacity (mg/g),m is the weight of adsorbent (g)and C is the solution concentration at the end of the adsorption (mg/L).
3 Results and discussion
3.1 Characterization of ozone treated rice husk carbon
The chemical characteristics of untreated and ozone treated rice husk carbon by EDAX analysis is presented in Table 1.From Table 1,it is observed that the amount of carbon was increased after treatment with ozone.This may be ascribed to the silica removal and carbon enrichment,resulting from loosened silica attached to the carbonaceous material during ozone treatment.

Table 1 Chemical characteristics of untreated and ozone treated carbonized rice husk.
The specific surface areas of the untreated and the ozone-treated rice husk carbons were found to be 20 and 380 m2/g,respectively.The increase in the surface area can be explained as following.Initially the surface of the carbon was plain and smooth.On ozone treatment,ozone exists as atomic oxygen and molecular oxygen.The molecular oxygen is not active.But,the atomic oxygen oxidizes the surface of the carbon into various functional groups,which made the surface of the carbon irregular and porous[16].
The surface morphology of the untreated and the ozone-treated rice husk carbon was observed using scanning electron microscopy.Fig.1 shows the SEM images of (a)the untreated and (b)the ozone-treated rice husk carbons.These images reveal the surface texture and porosity of the untreated and the ozone-treated rice husk carbons.The plain and smooth surface was observed from the image of the untreated rice husk carbon,whereas the rough surface with small pores was observed from the image of the ozone-treated one[16].

Fig.1 SEM images of (a)untreated and (b)ozone treated carbonized rice husk.
This confirms the change of surface texture,resulting from the ozone treatment on rice husk carbon.
Fig.2 shows the FT-IR spectra of the untreated and the ozone-treated rice husk carbons.The peaks at 3 415,2 147,1 563,1 420 and 652.81 cm-1were observed,which are assigned to OH broad stretching vibration,aldehydes,medium bending of alkynes and OH bond out of planes,for the untreated rice husk carbon,respectively.On ozone treatment,the peaks at 3 420 and 3 130 cm-1were shifted from the peaks at 3 415 and 2 147 cm-1,which are assigned to hydrogen bonded hydroxyls involved in phenolic and carboxylic groups,respectively.The peak at 2 350 cm-1is associated to the ketonic group,that at 1 641cm-1may be due to the presence of carbonyl group or quinone[16],and that at 787.2 cm-1may be due to peroxide but weakly active.Ozone exists as both molecular and atomic oxygen on the surface of carbon.Atomic oxygen is a powerful oxidizing agent,which oxidizes the carbon surface into acidic functional groups such as carboxylic,ketonic and phenolic on ozone treatment.These functional groups increased the uptake capacity of Cr (VI)ions.

Fig.2 FT-IR spectra of(a)untreated and(b)ozone treated carbonized rice husk.
Fig.3 shows the XRD pattern for the ozone-treated rice husk carbon.The XRD pattern reveals the bulk and amorphous structure of the carbon.The first peak at 2θ value of 23.17ois referred to the d002of graphitic carbon and the peak at 2θ value of 45.220oto d101of graphitic carbon.
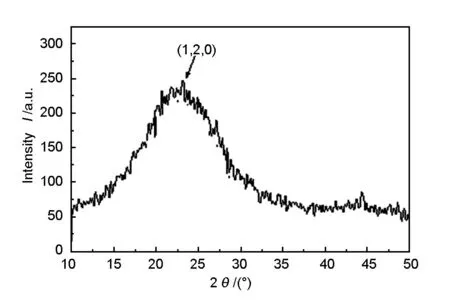
Fig.3 XRD pattern of ozone treated carbonized rice husk.
3.2 Effect of contact time
The effect of contact time on the adsorption capacity of Cr (VI)ions is given in Fig.4.It is observed that the adsorption capacity increased with contact time from 4.6 to 8.7 mg/g for the solution with an initial Cr (VI) ion concentration of 50 mg/L and from 9.8 to 13.1 mg/g for the solution with an initial Cr (VI)ion concentration of 100 mg/L.Maximum adsorption capacity was reached at 100 min,beyond which the adsorption capacity became constant,indicating that equilibrium was gradually established.

Fig.4 Effect of contact time on adsorption capacity of Cr (VI)ions(initial conc:100 mg/L and 50 mg/L,adsorbent dosage:0.2 g/50 mL,pH:2,agitation speed:300 r/min).
3.3 Effect of agitation speed
Fig.5 shows the effect of agitation speed on adsorption capacity of Cr (VI)ions by varying the agitation speed from 200 to 350 r/min for the solution with an initial Cr (VI)ion concentration of 50 mg/L at pH=2 and the adsorbent dosage of 0.2 g.The rate of adsorption and the adsorption capacity increased with agitation speed.The maximum adsorption capacity was obtained at 300 r/min.Hence the agitation speed was fixed at 300 r/min.

Fig.5 Effect of agitation speed on adsorption capacity of Cr (VI)ions (initial conc:50 mg/L,pH:2,adsorbent dosage:0.2 g/50 mL).
3.4 Effect of pH value
The adsorption of Cr (VI) ions is highly influenced by pH value of the solution.Fig.6 shows that the adsorption capacity was found to be higher at lower pH value,and lower at higher pH value.At pH value of 2,the Cr (VI)ions exist as hydrogen chromate(HCrO4-:90%),dichromate (Cr2O7-:5%)and chromic acid (H2CrO4:5%)[17].At pH value higher than 2,the predominant species of Cr(VI)ion is CrO42-.The surface positive functional groups of the carbon after the ozone treatment carried the oxyanions (negatively charged)of Cr(VI) ions by electrostatic force of attraction.Therefore,the adsorption capacity was higher at lower pH value.At higher pH value,the negative charges on the adsorbent surface is higher,which decreased the attraction of oxyanions of CrO42-on the adsorbent.Hence,the pH value was optimized at 2.0 for maximum uptake capacity of Cr (VI)ions.
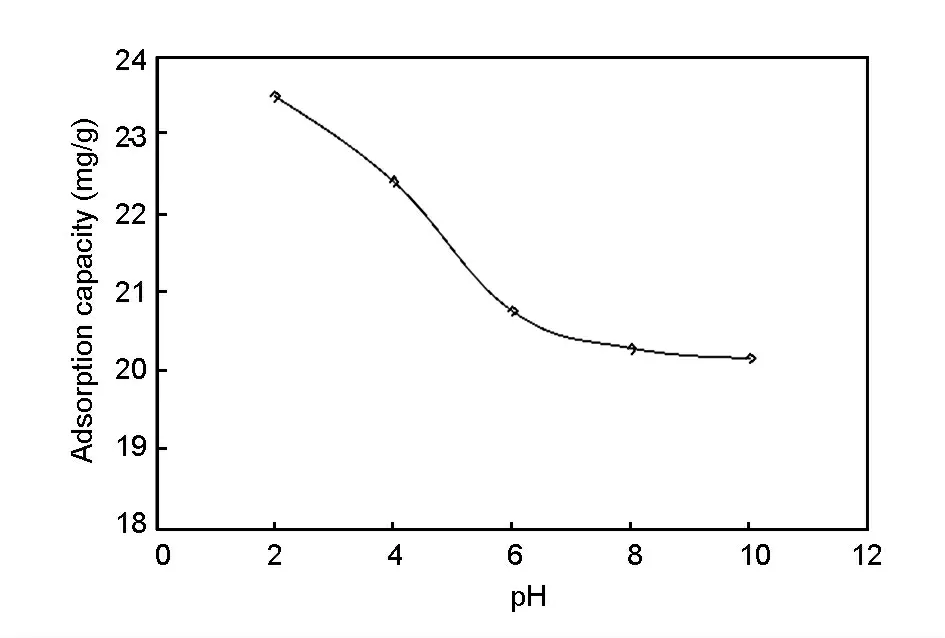
Fig.6 Effect of pH value on adsorption capacity of Cr (VI)ions(initial conc:100 mg/L,agitation speed:300 r/min,adsorbent dosage:0.2 g/50 mL).
3.5 Effect of adsorbent dosage
Effect of adsorption dosage on adsorption capacity and removal percentage of Cr (VI)ions was studied by varying the adsorbent dosage from 0.2 to 1g and the results are presented in Fig.7.

Fig.7 Effect of adsorbent dosage on adsorption capacity and percentage removal of Cr (VI)ions(initial conc:100 mg/L,agitation speed:300 r/min,pH:2).
From Fig.7,it is observed that the adsorption capacity decreased and removal percentage of Cr(VI)ions increased with adsorbent dosage.The similar trends were reported by other investigators[18,19].The decrease in adsorption capacity might be attributed to the shortage of metal ion concentration in the solution since the initial metal ion concentration was kept constant for all dosages investigated.
3.6 Effect of initial concentration
The initial Cr (VI)ion concentration was varied from 50 to 250 mg/L in order to evaluate the effect of Cr (VI)ion concentration on the uptake capacity and the removal percentage.The results are shown in Fig.8.The adsorption capacity of Cr(VI)ions increased and the removal percentage of Cr (VI) ions decreased with Cr (VI) ion concentration.The increase in adsorption capacity with Cr (VI)ion concentration may be ascribed to the higher utilization rates of active sites for the adsorption at higher concentrations.The decrease in the removal percentage may be attributed to the limited number of actives sites in the adsorbent above certain concentration[20].Thus the maximum adsorption capacity was 52.13 mg/g and the maximum removal percentage was 95% at an initial Cr (VI)ion concentration of 250 and 50 mg/L,respectively.

Fig.8 Effect of initial concentration on adsorption capacity and percentage removal of Cr (VI)ions (pH:2,agitation speed:300 r/min,adsorbent dosage:0.2 g/50 mL).
3.7 Effect of temperature
The effect of temperature on adsorption of chromium ions was investigated by varying the adsorption temperature from 30 to 70oC as shown in Fig 9.
The maximum adsorption capacity was observed at 35oC.This may be ascribe to the fact that the kinetic energy of oxyanions[21,22]such as HCrO4-,H2CrO4and Cr2O7-at lower temperatures are lower,which helps to contact sufficiently between the adsorbate and adsorbent.However,the kinetic energy is higher than the electrostatic attraction between the adsorbent and the Cr (VI)ions at higher temperature,which lead to a decrease of the adsorption capacity with temperature.The removal percentage decreased with temperature may be attributed to the fact that desorption of Cr (VI)ions caused by an increase in thermal energy is high.

Fig.9 Effect of temperature on adsorption capacity of Cr (VI)ions(pH:2,agitation speed:300 r/min,adsorbent dosage:0.2 g/50 mL,initial concentration:100 mg/L).
3.8 Adsorption isotherms and Chi square analysis
Various isotherms like Freundlich,Langmuir and Temkin isotherms[23]were applied to describe the equilibrium characteristics of adsorption of Cr(VI)ions by the ozone-treated rice husk carbon.In the Langmuir equation,θ (mg/g)is the measure of adsorption capacity under the experimental conditions and b is a constant related to the energy of adsorption.In Freundlich isotherm,n is indicative of bond energies between metal ion and the adsorbent and Kfis related to bond strength.a and b are Temkin constants.
The non linear Chi square analysis[23]was used to compare all the isotherms.The mathematical equation was given by Eq.(3)
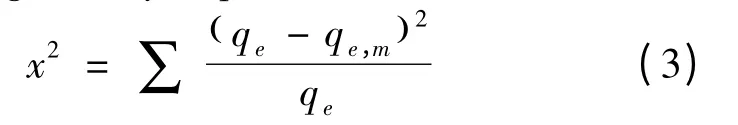
Where qe,mis the equilibrium capacity obtained by calculation from model (mg/g)and qeis the equilibrium capacity (mg/g)determined from the experimental data.If data from model are similar to the experimental data then x2would be a small number and vice versa[23].In linear analysis the different forms of equation would affect the regression coefficient (r) and coefficient of determination (r2)value significantly,which will affect the final results.This can be avoided by using nonlinear Chi square test analysis.
The linearized form of isotherms,values of constants,coefficient of determination (r2),regression coefficient (r) and Chi square test analysis (x2)are given in Table 2.
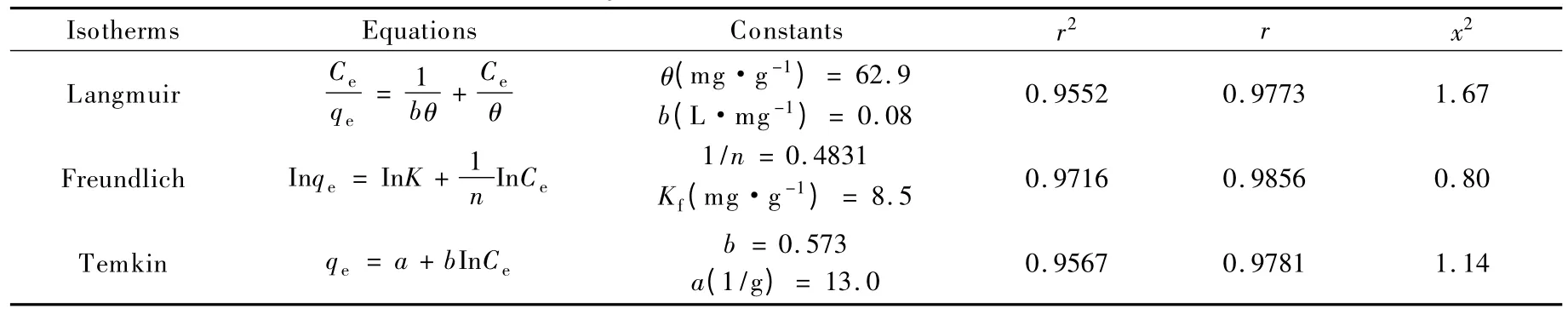
Table 2 Summary of constants for various isotherm models.
Fig.10 shows the comparison between theoretical isotherms and experimental data obtained for adsorption of Cr (VI)ions using the ozone-treated rice husk carbon.It is observed that the Freundlich isotherm seemed to be best when the experimental data were fitted by models.Correspondingly,the Chi square test analysis also showed that the x2value for Freundlich is very low compared with Langmuir and Temkin isotherms.This reveals that the multilayer adsorption of Cr(VI)ions on the ozone-treated rice husk carbon is impossible.
3.9 Adsorption kinetics
In order to investigate the rate of adsorption of chromium by the ozone-treated rice husk carbon,three different kinetic models,the pseudo first order kinetics,the pseudo second order kinetics and the simple elovich kinetics were tested[6].The linearized form of adsorption kinetics and their constants are given in Table 3.
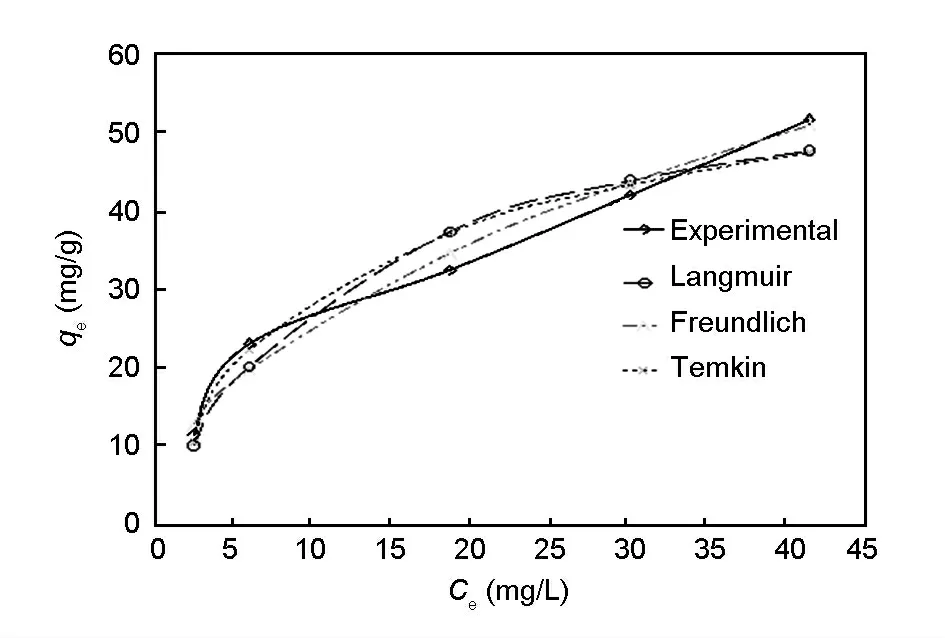
Fig.10 Theoretical isotherms and experimental data for adsorption of Cr (VI)ions using ozone treated rice husk carbon.
In the pseudo first order kinetics,qtis the adsorption capacity at time t (mg/g)and K1adis the pseudo-first-order rate constant in min-1.In the pseudo second order kinetics,K2adis the pseudo-second-order rate constant in 1/mol·s.In the simple elovich kinetics,α and β are the simple elovich kinetic constants.
From Table 3,it is confirmed that the adsorption of Cr (VI)ions using the ozone-treated rice husk carbon followed the pseudo second order kinetics and is shown in Fig.11.The pseudo second order kinetics model indicates that the adsorption of Cr (VI)ions on the surface of the ozone-treated rice husk carbon represented two phase reaction,a rapid adsorption for a short duration in the initial stage followed by a slow adsorption for a long duration at late stage.Several investigators[24,25]reported that the fast reaction may be attributed to chemisorption involving valence forces through exchange or sharing of electron between the adsorbent and adsorbate.The slow reaction is ascribed to the diffusion of ions into the adsorbent.Hence the rate limiting step is chemisorption.
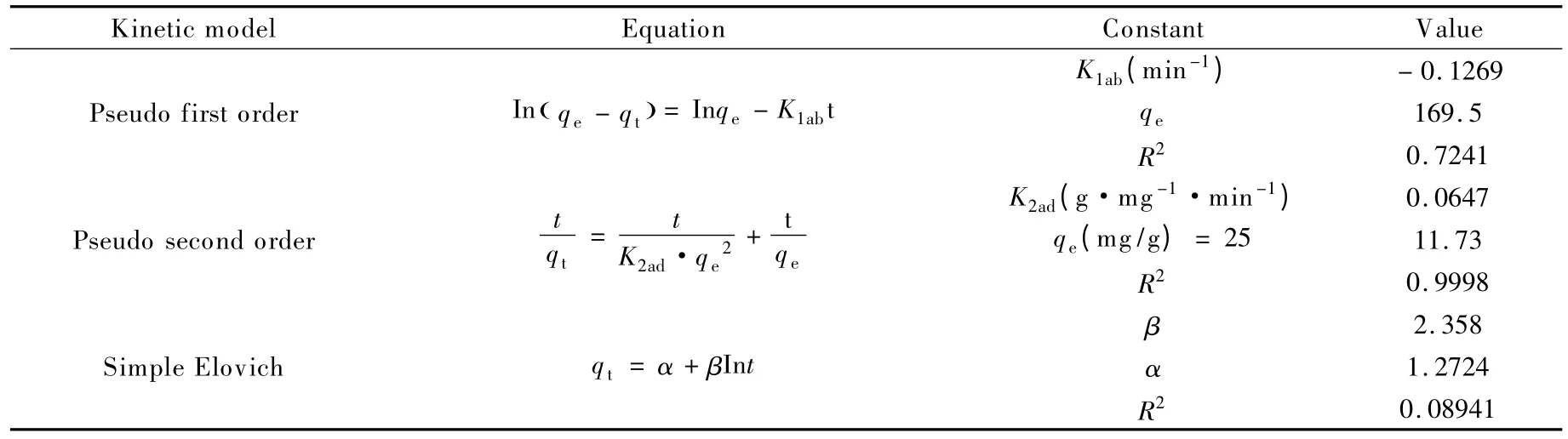
Table 3 Summary of constants for various kinetic models.
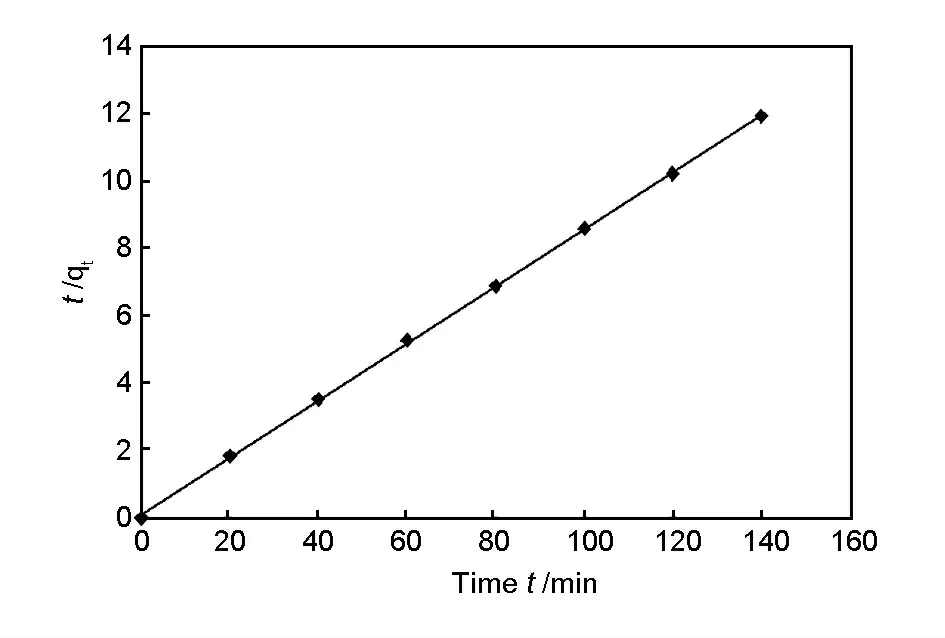
Fig.11 Pseudo second order kinetics for adsorption of Cr (VI)ions using Ozone treated rice husk carbon.(C0=50 mg/L,m=0.2 g,speed=300 r/min,pH:2).
3.10 Intraparticle diffusion
The possibility of intra-particle diffusion[6]was explored by using the intra-particle diffusion model as given in Eq.(4).

Where Kidis the intra-particle diffusion rate constant in mg·g-1min-1/2and C is the intercept.According to Eq.(4),a plot of qtversus t1/2provides a straight line with a slope Kidand an intercept C when an adsorption follows the intra-particle diffusion mechanism.
From Fig.12,it is observed that there are two separate regions,i.e.the initial portion is attributed to the bulk diffusion (Kid,1)and the final portion to the intra-particle diffusion (Kid,2),which is also evident from the experimental data followed by Freundlich isotherm.The constants are given in Table 4.The values of Kid,1Kid,2,C1and C2using Eq.(4)were found to be 3.313 6 mg·g-1·min-1,3.099 9 mg·g-1·min-1,33.27 and 19.60,respectively.

Fig.12 Intraparticle diffusion for adsorption of Cr (VI)ions using Ozone treated rice husk carbon(C0=100 mg/L,m=0.2 g,speed=300 r/min,pH:2).

Table 4 The constants of intraparticle diffusion.
3.11 Mass transfer studies
The mass transfer equation[26]is generally expressed by Eq.(5)

Where C0is the initial metal ion concentration(mg·dm-3),Ctis the metal ion concentrationat time t,D is a fitting parameter,K0is the adsorption constant in min-1,which is related to the mass transfer adsorption coefficient K,K0=Km,where m is the mass of the adsorbent (g).
A linearized form of the equation is given in Eq.(6)

A plot of In(C0-Ct)versus time should give a linear relationship,from which the constants D and K0can be determined from the intercept and slope of the plot,respectively.
Fig.13 shows a plot of In(C0-Ct)versus time.The fitting parameter InD,the adsorption constant K0and the mass transfer adsorption coefficient value K computed from the slope and intercept are 92.7,0.0001 min-1and 2.5 ×10-5(g·L-1·min-1),respectively.
3.12 Thermodynamic parameters
The thermodynamic parameters[27]for the adsorption of Cr (VI)ions were determined using the following equations (7),(8)and (9).
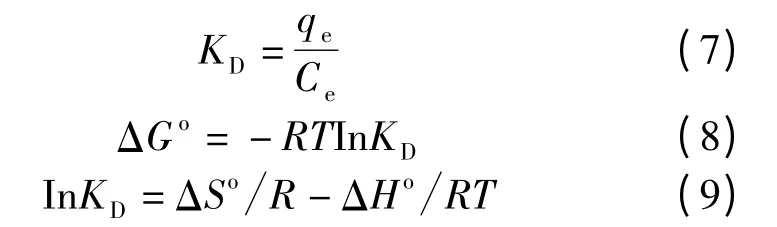
Where KDis the distribution coefficient for the adsorption in g/L,ΔGois the Gibbs free energy in J/mol,R is the gas constant in J/(mol·K),T is the absolute temperature in K,T is the entropy change in J·mol-1·K-1and ΔHois the enthalpy change in kJ/mol.
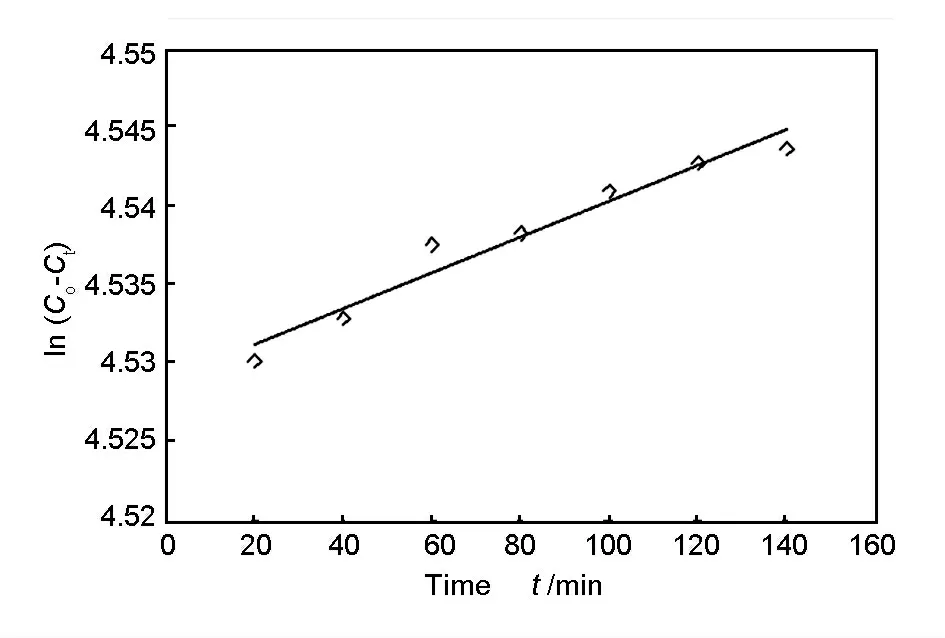
Fig.13 Mass transfer kinetics for adsorption of Cr (VI)ions using Ozone treated rice husk carbon(C0=100 mg/L,m=0.2 g,speed=300 r/min,pH:2).
The thermodynamic constants were determined from Fig.14 and the results for various temperatures are listed in Table 5.The negative values of ΔHofor all the temperatures indicate that the reaction is exothermic.The positive value of ΔSoindicate the structural change occurs on the surface of the adsorbent and the increased randomness during the adsorption.The negative values of Gibbs free energy ΔGoindicate that the adsorption is spontaneous.
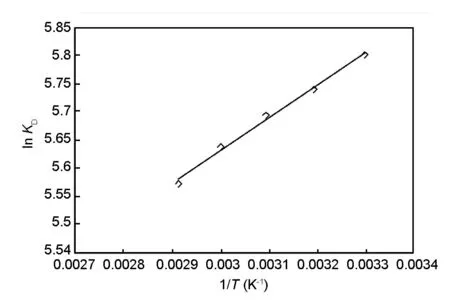
Fig.14 Determination of thermodynamic parameters(C0=100 mg/L,m=0.2 g,speed=300 r/min,pH:2).
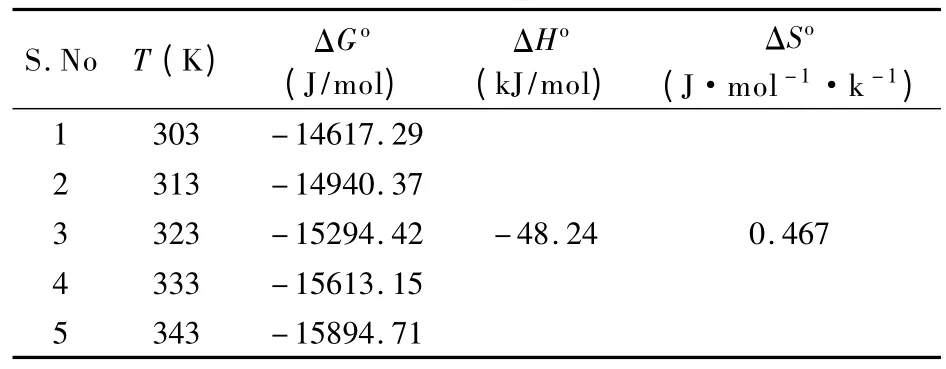
Table 5 Thermodynamic parameters for the adsorption of Cr (VI)ions using Ozone treated rice husk carbon at different temperatures.
3.13 Desorption studies
Desorption studies were also conducted to explore the feasibility of recycling the adsorbents and recovery of the Cr metal.NaOH was used for stripping the adsorbates.Desorption experiments were conducted by mixing 0.6 g of Cr (VI)loaded ozone-treated rice husk carbon with 50 mL of 0.1 mol/L NaOH.In order to determine the reusability of the adsorbent,the adsorbent was taken out from the solution and washed with double distilled water.Adsorption and desorption studies were repeated three times by using the same adsorbent.Desorption of ions previously deposited on the adsorbent was generally low.65%-75% removal of Cr (VI)ion was obtained on regeneration of the adsorbent,which is comparable with the reported value of 61%[28].
4 Conclusions
The ozone-treated rice husk carbon is an effective adsorbent for the removal of Cr (VI)ions,owing to the surface modification by the atomic oxygen in ozone treatment.The maximum removal percentage of 94% Cr (VI)ions from the aqueous solution of 100 mg/L was obtained at optimized conditions of pH:2.0,adsorbent dosage of 0.2 g,time of 2.5 h and speed of 300 r/min.Freundlich isotherm reveals the chemisorption of Cr (VI)ions on the ozone-treated carbon.The rate of adsorption follows the pseudo second order kinetics.The adsorption process is spontaneous and exothermic in nature.Desorption studies reveal that the recovery of Cr (VI)ions from the ozone-treated rice husk carbon is possible.
Acknowledgements
The author gratefully acknowledges those who have been instrumental in the successful completion of this project.
[1]Utrilla J R,Toledo I B,Gercia M A F,et al.Bioadsorption of Pb(II),Cd(II)and Cr (VI)on activated carbon from aqueous solutions[J].Carbon,2003,41:323-330.
[2]Garg U K,Kaur M P,Sud D,et al.Removal of hexavalent chromium from aqueous solution by adsorption on treated sugarcane bagasse using response surface methodological approach[J].Desalination,2009,249:475-479.
[3]Owald M,Aroua M K,Daud W A D W,et al.Removal of hexavalent chromium-contaminated water and wastewater:A Review[J].Water Air Soil Pollut,2009,200:59-77.
[4]Monser L,Adhoum N.Modified activated carbon for the removal of copper,zinc,chromium and cyanide from wastewater[J].Sep Purif Technol,2002,26:137-146.
[5]Attia A A,Khedr S A,Elkholy S A.Adsorption of chromium ion (VI)by acid activated carbon [J].Braz J Chem Eng,2010,27:183-193.
[6]Nomanbhay S M,Palanisamy K.Removal of heavy metal from industrial waste water using chiotsan coated oil palm shell charcoal[J].E J Biotechnol,2005,8:43-53.
[7]Thirunavukkarasu E,Palanivelu K.Biosorption of Cr(VI)from plating effluent using marine algal mass[J].Indian Journal of Biotechnology,2007,16:359-364.
[8]Yigitoglu M,Arslan M.Adsorption of hexavalent chromium from aqueous solutions using 4-vinyl pyridine grafted poly (ethylene terephthalate)fibers[J].Polym Bull,2005,55:259-268.
[9]Shafey E I.Behaviour of reduction-sorption of chromium (VI)from an aqueous solution on a modified sorbent from rice husk[J].Water Air Soil Pollut,2005,163:81-102.
[10]Jaman H,Chakraborty D,Saha P.A study of the thermodynamics and kinetics of copper using chemically modified rice husk[J].Clean,2009,37:704-711.
[11]Jia Y F,Thomas K M.Adsorption of cadmium ions on oxygen surface sites in activated carbon[J].Langmuir,16:2002,1114-1122.
[12]Valdes H,Polo M S,Utrilla J R,et al.Effect of ozone treatment on surface properties of activated carbon[J].Langmuir,2002,18:2111-2116.
[13]Manchester S,Wang X,Kulaots I,et al.High capacity mercury adsorption on freshly ozone-treated carbon surfaces[J].Carbon,2008,46:518-524.
[14]Chiang H L,Chiang P C,Huang C P.Ozonation of activated carbon and its effect on the adsorption of VOCs exemplified by methylethylketone and benzene[J].Chemosphere,2002,47:267-275.
[15]Bhatti I,Qureshi K,Kazi R A,et al.Preparation and characterization of chemically activated almond shells by optimization of adsorption parameters for removal of chromium (VI)from aqueous solutions[J].World Academy of Science Engineering and Technology,2007,34:199-204.
[16]Subrahmanyam C,Bulushev D A,Minsker L K.Dynamic behavior of activated carbon catalysts during ozone decomposition at room temperature[J].Appl Catal B,2008,61:98-106.
[17]Christine M,Sala U F,Sala,U P.Quantitative determination of hexavalent chromium in aqueous solutions by UV-Vis spectrophotometer[J].Central European Journal of Chemistry,2007,5:1083-1093.
[18]Rane N M,Sapkal R S,Sapkal V S,et al.Use of naturally available low cost adsorbents for removal of Cr (VI)from waste water [J].International Journal of Chemical Sciences and Applications,2010,1(2):65-69.
[19]Tazrouti N,Amrani M.Chromium (VI)adsorption onto activated kraft lignin produced from Alfa grass (stipa tenacissima)[J].BioResources,2009,4:740-755.
[20]Mor S,Ravindra K,Bishnoi N R.Adsorption of chromium from aqueous solution by activated alumina and activated charcoal[J].Bioresour Technol,2007,98 (4):954-957.
[21]Gupta S,Babu,B V.Removal of toxic metal Cr (VI)from industrial waste water using sawdust as adsorbent:Equilibrium,kinetics &regeneration studies[J].Chem Eng J,2009,150:352-365.
[22]EI-Ashtoukhy E S Z,Amin N K,Abdelwahab O.Removal of lead (ii)and copper (ii)from aqueous solution using pomegranate peel as a new adsorbent[J].Desalination,2008,223:162-173.
[23]Ho Y S,Selection of optimum sorption isotherm[J].Carbon,2004,42:2115-2116.
[24]Saueprasearsit P.Adsorption of chromium (Cr+6)using durian peel[C].International Conference on Biotechnology and Environment Management IPCBEE,2011,18.
[25]Jun L H,Tian L M,Li Z J.A kinetic study on the adsorption of Cr (VI)onto a natural material used as land fill liner[J].Electronic Journal of Geotechnical Engineering,2009,14:1-10.
[26]Augustin A A,Orike B D,Edidiong A D.Adsorption kinetics and modeling of Cu (II)ion sorption from aqueous solution by mercaptoacetic acid modified cassava (manihot sculenta cranz)wastes[J].Electronic Journal of Environmental Agricultural &Food Chemistry,2007,6:2221-2234.
[27]Sadaoui Z,Hemidouche S,Allalou O.Removal of hexavalent chromium from aqueous solutions by micellar compounds[J].Desalination,2009,249:768-773.
[29]Gholipour M,Hashemipour H,Mollashahi M.Hexavalent chromium removal from aqueous solution via adsorption on granular activated carbon:Adsorption,desorption,modeling and simulation studies[J].APRN Journal of Engineering and Applied Sciences,2001,6:10-18.

