Studying neurological disorders using induced pluripotent stem cells and optogenetics
Studying neurological disorders using induced pluripotent stem cells and optogenetics
Neurological disorders are amongst the most widely studied human aliments. Yet, they are also one of the most poorly understood. Although most of these disorders are polygenic, genotype still plays an important role in their etiologies. For example, in schizophrenia and autism spectrum disorders, there is a 40–60% concordance rate in monozygotic twins, with 60–90% heritability (Burmeister et al., 2008). However, the mechanisms by which multiple genes and their genomic variations infl uence the phenotypes of the disorders remain to be understood. The complexities of the disorders are further compounded by the individual rarity of the genomic variations and their variable penetrance (Cook and Scherer, 2008). Thus, conventional disease modeling, such as gene knockout in cells or in animals, to attain the desired disease genotype may not be the most suitable platform for tackling most neurological disorders.
With the advent of induced pluripotent stem cell (iPSC) technology, there presents a revolutionizing method for modeling complex human disorders. iPSCs are somatic cells that have been reprogrammed through the use of transcription factors to restore pluripotency (Takahashi and Yamanaka, 2006). One of the ultimate goals for iPSC technology is to obtain somatic cells of specifi c lineages via directed diff erentiation. Cells diff erentiated from iPSCs can be used to model patient-specifi c disease mechanisms in vitro, for transplantation to replace lost cells, or for drug screening. This technology is especially crucial for neuroscience research, where brain cells from human patients are not easily obtainable. Thus, human iPSC-derived neurons make an ideal model system for the study of neurological disorders and the development of neural functionality and plasticity (Johnson et al., 2007; Ladewig et al., 2008). In the event of neurodegeneration, they can act as a therapeutically relevant source of cells for replacement purposes (Sonntag et al., 2005). In order for these ends to be met, several hurdles would have to be crossed (illustrated in Figure 1). Firstly, the iPSC colonies have to be generated via reprogramming and maintained for diff erentiation into the desired neuronal subtypes. Next, the functional maturity of the derived neurons has to be verifi ed. Lastly, the ability of the iPSC-derived neurons to integrate functionally into an existing neuronal network has to be probed.
In Takahashi and Yamanaka’s experiments, iPSCs were generated from retrovirus-mediated introduction of four transcription factors (Oct-3/4, Sox2, c-Myc, and Klf4) into mouse embryonic and adult fi broblasts (Takahashi and Yamanaka, 2006). The iPSCs exhibited morphology and growth properties similar to that of embryonic stem cells; they also expressed embryonic stem cell markers. The researchers then repeated the experiment to similar success with adult human dermal fi broblasts (Takahashi et al., 2007). Since then, other groups have jumped onto the iPSC bandwagon, either experimenting with the cocktail of transcription factors for inducing pluripotency, or tinkering with the generation of iPSCs from novel cell types, or trying to elucidate the exact mechanisms through which the transcription factors induce pluripotent stem cells. An important development in the reprogramming process is in the delivery of reprogramming factors into the somatic cells. Retroviral and lentiviral vectors have been widely used, while virus-free methods are catching on. The latter are preferred if one needs the iPSCs to be free of vector and transgene sequences. These virus-free methods include the use of episomes, RNA and protein transfection, small molecule carriers, and cell-penetrating peptides to deliver the reprogramming factors (Compagnucci et al., 2014).
In addition to skin fi broblasts, other cell types have been used for iPSC derivation. These include keratinocytes, neural cells, mature B and T cells, hepatocytes, amniotic cells, and hair follicular cells, and cells derived from adipose tissue (Compagnucci et al., 2014). Many of these somatic cells can be sampled with minimal invasiveness to patients. This is another plus point for the use of iPSC technology to model patient-specifi c diseases. On the other hand, iPSCs derived from diff erent somatic cells may habour intrinsic potential to preferentially diff erentiate into specifi c cell lineages. Thus, further studies are needed to examine the diff erences between iPSCs derived from diff erent cell types and how the diff erent sources of somatic cells aff ect the effi ciency of pluripotency induction and subsequent directed diff erentiation.
Besides optimizing the procedure for iPSC generation, diff erent protocols have been conceived for the induction of specifi c cell types. There are various well-established protocols for obtaining specifi c cell types, as well as customized ones that have been fi ne-tuned by individual research groups for obtaining specifi c neuronal cell types (reviewed in Compagnucci et al., 2014). These protocols vary in several parameters, such as the types and amounts of growth factors and supplements added to direct diff erentiation, the frequency and length of time for which they are used, or the type of culture media used. It has also been reported that the presence of other cells, such as astrocytes and oligodendrocytes, can aff ect diff erentiation effi ciency and neuronal maturation. All the aforementioned factors aff ect the diff erentiation effi ciency of the iPSCs and the length of time required to attain the desired cell type. Given that diff erentiation effi ciency is sensitive to the slightest variation in culture conditions, obtaining a robust diff erentiation reproducibly is considered to be the most challenging obstacle in establishing an iPSC culture protocol.
After obtaining an iPSC-differentiated neuron culture, the next step is to establish neuronal identity and functional maturity. Morphological analyses, RNA and protein profi ling, as well as immunostaining for neural cell markers are normally used to confi rm neuronal identity. Subsequently, electrophysiological techniques and analysis are essential to demonstrate functional identity and maturity. For instance, the ability to fi re action potentials and the presence of postsynaptic currents (PSCs) typically indicate that the newly-derived neurons have matured functionally and are capable of communicating with other neurons. The functionalproperties of iPSC-derived neurons should then be compared to the intrinsic properties of the neuronal subtypes that they aim to model or replace, to ensure the generation of relevant cell types from the iPSCs.
To more accurately mimic the complexity of in vivo conditions when modeling neurological disorders, a co-culture system of iPSC-derived neurons and other cell types should be used. It is then possible to assess the ability of iPSC-derived neurons to physically interact with or synaptically connect to the other cell types in the system, and if these synapses are functional. There are at least four possible ways for cell-cell interactions and connections in the co-culture system. First, the iPSC-derived neurons communicate only with each other, and not with the other cell types. Second, they receive input from the other cell types, but do not send any reciprocal output. Third, the iPSC-derived neurons give input to the other cells, but do not receive any reciprocal input. Fourth, the signal transmission between the iPSC-derived neurons and the other cell types in the system can be bidirectional. Under most normal circumstances, only the iPSC-derived neurons described in the fourth instance are considered functionally integrated into the circuitry.
The degree of functional integration of iPSC-derived neurons to their co-culture systems is still largely unknown. Conventionally, evaluation of functional synaptic integration is based on morphological parameters and receptor binding studies (Tønnesen and Kokaia, 2012). Electrophysiological characterization remains the gold standard for determining functional integration, but it can be relatively complex since identifi cation and selective activation of specifi c cell types in a co-culture system can be problematic. Extracelluar fi eld stimulations have been used to investigate synaptic integration in several studies involving grafts of stem cell-derived neurons (Tønnesen and Kokaia, 2012). However, data from such stimulations do not allow for identification of the origin of synaptic inputs recorded, due to the nonspecifi c nature of the stimulation. Another possible solution is dual whole-cell recordings, but such an approach is limited by its diffi cult technique and the low probability of synaptic coupling between recorded cells. Since the probability of the two recorded cells being contacted by a common third cell is certainly higher than locating two directly connected cells, coincidence detection of postsynaptic events may be employed to test for functional integration. Unfortunately, it can be a time-consuming endeavor due to the trial-and-error process, and there remains the issue of it being an indirect way of examining functional integration.
Recent developments in the optogenetics fi eld have provided reasonable solutions to the conundrum of functional integration of iPSC-derived neurons with the other cells in a co-culture system. The genetic introduction of optically-gated membrane proteins into cells allows the alteration of membrane potentials with high temporal resolution. Thus, control over activity in selected cell populations can be attained (Boyden et al., 2005; Zhang et al., 2007). Therefore, it becomes relatively uncomplicated to establish functional integration between iPSC-derived neurons and other cell types, given the selective control allowed for activating or silencing diff erent cell populations independently of each other.
One of the most widely used in the optogenetics toolbox is channelrhodopsin-2 (ChR2), a blue light (around 470 nm)-activated depolarizing cation channel protein (Boyden et al., 2005). Numerous studies have used the light-gated channel to enable stimulation of cells in complex neuronal networks, both in vitro and in vivo. ChR2-based stimulation allows for specifi city, as only cells expressing the light-gated channel would respond to the light stimulus. Such a response consists of light-induced depolarization of the neuronal membrane. The depolarization would have to reach the fi ring threshold in order to elicit an action potential. For reliable and robust action potential induction, ChR2 expression level in the cell has to be high, in order to compensate for its small single-channel conductance. As expression levels may vary between individually transfected cells, some ChR2-expressing neurons may experience only subthreshold depolarization during light stimulation. Hence, it is important to introduce ChR2 to neurons using methods that render high effi ciency in transduction and protein expression. Using variants of ChR2, such as the H134R mutant which enables enhanced photocurrents, can circumvent weak action potential fi ring due to low ChR2 expression levels.
An integrated approach combining iPSC and optogenetics technologies is essential for interrogating functional synaptic connections in vitro (Figure 1). One such example of an in vitro optogenetics system is a triple co-culture consisting of iPSC-derived neurons, primary cortical neurons, and astrocytes diff erentiated from neural progenitor cells (Su et al., 2015). The presence of primary neurons can enhance differentiation and maturation of human iPSC-derived neurons, while the astrocytes support the growth of the neurons. The primary neurons were transduced with lentivirus that expresses ChR2 and thus, they can be optically activated. Whether the iPSC-derived neurons that are also in the co-culture form functional synapses with these primary neurons can be investigated by detecting for PSCs upon blue light stimulation (Figure 1). iPSC-differentiated neurons were shown to exhibit an increase in PSC frequency upon photostimulation of the ChR2-expressing primary neurons (Su et al., 2015). With such a system for the study of neurological disorders, any patient- or disease-specifi c iPSC-derived neurons can be used to investigate their respective disease mechanics. The disease status of any of the component cell types can be manipulated to examine their contributive eff ects to the neurological disorder in question. In addition, it would be possible to modify the components of the system in a mix-and-match manner to permit unique questions to be addressed. Furthermore, the co-culture may be a better representation of a system than lone cell types for the study of pharmacodynamic eff ects in the screening of drug compounds.
Both the iPSC and optogenetics technologies have been established for over a decade, but their combined potential is only starting to be realized. The integrated in vitro approach combining both technologies provides the means to understand and resolve underlying mechanisms in complex neurological disorders. It is also hoped that advancements in both these technologies continue to shed light towardsthe understanding and therapeutics of complex neurological disorders.
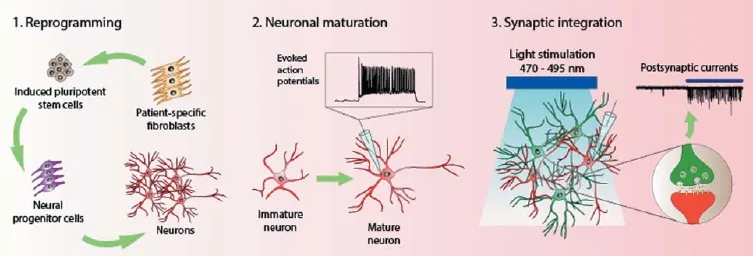
Figure 1 An integrated in vitro approach of induced pluripotent stem cell (iPSC) and optogenetics technologies for studying neurological disorders.
Eunice W.M. Chin, Eyleen L.K. Goh*
Program in Neuroscience and Behavioral Disorders, Duke-NUS Graduate Medical School, Singapore, Singapore (Chin EW, Goh EL)
NUS Graduate School for Integrative Singapore, Sciences and Engineering, National University of Singapore, Singapore, Singapore (Chin EW)
Department of Physiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore (Goh EL) KK Research Center, KK Women’s and Children’s Hospital,
Singapore, Singapore (Goh EL)
*Correspondence to: Eyleen L.K. Goh, Ph.D., eyleen.goh@duke-nus.edu.sg.
Accepted: 2015-08-18
orcid: 0000-0002-8244-6959 (Eyleen L.K. Goh)
Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K (2005) Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8:1263-1268.
Burmeister M, McInnis MG, Zollner S (2008) Psychiatric genetics: progress amid controversy. Nat Rev Genet 9:527-540.
Compagnucci C, Nizzardo M, Corti S, Zanni G, Bertini E (2014) In vitro neurogenesis: development and functional implications of iPSC technology. Cell Mol Life Sci 71:1623-1639.
Cook EH Jr., Scherer SW (2008) Copy-number variations associated with neuropsychiatric conditions. Nature 455:919-923.
Johnson MA, Weick JP, Pearce RA, Zhang SC (2007) Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J Neurosci 27:3069-3077.
Ladewig J, Koch P, Endl E, Meiners B, Opitz T, Couillard-Despres S, Aigner L, Brüstle O (2008) Lineage selection of functional and cryopreservable human embryonic stem cell-derived neurons. Stem Cells 26:1705-1712.
Sonntag KC, Simantov R, Isacson O (2005) Stem cells may reshape the prospect of Parkinson’s disease therapy. Brain Res Mol Brain Res 134:34-51.
Su CT, Yoon SI, Marcy G, Chin EW, Augustine GJ, Goh EL (2015) An optogenetic approach for assessing formation of neuronal connections in a co-culture system. J Vis Exp 96:e52408.
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fi broblast cultures by defi ned factors. Cell 126:663-676.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fi broblasts by defi ned factors. Cell 131:861-872.
Tønnesen J, Kokaia M (2012) Chapter 6 - Electrophysiological investigations of synaptic connectivity between host and graft neurons. In: Progress in Brain Research (Dunnett SB, Björklund A, eds), pp97-112. Elsevier.
Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K (2007) Multimodal fast optical interrogation of neural circuitry. Nature 446:633-639.
10.4103/1673-5374.169607 http://www.nrronline.org/
Chin EW, Goh EL (2015) Studying neurological disorders using induced pluripotent stem cells and optogenetics. Neural Regen Res 10(11):1720-1722.
- 中国神经再生研究(英文版)的其它文章
- Brain protein oxidation: what does it refl ect?
- The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system
- Intracellular sorting pathways of the amyloid precursor protein provide novel neuroprotective strategies
- VEGF in the nervous system: an important target for research in neurodevelopmental and regenerative medicine
- Ef cacy of glucagon-like peptide-1 mimetics for neural regeneration
- Compliant semiconductor scaf olds: building blocks for advanced neural interfaces

