A competition mechanism between CO23- and C12 H 25SO4-in the interlayer gallery of Mg/Al layered double hydroxides
LI Haojie,WU Dingwei,JIA Xinliang,LI Biao,CHEN Miao,WANG Linhong,LIU Xiaojuan,JIA Chunling,PENG Peng
(College of Chemistry and Chemical Engineering,Zhoukou Normal University,Zhoukou 466001,China)
Intercalation of organic guest species into layered inorganic solids is a way of producing ordered inorganic-organic assemblies with unique microstructures controlled by host-guest and guest-guest interactions[13].Among possible layered materials,compounds in the anion-exchange group are relatively rare.Layered double hydroxides(LDHs)are a class of layered materials consisting of positively charged brucite-like layers and the interlayer exchangeable anions.The interlayer anions can exchange with diversified anions,which makes LDHs become supermolecule intercalation structured material with different application functions.In recent years,much attention has been paid to delamination of LDHs,which should produce positively charged sheets in contrast to the majority of polyanionic nanosheets derived from cation-exchangeable layered materials.
The popular delamination method is intercalation of organic guest molecule into the interlayer gallery,which also is a more direct and simple method.Generally,sodium dodecyl sulfate(NaDS)is chosen as the guest or gallery anion[4-6],which is a long surfactant anion and leads delamination of LDHs by a convenient physical method.It is known that CO23-ions have a very strong affinity to LDHs[7].According to the guest selectivity sequences determined by the charge and size of the anions,the selectivity of CO23-should be very high.Eliminating CO23-from the reaction system is found to be essential for successfully synthesizing LDHs.The influence of CO23-to synthesize LDHs containing organic guest molecule is inevitable and has big significance for us to discuss.However,few articles are focused on the completive relationship between monovalent and divalent anions.CO23-as most common strong affinity ion,three advantages are of real significance in the research of its competed relationship with monovalent macromolecule.The first is link the research between monovalent and divalent anions,which is easy to investigate their competition relation in the interlayer gallery of LDHs.The second is highly effective in preventing the influence of CO23-during the experimental process and simplify the experimental program.The third is useful for the study of structural topological variations in the interlayer gallery.In our work,we also choose sodium dodecyl sulfate as a gallery anion and synthesize Mg3Al(OH)8(C12H25SO4)(named LDH-DS)by a spontaneous self-assembly method.Herein,the influence of CO23-to synthesize LDH-DS is discussed and the competition mechanism between CO23-and C12H25SO4-(denoted DS)in the interlayer gallery is expounded.
1 Experiment
1.1 Synthesis of samples
The LDH-DS was synthesized by a spontaneous self-assembly method according to the literature[7].A solution containing Mg/Al(R=3),C12H25SO4Na(0.011 2 mol),and NaCO3was prepared and p H value was adjusted to about p H=10 with 1 mol/L NaOH aqueous solution.The content of NaCO3was varied as indicated in Table 1.The obtained slurry was aged for 3 days at 80℃and the products were denoted as LDHDS,CS1,CS2,CS3,and CS4.The resulting LDH-DS was shaken in formamide for 0.5 h to produce a colloidal suspension of exfoliated single layers.LDH-CO3reconstructed by adding an aqueous solution of NaCO3to a dispersion of exfoliated LDHs layers[8].
1.2 Characterization
Powder X-ray diffraction(XRD)patterns were measured on a Rigaku D/max 2 500 V/PC diffractometer using Cu K αradiation(λ =1.540 56Å,40 k V,100 m A).The proportion of volatile elements C,H,and N were analyzed by using a Vario EL3 elemental analyzer(CHNS analysis).FTIR spectra were recorded by a Parkin-Elmer 1 750 spectrophotometer.
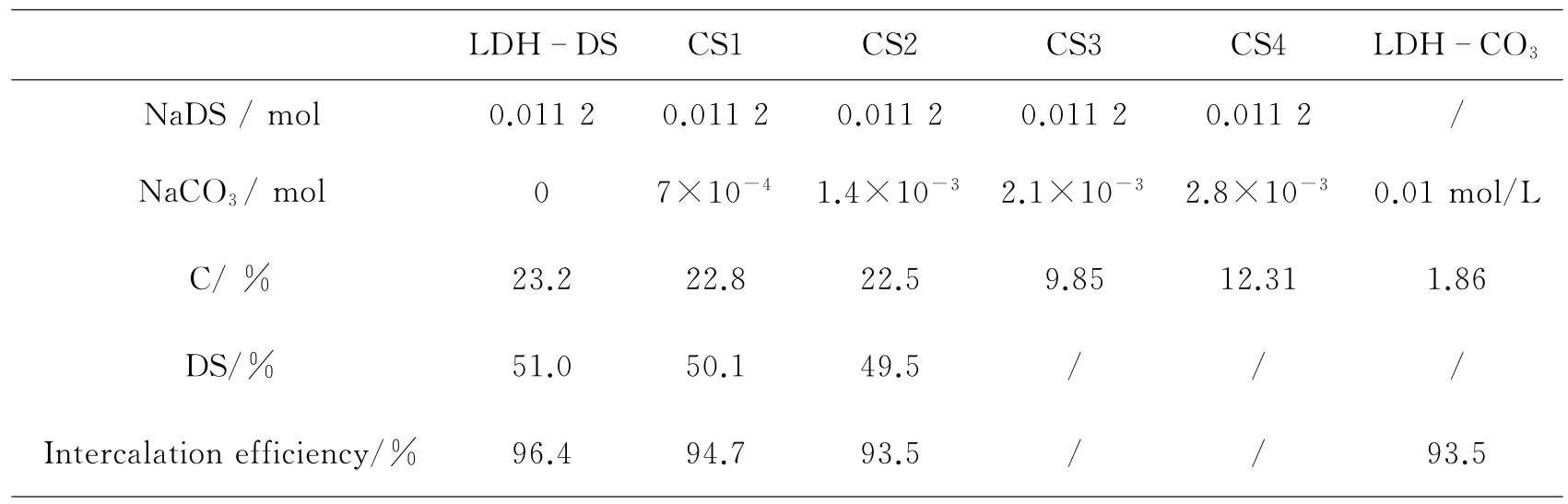
Table 1 Elemental analysis results and the intercalation percentage of DS-in LDH-DS
2 Results and discussion
2.1 CNH analysis of samples
Table 1 compares the results of elemental contents of the resulting nanocomposites,and intercalation efficiency of DS is given.This value is calculated by the assumption that DS contains 45.5%carbon,a result obtained from CHNS analysis.LDH-DS has a high capacity for DS intercalation.In samples CS1 and CS2,the amount of carbon is not changed much,indicating that LDHs also has a high capacity for DS intercalation when a little CO23-is presented in solution.In the sample CS3 and CS4,the carbon content lies between that of LDH-DS and LDH-CO3.The reason may be associated with the competitive mechanism of DS and CO23-.Because of increased CO23-concentration,many DS and CO23-anions of dissociative state are present in the interlayer gallery except for CO23-connecting layers.
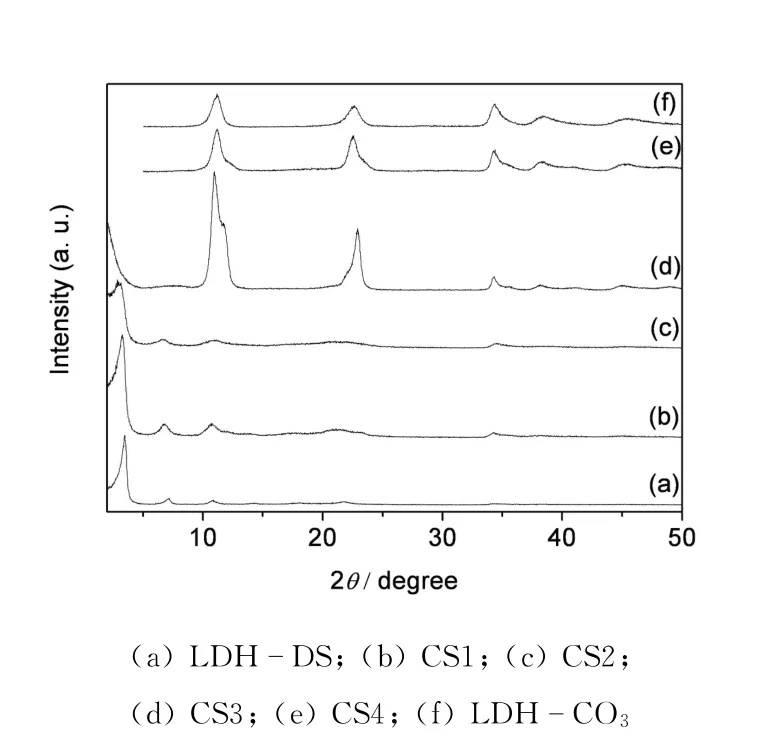
Fig.1 XRD patterns of the as-synthesized samples
2.2 Competition intercalation between CO23- and C12 H25 SO4-
It has been generally acknowledged that the d-spacing(d003)of a DS-intercalated LDHs sample is about 26Å(2.6 nm),which coincides with XRD pattern shown in the Fig.1a.The host framework,which contains stacking of[Mg3Al(OH)8]+layers,has a rhombohedral structure with an a=b and c=3d003unit cell.The structure in Fig.1a can thus be indexed using a=b=2d110=3.04Åand c=3d003=78Åfor a rhombohedral symmetry unit cell.As proved by XRD in Fig.1f,layered double hydroxide containing CO23-(LDH-CO3)was produced and it has a basal spacing of 7.9Å.As shown in the Fig.1,the identical powder XRD patterns between samples CS and LDH-DS reveal that CS1 and CS2 are layered double hydroxides containing DS.No impure peaks are observed in the XRD of samples CS1 and CS2 when 0.001 4 mol or less CO23-is added,indicating that a relatively small amount of CO23-cannot affect the DS intercalation.As the amount of CO23-increased in solution,the spacing and the width of the(003)reflection tend to increase.Samples CS1 and CS2 have bigger spacing and greater width than sample LDH-DS,indicating that the Mg/Al layers do not integrate tightly and they have a low-crystallinity.A little CO23-takes up the interlayer space,which affects combination between DS and layers.In the Fig.1d,XRD pattern of LDH-CO3becomes the predominated form when the amount of CO23-is too large,and impure peaks can be seen.As the concentration added again,XRD pattern of sample CS4 is totally in accordance with that of sample LDH-CO3.Thus,the synthesis of LDH-DS is not affected when a little CO23-is present in the solution,which can also be seen from FTIR spectra.

Fig.2 FTIR spectra for DS
Fig.2 shows the FTIR spectrum of DS,showing two main doublet absorption bands in the region of 2 850~2 950 cm-1and another band at 1 210~1 240 cm-1.The former indicates the presence of aliphatic group and latter due to the presence of sulfate group in DS.The absorption bands at around 1 469 cm-1indicates C-H bending vibration.

Fig.3 FTIR spectra of the as-synthesized samples
Fig.3 shows FTIR spectra of the as-synthesized samples prepared under different proportions of chemical reagents.The absorption band at around 3 500 cm-1,is attributed to OH stretching due to the presence of hydroxyl group of LDHs.The H-O-H bending vibration(vH-O-Hbend)and O-H symmetric stretching vibration(vO-Hsym)in these nanocomposites appear in the ranges 1 610~1 630 and 3 440~3 470 cm-1,respectively.The lower values of vO-Hsymin these nanocomposites compared to that of free OH groups(>3 650 cm-1)indicates that all the OH groups are involved in hydrogen bonding with DS(or CO23-)and the Mg/Al double metal hydroxide interlayer.The dominance of the bands at around 2 850~2 950 cm-1and 1 210~1 240 cm-1clearly indicates the presence of DS in the interlayer of LDH-DS.C-H bending at around 1 469 cm-1also confirms the presence of the DS molecules in the sample LDH-DS.The band at 1 380 cm-1may be characteristic of CO23-ion,which suggests the carbonate contained in the samples.It contains in all samples and becomes more prominent as the concentration of CO23-increased,which is consistent with character peak of sample LDH-CO3.The band of CO23-is very weak in the samples LDH-DS,CS1,and CS2,and functional group DS is prominent in the compounds,although it can't totally hinder the insertion of CO23-.Impure peak of CO23-is very weak in FTIR spectra of Fig.3a and isn’t detected in the XRD of Fig.1a.So the sample LDH-DS comes up to the standard for various applications and the influence of CO23-can be ignored.In samples CS3 and CS4,The peak intensity of CO23-is stronger,and CO32-surpasses DS as the main interlayer ions.DS bands at around 2 850~2 950 cm-1and 1 210~1 240 cm-1become weaker.The phenomenon is caused by the sudden decrease of the combined DS,however,DS of the free state doesn’t reduce much in the interlayer gallery.As seen from FTIR spectra and XRD patterns,the synthesis of LDH-DS is not affected when a little CO23-is present in solution,although the band of CO23-is widely present in the samples.
2.3 Probable competition mechanism
As the sum of the length of DS molecule(21.3Å)and the layer thickness(about 4.8Å)almost coincides with the spacing values(27Å)on the basis of the XRD data(Fig.1a),a monolayer of surfactant chains oriented perpendicularly and interdigitated interactions between the inorganic brucite-like layers is envisioned as in Fig.4a.A simple summation of short side length of CO23-(4.76Å)and the thickness of brucitelike layer will give a basal spacing of 9.56Å,or 1.66Åmore than the observed value 7.9Åfor LDH-CO3.So there is a flat-lying mode in the gallery as shown in fig.4b.CO23-anions are evenly located around the middle of the gallery and their molecular planes are parallel to the brucite-like layers[9].Due to the charge balance,the number of the divalent anions CO23-in LDH-CO3is only half of that of the monovalent anions DS in LDH-DS.When anions DS and CO23-coexist in solution and CO32-presents at a very low concentration,CO23-is too less to fit every cavity on the surface of the brucite-like layers.When LDHs is synthesized by a spontaneous self-assembly method,layers combine with DS via van der Waals forceto keep charge balance.LDH-DS has a larger basal spacing and DS occupies a dominant position in the interlayer gallery.CO23-cannot connect both layers in the gallery of these compounds.As the concentration of CO32-increased,CO23-begins to occupy more space in the gallery.Because of CO23-ions exist in the gallery,layers and DS do not integrate tightly and the basal spacing becomes larger.If the concentration of CO32-increased again,it should react with the layers first and LDH-CO23-produces.In this circumstance,many DS of free state may exist horizontally in the interlayer gallery,and the combined DS cannot present in the narrow gallery of LDH-CO23-.

Fig.4 Schematic drawing for the microstructure of the LDH-DS(a)and LDH-CO3(b).
3 Conclusion
The competition mechanism between DS and CO23-in the interlayer gallery is validated and discussed.LDH-DS can be effectively synthesized and meets the requirements of experimental quality by a spontaneous self-assembly method,although CO23-inevitably affects the synthesis process.
[1]Ogawa M,Kuroda K.Photofunctions of intercalation compounds[J].Chem.Rev.,1995,95:399-438.
[2]Ogawa M,Kuroda K.Preparation of inorganic–organic nanocomposites through intercalation of organoammonium ions into layered silicates[J].Bull.Chem.Soc.Jpn.,1997,70:2593-2618.
[3]Hibino T,Jones W.New approach to the delamination of layered double hydroxides[J].J.Mater.Chem.,2001,11:1321-1323.
[4]Chen W,Feng L,Qu B.Preparation of nanocomposites by exfoliation of Zn/Al layered double hydroxides in nonpolar LLDPE solution[J].Chem.Mater.,2004,16:368-370.
[5]Hu G,O'Hare D.Unique layered double hydroxide morphologies using reverse microemulsion synthesis[J].J.Am.Chem.Soc.,2005,127:17808-17813.
[6]Venugopal B R,Shivakumara C,Rajamathi M.Effect of various factors influencing the delamination behaviour of surfactant inter calated layered double hydroxides[J].J.Colloid Interface Sci.,2006,294:234-239.
[7]Miyata S.Anion-exchange properties of hydrotalcitelike compounds[J].Clays Clay Miner.,1983,31:305-311.
[8]Wu Q,Olafsen A,Vistad B,et al.Delamination and restacking of a layered double hydroxide with nitrate as counter anion[J].J.Mater.Chem.,2005,15:4695-4700.
[9]Xu Z,Zeng H.Abrupt structural transformation in hydrotalcite-like compounds Mg1-xAlx(OH)2(NO3)x·n H2O as a continuous function of nitrate anions[J].J.Phys.Chem.B.,2001,105:1743-1749.

