隔药灸天枢和气海对慢性炎性内脏痛大鼠痛行为和痛情绪的影响
Huang Yan (黄燕), Yang Yan-ting (杨延婷), Liu Xiao-xu (刘晓旭), Zhao Yan (赵嬿), Feng Xiao-ming (丰晓溟),Zhang Dan (张丹), Wu Huan-gan (吴焕淦), Zhu Yi (朱毅), Huang Wen-yan (黄文燕), Ma Xiao-peng (马晓芃)
1 Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China
2 Beijing University of Chinese Medicine, Beijing 100029, China
3 Shanghai Research Institute of Acupuncture and Meridian, Shanghai 200030, China
Chronic visceral pain is a common symptom in clinic.Chronic pain can be neuropathic or inflammatory according to the characteristics. Inflammatory visceral pain is a common syndrome that drives patients to the clinic. The long duration of inflammatory visceral pain brings both suffer and economic burden to patients.With the development of pain medicine, people have realized that pain, especially chronic pain is not just a simple pathological phenomenon, but also accompanied by changes of psychology, cognition and behavior.
International Association for the Study of Pain (IASP)has proposed a definition: pain is an unpleasant sensory and emotional experience associated with either actual or potential tissue damage, or described in terms of such damage. Recently more and more clinical observations demonstrate that, the malignant moods such as anxiety, depression and fear in patients with chronic pain would bring more severe harm than pain itself[1].
As an important component of traditional Chinese medicine, acupuncture is superior in treating pain conditions. Experimental researches have found that in a rat model of complete Freund’s adjuvant (CFA)-induced arthritis, acupuncture could relieve chronic inflammatory pain as well as the depression/anxiety state[2]. So far there are rare reports of moxibustion effect on chronic inflammatory visceral pain. The present study used a rat model of chronic inflammatory visceral pain induced by Trinitrobenzene Sulfonic Acid(TNBS) and observed the effect of herbal-partitioned moxibustion (HPM) on the pain behavior and emotion in the model, to explore the analgesic effect of moxibustion from behavioral and affective aspects.
1 Matierial and Methods
1.1 Animals and grouping
Twenty-four healthy male adult Sprague-Dawley (SD)rats, weighing (150±20) g, were purchased from Shanghai Sippr-BK Experimental Animal Company. The certificate number is SCXK (SH) 2013-0016. The rats were housed in a light-dark (12:12) room, with the RT of(20±1) ℃ and the humidity of about 50%. One week later rats were randomly divided into a normal group, a model group and an HPM group, with eight in each group.
1.2 Instruments and agents
5% TNBS (Sigma, American); absolute ethyl alcohol,pentobarbital sodium (Sinopharm Chemical Reagent Co.,Ltd., China); 7420/7445 rat autonomous behavior equipment (Ugo Basile,Italy); Ethovision 3.1 EPM behavior analyze system (Noldus, Netherlands); Von Frey Filaments (Stoelting, America); BME2410A thermal stimulus equipment (Biological Engineering Institute, China Academy of Medical Sciences, China).
1.3 Model preparation
According to the method described by Morris GP,et al[3], the rat model of chronic inflammatory visceral pain was prepared using TNBS. Rats were fasting but could drink water for 24 h before modeling; 50%ethanol was mixed with equal volume ethanol and double distilled water; 5% TNBS and 50% ethanol were mixed at 2:1 volume ratio into TNBS enema and 2%Pentobarbital Sodium [30 mg/(kg·BW)]was intraperitoneally injected for anesthesia. The rats received TNBS [3 mL/ (kg·BW)]via enema for modeling. A 1 mL syringe was connected to a clyster needle and the enema was extracted. The rat’s tail was hung up, when the needle was slowly inserted into the anal for 6-8 cm and the enema was injected. After the needle was pulled out the rat was kept still for 1 min to prevent enema overflow. Enema was given once every 7 d, 4 times in total. Abdominal withdrawal reflex (AWR) score,mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) were detected before and after modeling to evaluate the visceral and somatic pain.The successfully modeling rats displayed weight loss,diarrhea or bloody feces[4], and the abnormal changes of AWR, MWT and TWL.
1.4 Grouping and interference
1.4.1 Normal group
Rats in the normal group were only fixed without modeling or any interference.
1.4.2 Model group
Rats in the model group were modeling and fixed as the HPM group.
1.4.3 HPM group
Rats in the HPM group were modeling followed by HPM treatment.
Acupoints and locations: Bilateral Tianshu (ST 25)and Qihai (CV 6) were selected. The locations of acupoints were according to the rats’ standard acupoints in the Experimental Acupuncture Science[5]and relevant literature[6-7]. Tianshu (ST 25) points are 5 mm horizontally away from Shenque (CV 8) point(the intersection point of upper 2/3 and lower 1/3 along the line from xiphoid process to upper edge of pubic symphysis), one on each side. Qihai (CV 6) point is located 10 mm below the umbilicus [Shenque (CV 8)point], i.e, in the abdominal midline, at the midpoint between Shenque (CV 8) and Guanyuan (CV 4).
HPM method: Herb cake was a mixture of herb powder and yellow rice wine, and moulded to a cake of 10 mm in diameter. A moxa cone was moulded with 90 mg weight. Two cones for each acupoint, once a day for 7 d (Figure 1).
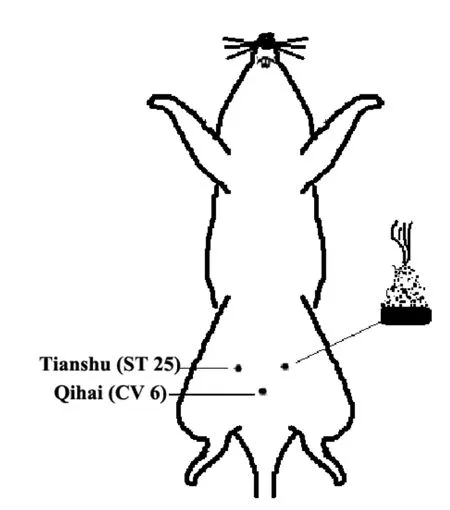
Figure 1. HPM schematic diagram
1.5 Index and methods
1.5.1 Pain behavior assessment
Visceral sensitivity detection: AWR score was adopted according to literature[8]. Colorectal distension(CRD) was applied through a self-made balloon which is connected with a sphygmomanometer and a syringe through a three-way-valve. Animals were fasted with water ad lib 8-12 h before scoring to reduce feces.Before stimulation the balloon head end touched the anus to promote defecation. The balloon was slowly inserted to the descending colon site along the rectum physiological curvature, and the syringe was pushed to give constant pressure, by connecting to a three-wayvalve on the sphygmomanometer to control the size of balloon, and 20 mmHg, 40 mmHg, 60 mmHg, 80 mmHg pressure levels were respectively given.
The balloon expansion lasted for 20 s, each time with 5 min interval. Each rat received 3 times expansion, and the mean score was recorded.
The AWR score was assigned as follows.
1 point: Normal behavior without response.
2 points: Contraction of abdominal muscles.
3 points: Lifting of abdominal wall.
4 points: Body arching and lifting of pelvic structures.MWT detection: Von Frey filament (2.0 g, 4.0 g, 6.0 g,8.0 g and 15.0 g) and up-down method were used to calculate 50% positive response paw withdrawal threshold[9]. A plexiglass box (22 cm × 12 cm × 22 cm)was placed on a metal mesh floor. After rats were adapted for 15 min in the box, von Frey filaments were perpendicularly applied to the mid plantar surface for less than 4 s. The 50% withdrawal threshold was determined using the up-down method of Dixon (1980).A testing regime described by Chaplanetal (1994) was used. A response was regarded as positive if the rat showed hind paw withdrawal with clear signs of aversion against the stimulus (i.e. licking the paw, lifting paw or maintaining the paw up). If a positive response was elicited, the next weaker filament was chosen for the next measurement. In the absence of positive response, a stronger filament was presented. This consecutive way was continued until three responses in the immediate vicinity of the 50% threshold were obtained. The resulting sequence of positive and negative responses was used to interpolate the 50%withdrawal threshold. The cut-off threshold is 15.0 g.
TWL detection: The plexiglass box was arranged on the 3 mm thick glass plate and rats were placed in the box. A heat stimulator shed light on the plantar surface until the rat avoidance response as lifting leg appeared,and the time was recorded[10]. The cut-off time was 20.1 s to prevent tissue damage. Each animal was measured for 5 times with 3 min interval. The maximum and minimum values were dismissed and the average of the rest 3 times was recorded as TWL.
1.5.2 Pain affection assessment
Open field test (OFT)[11-12]: The rats were placed in operation box (90 cm × 90 cm × 90 cm). The counter connected to computer automatically recorded the horizontal and vertical activity values for 5 min. The total number of vertical activity was statistical analyzed.
Elevated plus-maze text (EPMT)[13]: the rats were placed in elevated plus maze at the center of the platform and its head was on one open arm. Then the entry numbers and dwell time were recorded for 5 min,i.e., open arm entry (OE), open arm time (OT), close arm entry (CE) and close arm time (CT). According to the above data, the percentage of open arm entries OE% = OE ÷ (OE + CE) × 100%; the ratio of the residence time of the open arms OT% = OT ÷ (OT +CT) × 100%.
1.5.3 Colon histopathological observation
The colon tissue was through HE staining and observed through light microscopy.
1.6 Statistical methods
The SPSS 16.0 version statistical software was used for statistical analysis. The data were tested for normal distribution and homogeneity of variance. If the data followed normal distribution, they were showed as mean ± standard deviation (). One-way ANOVA was used to test the difference between groups, least significant difference (LSD) for homogeneity of variance data and Games-Howell differences for heterogeneity of variance data. If the data were not normally distributed,they were shown as median and quartiles, and Wilcoxon test was used to compare differences between groups. A P value <0.05 was regarded a significant difference.
2 Results
2.1 Histopathological changes of rat colon
The epithelium structure of normal rat colon was clear and complete, with seldom inflammatory cell infiltration in lamina propria and no congestion, edema or ulceration. The tissue structure was unclear in rat colon of the model group, with some mucous layer missing, the glands destroyed, a large number of neutrophils and lymphocytes infiltration, and severe edema. In the HPM group, the epithelium mucosa was intact rat’s colon with thickened lamina propria, many inflammatory cells infiltrated, and slight submucosal tissue edema (Figure 2).
2.2 AWR score
When colorectal distension was given to rats in each group, the AWR score of rats in the model group was significantly higher than that in the normal group(P<0.01) at the pressures of 20 mmHg, 40 mmHg,60 mmHg and 80 mmHg respectively. However, the AWR of the HPM group was significantly decreased as compared with the model group at each pressure respectively (P<0.01), (Table 1).
2.3 MWT
Before modeling, there were no statistical differences in MWT among groups (P>0.05).
After modeling, both results of MWT in the model group and the HPM group were decreased significantly compared with the normal group respectively(P<0.05).
After treatment, MWT in the HPM group was significantly increased compared with that in the model group (P<0.05).
Within-group comparison showed that there was no difference in MWT in the model group before and after treatment (P>0.05). However, MWT in the HPM group after treatment was increased significantly compared with that before treatment (after modeling), (P<0.05),(Table 2).
2.4 TWL
Before modeling, there were no statistical differences in TWL among groups (P>0.05).
After modeling, both results of TWL in the model group and the HPM group were decreased significantly compared with that in the normal group respectively(P<0.05).
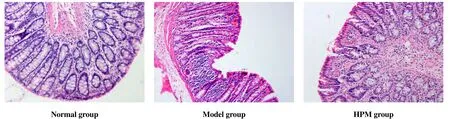
Figure 2. Histopathological changes of rat’s colon in each group

Table1.Effect of HPM on AWR score in rats with chronic inflammatory visceral pain [M (min-max)]
Table2.Effect of HPM on MWT in rats with chronic inflammatory visceral pain (, g)

Table2.Effect of HPM on MWT in rats with chronic inflammatory visceral pain (, g)
Note: Compared with the same group before modeling, 1) P<0.05; compared with the normal group at the same time point, 2) P<0.05;compared with the same group after modeling, 3) P<0.05; compared with the model group at the same time point, 4) P<0.05
Group n Before modeling After modeling After treatment Normal 8 14.07±4.39 14.52±0.25 14.92±1.22 Model 8 15.00±0.00 10.00±5.251)2) 8.18±3.201)2)HPM 8 15.00±0.00 10.18±3.171)2) 13.22±1.573)4)
TWL in the HPM group after treatment was significantly increased compared with that in the model group (P<0.05).
Intra-group comparison showed that there was no difference of TWL in the model group before and after treatment (P>0.05). However, TWL in the HPM group after treatment was increased significantly compared with that before treatment (after modeling), (P<0.05),(Table 3).
Table3.Effect of HPM on TWL in rats with chronic inflammatory visceral pain (, s)

Table3.Effect of HPM on TWL in rats with chronic inflammatory visceral pain (, s)
Note: Compared with the same group before modeling, 1) P<0.05; compared with the normal group at the same time point, 2) P<0.05;compared with the same group after modeling, 3) P<0.05; compared with the model group at the same time point, 4) P<0.05
Group n Before modeling After modeling After treatment Normal 8 19.20±1.95 19.01±0.47 19.24±0.60 Model 8 18.38±3.62 12.55±0.751)2) 13.33±0.301)2)HPM 8 18.32±2.87 13.37±1.921)2) 16.78±1.403)4)
2.5 OFT
The OFT results showed that the total distance(horizontal activity value) in rats of the model group was significantly reduced compared with that in the normal group (P<0.01). The horizontal activity distance in the HPM group was increased significantly compared with that in the model group with a statistical difference(P<0.01), (Figure 3).
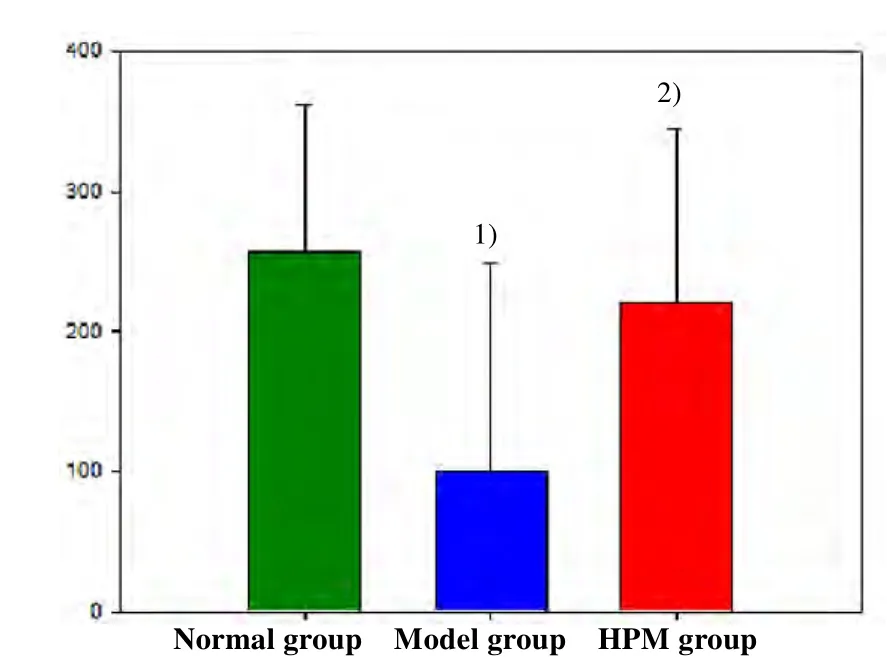
Figure3. Comparison of horizontal activity value of OFT after treatment [Note: Compared with the normal group, 1)P<0.01; compared with the model group,2) P<0.01)]
The number of upright (vertical activity value) in rats of the model group was significantly reduced compared with that in the normal group (P<0.01); the vertical activity value of rats in the HPM group increased compared with that in the model group with a statistically significant difference (P<0.01), (Figure 4).
2.6 EPMT
Compared with the normal group, OE% and OT% in the model group were significantly decreased with statistically significant differences (P<0.01). OT% and OE% levels in the HPM group increased significantly compared with those in the model group (P<0.01),(Figure 5).

Figure 4. Comparison of vertical activity value of OFT after treatment [Note: Compared with the normal group, 1)P<0.01; compared with the model group,2) P<0.01)]
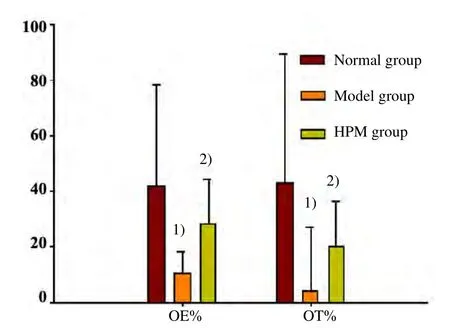
Figure 5. Comparison of EPMT after treatment[Note: Compared with the normal group, 1) P<0.01;compared with the model group,2) P<0.01)]
3 Discussion
Chronic visceral pain is a common symptom of many gastrointestinal diseases, such as inflammatory bowel disease, irritable bowel syndrome and chronic enteritis.The recognition of chronic visceral pain mechanism is far behind of physical pain, due to its various causes and manifestations. So far the occurrence and duration in chronic visceral pain is a very complicated process with pathological changes in both peripheral nociceptors and central neuronal sensitization, and is associated with the peripheral-central passway of sensation.
Currently, the treatment of chronic visceral pain is mainly with drugs and neuromodulation therapy, but the effect is far from satisfactory. Analgesic effect of moxibustion therapy has been confirmed by many clinical studies, showing that it can effectively relieve abdominal pain, stomachache, and dysmenorrhea[14-17].Tianshu (ST 25) and Qihai (CV 6) are a pair of acupoints prescription summarized out of clinical practice for gastrointestinal diseases by our group. Tianshu (ST 25) is the Front-Mu point of the large intestine. It functions as promoting qi movement and digestion to stop diarrhea and pain. Qihai (CV 6) functions as soothing intestinal stagnation, strengthening qi and regulating blood.Combination of the two points could effectively treat abdominal pain, diarrhea and other diseases[18-22].Therefore, this study selected moxibustion at Tianshu(ST 25) and Qihai (CV 6) to treat chronic inflammatory visceral pain.
Patients with chronic visceral pain are often accompanied by physical pain, such as back pain,headaches and muscle pain. This phenomenon may be due to central sensitization induced by peripheral nociceptive stimuli, or cross-talk in central nervous system of visceral afferent fibers and limb efferent fibers[23]. Animal experiments showed that visceral noxious stimulation often leads to both visceral and somatic hypersensitivity[24]. Some studies show that in a rat model of TNBS-induced intestinal inflammation,somatic hyperalgesia was also observed besides visceral hyperalgesia, and the somatic hyperalgesia reflected the degree of intestinal inflammation signals transmitted to the central nervous system. It was deduced that many spinal neurotransmitters might be involved in this process[23]. The present study observed the effect of moxibustion at Tianshu (ST 25) and Qihai (CV 6) on visceral and somatic pain in a rat model of TNBS-induced visceral pain. It was found that, AWRs at each pressure of the model group were significantly higher than those of the normal group, indicating that hyperalgesia and allodynia were presented in the rat model of TNBS-induced chronic inflammatory pain.
In addition, the MWT and TWL in model rats also decreased significantly compared with the normal group, suggesting the presence of physical (mechanical and thermal) hyperalgesia in the model. In the HPM group, the MWT and TWL were significantly increased compared with those in the model group, indicating that moxibustion has analgesic effect on somatic pain in the model. The existence of both visceral and somatic pain in visceral pain model rats, so far, hasn’t clear explanation. Some scholars speculated that the pathogenesis of visceral pain was not limited to the organs but may involve levels of the spinal cord or brain[24]. The visceral inflammation caused peripheral sensitization which transmitted more signals to central nervous system, resulting in neuronal plasticity and central sensitization. The descending inhibitory passway was suppressed, leading to somatic and visceral hyperalgesia. Whether the analgesic effect of moxibustion on visceral pain is associated with modulating the peripheral neurotransmitters needs further study.
Sensory differentiation and emotional experience are two components of pain. Clinical observations show that patients with chronic visceral pain often suffer from severe emotional disturbance, which in turn enhances feelings of pain[25]. Therefore, the malignant sentiment should also be an indicator of pain observation and effectiveness of pain treatments. Therefore, this study used open-field test and elevated plus maze test to observe the effect of moxibustion therapy on emotions in visceral pain model. OFT could record locomotor activity and exploratory behavior as a major tool to detect depression and anxiety[11]. EPMT is an unconditioned reflex test to evaluate the anxious behavior of animals. It is based on spontaneous behavior and can be more objective reflect the animal's anxiety state[26]. The results showed that TNBS induced chronic inflammatory visceral pain as well as malignant sentiments in rats. After modeling, the level of OFT activities, OE% and OT% significantly decreased and HPM elevated OFT activities, OE% and OT% value,indicating that moxibustion could relieve anxiety and depression in visceral pain.
Studies have shown that moxibution could downregulate serum interleukin-2 (IL-2), IL-6 and tumor necrosis factor-α (TNF-α) levels in a rat model of depression[27]. Other studies show that moxibustion reversed the decrease of brain derived neurotrophic factor (BDNF) in cortical and hippocampal neurons in depression rats, increased the number of BDNF positive neurons and BDNF mRNA expression levels, suggesting that protection of cortical and hippocampal neurons might be the mechanism of moxibustion treatment on depression[28]. While acupuncture plays its regulating role by acupoints stimulation[29-31], the mechanism of moxibution on visceral pain, which might refer to multiple levels, remains further exploration[32-34].
In the present study, model rats showed not only somatic and visceral hyperalgesia but also malignant emotion as anxiety and depression. We used both tools to detect the perception and affection of pain state, as well as effectiveness of moxibustion treatment.Herb-partitioned moxibustion can significantly relieve visceral pain in rats with chronic inflammatory pain, and can improve the anxiety and depression state.
Conflict of Interest
The authors declared that there was no potential conflict of interest in this article.
This work was supported by National Natural Science Foundation of China (No.81273843); National Basic Research Program of China (973 Program, No.2009CB522900); Project of Shanghai Municipality Health Bureau (No. 20144Y0153, No. 20124Y004).
Statement of Informed Consent
The treatment of animals conformed to the ethical criteria in this experiment.
[1]Zhang YQ. Neural mechanism of pain affection and relative memory development. Ziran Kexue Jinzhan, 2005,15 (12): 1409-1415.
[2]Sun J, Wen J, Wei HF, Zhong DK, Zeng PJ, Li Y, Yang L,Zhang LF. Experimental observation on psychological and behavioral changes by electroacupuncture at different acupoints in model rats of chronic inflammatory pain.Xiandai Shengwu Yixue Jinzhan, 2011, 11(20): 3820-3825.
[3]Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon.Gastroenterology, 1989, 96(3): 795-803.
[4]Chen X, Yang SZ, Chi BR. Reproduction of rat inflammatory bowel disease model and intervention effect of Changkangyin on it. Jilin Daxue Xuebao: Yixue Ban,2008, 34(2): 262-265.
[5]Guo Y. Experimental Acupuncture Science. Beijing: China Press of Traditional Chinese Medicine, 2008: 415.
[6]Li CR, Hua XB, Zhou HL, Song DL, Hu YL. Development of acupuncture points diagram of guinea pigs. Shanghai Zhenjiu Zazhi, 1992, 11(2): 28-30.
[7]Wang HJ, Ji LX. Discussion of localization of rat Shenque(CV 8) point. Zhen Ci Yan Jiu, 2007, 32(5): 312.
[8]Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development.Gastroenterology, 2000, 119(5): 1276-1285.
[9]Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL.Quantitative assessment of tactile allodynia in the rat paw.J Neurosci Methods, 1994, 53(1): 55-63.
[10]Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain, 1988, 32(1): 77-88.
[11]Shin J, Gireesh G, Kim SW, Kim DS, Lee S, Kim YS,Watanabe M, Shin HS. Phospholipase C beta 4 in the medial septum controls cholinergic theta oscillations and anxiety behaviors. J Neurosci, 2009, 29(49): 15375-15385.
[12]Yang S. Study on the mechanism of acupuncture on visceral hypersensitivity and affection regulation in a rat model of IBS. Master thesis of Beijing University of Chinese Medicine, 2013.
[13]Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl), 1987, 92(2):180-185.
[14]Zhang J, Zhang Z. Cuckoo-bud moxibution treatment in 56 cases of stomachache. Zhongguo Zhen Jiu, 2006, 26(10):744.
[15]Zhang BM, Wu HG. Application progress of moxibution on painful diseases of soft tissues. Shanghai Zhenjiu Zazhi,2005, 24(2): 43-46.
[16]Zhou EH, Liu HR, Wu HG, Shi Y, Wang XM, Tan LY, Yao LQ, Zhong YS, Jiang Y, Zhang LL. Suspended moxibustion relieves chronic visceral hyperalgesia via serotonin pathway in the colon. Neurosci Lett, 2009,451(2): 144-147.
[17]Zhang W. Study on correlation between time, sensation and efficacy of moxibustion Guanyuan (CV 4) in primary dysmenorrhea. Shizhen Guoyi Guoyao, 2014, 25(5):1148-1150.
[18]Liu HR, Hua XG, Yang Y, Wu HG. Clinical research on 5-HT expression in colon mucosa of diarrhea predominant patients and acupuncture treatment. Liaoning Zhongyi Zazhi, 2006, 33(8): 953-954.
[19]Wu HG, Shi Z, Zhu Y, Ma XP, Yao Y, Cui YH, Zhao TP,Liu HR. Clinical research of herb-partitioned moxibustion treatment on ulcerative colitis. Shanghai Zhenjiu Zazhi,2007, 26(4): 3-4.
[20]Shi Y, Wu HG. Clinical research of herb-partitioned moxibustion treatment on Crohn’s disease. Jiangxi Zhongyiyao, 2003, 34(8): 16-17.
[21]Qi L, Li N, Liu HR, Ma XP, Wu LY, Wang XM, Zhou CL,Wu HG. Clinical and experimental studies on moxibustion for treatment of irritable bowel syndrome. CJTCMP, 2010,25(12): 2224-2227.
[22]Liu HR, Qi L, Wu LY. Effects of moxibustion on dynorphin and endomorphin in rats with chronic visceral hyperalgesia. World J Gastroenterol,2010,16(32): 4079-4083.
[23]Zhou QQ, Price DD, Caudle RM, Verne GN. Visceral and somatic hypersensitivity in TNBS-induced colitis in rats.Dig Dis Sci, 2008, 53(2): 429-435.
[24]Tian XL, Wang YF, Chen SL, Mo JZ, Chen WH, Cao ZJ.Intestinal inflammation induces visceral and somatic hypersensitivity. Gastroenterology, 2012, 17(7): 399-403.
[25]Zhang YQ, Zhao ZQ, Ji RR. Emotional distress and related memory of pain: a neurobiological review. Neurosci Bull,2005, 21(1): 10-17.
[26]Wang X, Xie M. Comment on the dose-effect relationship of Suanzaoren decoction on behavior of EPM rat.Zhongguo Shiyan Fangjixue Zazhi, 2004, 10(1): 35-37.
[27]Li LP, Hua JS, Sun ZR, Sun DW, Huang L. Influence of moxibustion at Baihui (GV 20) and Taichong (LR 3) on cytokines in a rat model of chronic stress induced depression. Zhongyiyao Xuekan, 2006, 24(9): 1757-1760.
[28]Li LP, Bi Y. Effect of acupuncture and moxibustion on brain-derived neurotrophic factor in the chronic mild unpredictable stressors depression rats. Zhonghua Zhongyiyao Xuekan, 2008, 26(10): 2287-2290.
[29]Zhou M, Wang XY, Li WM. Progress on basic study of acupuncture for visceral pain. Shanghai Zhenjiu Zazhi,2007, 26(10): 45-48.
[30]Ma XP, Hong J, An CP, Zhang D, Huang Y, Wu HG, Zhang CH, Meeuwsen S. Acupuncture-moxibustion in treating irritable bowel syndrome: How does it work? World J Gastroenterol, 2014, 20(20): 6044-6054.
[31]Takahashi T. Effect and mechanism of acupuncture on gastrointestinal diseases. Int Rev Neurobiol, 2013, 111:273-294.
[32]Bao CH, Wu LY, Wu HG, Xu B, Lu Y, Liu HR, Yu SG,Zhao TP, Zhao JM, Zhao BX, Hu L, Chang XR. Study on effect and mechanism of moxibustion inhibit visceral pain of intestinal disease. Zhonghua Zhongyiyao Zazhi, 2014,29(2): 419-422.
[33]Huang RJ, Zhao JM, Wu LY, Dou CZ, Liu HR, Weng ZJ,Lu Y, Shi Y, Wang XM, Zhou CL, Wu HG. Mechanisms underlying the analgesic effect of moxibustion on visceral pain in irritable bowel syndrome: a review. Evid Based Complement Alternat Med, 2014: ID895914.
[34]Wu HG, Ma XP, Zhou CL, Bao CH, Dou CZ. Current research status and developing strategy of moxibustion.Shijie Zhongyiyao, 2013, 8(8): 845-851.
 Journal of Acupuncture and Tuina Science2015年1期
Journal of Acupuncture and Tuina Science2015年1期
- Journal of Acupuncture and Tuina Science的其它文章
- 电针对脑缺血再灌注模型大鼠血清白介素的影响
- 复式针刺补泻对臀大肌挛缩术后髋关节和膝关节屈伸角度的影响
- Treatment of ankylosing spondylitis by fire-needle therapy plus tuina manipulations
- Subtle adjustment of the cervical spine combined with Shu Jing Ding Xuan Decoction for cervical vertigo
- Electroacupuncture combined with traction and tuina for lumber intervertebral disc herniation
- Observation on therapeutic effect of electroacupuncture plus acupoint-injection for nerve root sciatica
