Hydrothermal Liquefaction of Wheat Straw in Sub-critical Water/Ethanol with Ionic Liquid for Bio-oil Production
Wang Baofeng; Han Shaohua; Zhang Jinjun
(1. Institute of Resources and Erovironment Engineering, Shanxi University, Taiyuan 030001; 2. School of Chemistry & Material Science, Shanxi Normal University, Linfen 041004)
Hydrothermal Liquefaction of Wheat Straw in Sub-critical Water/Ethanol with Ionic Liquid for Bio-oil Production
Wang Baofeng1,2; Han Shaohua2; Zhang Jinjun2
(1. Institute of Resources and Erovironment Engineering, Shanxi University, Taiyuan 030001; 2. School of Chemistry & Material Science, Shanxi Normal University, Linfen 041004)
Hydrothermal liquefaction of wheat straw in sub-critical water with ionic liquid was investigated in an autoclave. The product distribution at different temperatures and pressures was studied. The liquid oil and the residuals were tested by1H NMR, FTIR and SEM techniques. The results indicated that under the same conditions, the oil yield from liquefaction of wheat straw in water/ethanol was higher than that in sub-critical water. The result also showed that under the investigated conditions, adding 1-butyl-3-methylimidazolium chloride ([Bmim]Cl) could increase the total conversion and gas yield, while at the same time the yield of n-hexane insoluble fraction and the tetrahydrofuran soluble fraction was reduced. Moreover, the results also showed that upon adding [Bmim]Cl the contents of the aliphatic hydrogen and phenols in liquid oil also increased along with improved oil quality.
wheat straw; liquefaction; sub-critical water; ionic liquid
1 Introduction
Nowadays, more and more attention has been paid to the energy from biomass resources because of the increasing worldwide energy demand and low CO2emission requirements[1-2]. Liquefaction of biomass could yield valuable products, so there are many studies about liquefaction of biomass[3-19]. The studies include the following topics, viz.: the kinetic study on the liquefaction of wood[4], the hydrothermal liquefaction of beech wood using a natural calcium borate mineral[5], the co-deoxy-liquefaction of biomass and vegetable oil to hydrocarbon oil[6], the synergic effect study of methanol and water on pine liquefaction, etc[7-9]. As we have known, liquefaction of biomass with proper solvents and catalysts is a promising process to produce liquid biofuels and valuable chemicals, and there are also many studies on the effect of catalysts and solvents on liquefaction of biomass for production of biooils[1,10]. The solvents used in liquefaction of biomass covered water, ethanol, methanol, acetone, phenolic compounds, polyhydric alcohol and tetralin[11-19]. The catalysts used for liquefaction included alkalies, or alkaline salts, or stronger acidic salts, such as A1Cl3+HC1, (NH4)2SO4and CuSO4, Ni–Mo/Al2O3, K2CO3, H2SO4and NaOH, Ru/C catalyst, and so on[9-10,19-21]. Recently, ionic liquids are also used as solvents and catalysts in liquefaction process thanks to their ability to dissolve a large amount of cellulose under considerably mild conditions for obtaining a close to 100% recovery[22-24]. Researchers have been using 1-butyl-3-methyl-imidazolium chloride as the ionic liquid because of its special characteristics[25-26]. Previous study showed that hydrophilic ionic liquids could dissolve cellulose in biomass[25]. Since the ionic liquid 1-butyl-3-methyl-imidazolium chloride ([Bmim]Cl) is miscible with water, so the hydrothermal liquefaction of wheat straw in sub-critical water with the ionic liquid 1-butyl-3-methyl-imidazolium chloride for producing bio-oil is proposed and studied in this paper.
2 Material and Methods
2.1 Samples
Wheat straw (WS) we used was collected from Linfen city in Shanxi Province of China. The sample was at firstair-dried and then crushed, ground and sieved to obtain a sieve fraction ranging from 0.15 mm to 0.25 mm. Table 1 presents the ultimate analysis of the wheat straw which was analyzed by a Vario ELIII elemental analyzer (Elementar, Germany).
The ionic liquid used was 1-butyl-3-methylimidazolium chloride ([Bmim]Cl) with a purity of 99%. This ionic liquid was purchased from the Henan Lihua Pharmaceutical Co., Ltd. (China) and was used without further purification.
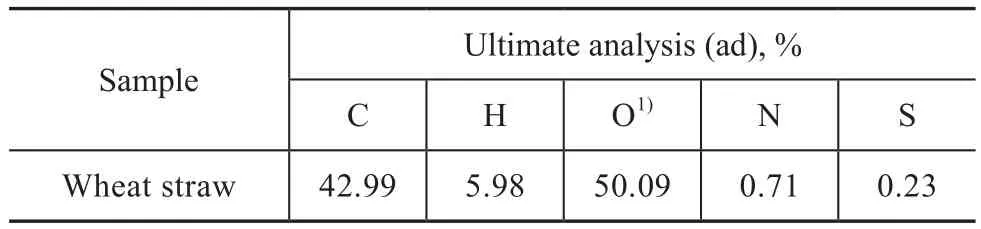
Table 1 The ultimate analysis of samples
2.2 Experimental setup and procedure
The hydrothermal liquefaction experiments were carried out in a 250-mL autoclave. Each time 10.00 g of wheat straw and 4.00 g of ionic liquid ([Bmim]Cl) were put into the reactor together with 80 ml of deionized water or a mixture of deionized water and ethanol (at a ratio of 1:1, v/v). Before the liquefaction experiment, the reactor was filled with nitrogen to the desired initial pressure (2.0—5.0 MPa) and sealed; then the reactor was heated to the desired temperature (260 ℃, 280 ℃, 300 ℃, and 320 ℃, respectively) at a temperature increase rate of 10 ℃/min by a furnace and then stayed at the required temperature for 30 min. After termination of the reaction the reactor was cooled down to room temperature and depressurized to atmospheric pressure. The solid and liquid products were discharged, separated, dried and sent for further analyses.
2.3 Fractionation of liquefaction products
The solid and liquid products were extracted by hexane, benzene and tetrahydrofuran, respectively. These chemical reagents (bought from the Guangfu Fine Chemical Research Institute of Tianjin, China) were all analytically pure reagents[27]. After extraction, the products were separated into the following fractions: the benzene soluble and hexane soluble fraction (oil, O), the hexane insoluble but benzene soluble fraction (HIF), the benzene insoluble but tetrahydrofuran soluble fraction (THFSF), and the tetrahydrofuran insoluble fraction (residue, R)[27-28].
2.4 Calculation of liquefaction products yield
The yields of liquefaction products were calculated as shown below[27]:

Hexame insoluble but benzene soluble fraction yield

Benzene insoluble but tetrahydrofuran soluble fraction

Gas yield (G, %)=100%-(O+HIF+THFIF+R)%
Total conversion (TC, %)=100%-R(%)
where wwis the mass of wheat straw, g; wOis the mass of the oil, g; wHIFis the mass of the hexane insoluble but benzene soluble fraction, g; wTHFSFis the mass of the benzene insoluble but tetrahydrofuran soluble fraction, g; and wRis the mass of the residue, g.
3 Results and Discussion
3.1 Hydrothermal liquefaction of wheat straw with ionic liquid at different temperatures
Figure 1 shows the distribution of liquefaction products at different temperatures (260 ℃, 280 ℃, 300 ℃, and 320 ℃, respectively). During the experiment, the initial nitrogen pressure was 2 MPa and the residence time was 30 min. It can be seen from Figure 1 (a) that during hydrothermal liquefaction of wheat straw in sub-critical water, when the temperature increased from 260 ℃ to 300 ℃, the oil yield and the residue yield also increased, while the gas yield and the total conversion decreased. When the temperature increased from 300 ℃ to 320 ℃, the oil yield decreased, while the gas yield and the total conversion increased. This outcome just coincidedwith the results of the previous study[19]. When the temperature increased from 260 ℃ to 320 ℃, the yield of hexane insoluble but benzene soluble fraction (HIF) and the yield of benzene insoluble but tetrahydrofuran soluble fraction (THFSF) changed irregularly. The reason might be attributed to the competitive reactions, hydrolysis and repolymerization during liquefaction process at high temperature[25]. It can also be seen from Figure 1 (b) that during hydrothermal liquefaction of wheat straw in the presence of sub-critical water with ionic liquid, when temperature increased from 260 ℃ to 320 ℃, the yields of the liquefaction products all changed, implying that the reaction temperature was a crucial parameter for effective conversion of biomass to liquid products[30]. Upon comparing Figure 1 (a) with Figure 1 (b), it can be noticed that addition of ionic liquid [Bmim]Cl changed the oil yield slightly except for the case at 300 ℃; moreover, addition of ionic liquid [Bmim]Cl also could increase the total conversion and gas yield obviously along with the decrease in yields of HIF and THFSF. This result implied that adding ionic liquid [Bmim]Cl was beneficial to the decomposition of wheat straw during hydrothermal liquefaction in the presence of sub-critical water. The reason might be ascribed to the fact that [Bmim]Cl was able to dissolve the long aliphatic chains and bridges, and could solubilize the trapped organics[25].
3.2 Hydrothermal liquefaction of wheat straw with ionic liquid at different pressure

Figure 1 Distribution of products from liquefaction of wheat straw in the presence of sub-critical water at different temperatures (at 2 MPa, 30 min)■—260 ℃;■—280 ℃;■—300 ℃;■—320 ℃
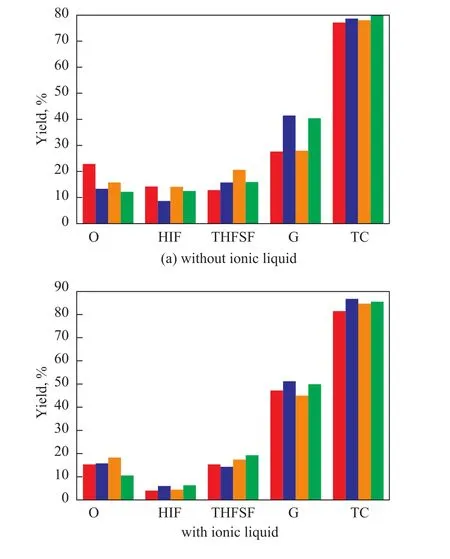
Figure 2 Distribution of products from liquefaction of wheat straw in the presence of sub-critical water under different pressures(at 320℃, 30min)■—1 MPa;■—2 MPa;■—3 MPa;■—4 MPa
Figure 2 shows the distribution of liquefaction products at different pressures with or without the ionic liquid [Bmim]Cl at a temperature of 320 ℃ and a residence time of 30 min. It can be seen from Figure 2(a) that during hydrothermal liquefaction of wheat straw in the presence of sub-critical water, when the initial nitrogen pressure increased from 1 MPa to 4 MPa, the products yields changed obviously, and it also can be seen that the maximum yield of oil was 22.7% at 1 MPa, and the maximumyield of gas was 41.3% at 2 MPa, while the maximum total conversion reached 80.4% at 4 MPa. It can be seen from Figure 2(b) that during hydrothermal liquefaction of wheat straw in the presence of sub-critical water with the ionic liquid [Bmim]Cl, when the initial nitrogen pressure increased from 1 MPa to 4 MPa, the products yields also changed obviously, and the maximum yield of oil reached 18.1% at 3 MPa; the maximum yield of gas and total conversion was 51% and 86.6%, respectively, at 2 MPa. Upon comparing Figure 2(a) with Figure 2(b), it can be seen that when the initial nitrogen pressure increased from 1 MPa to 4 MPa during hydrothermal liquefaction of wheat straw in the presence of sub-critical water, addition of ionic liquid [Bmim]Cl could increase the gas yield and the total conversion along with a decrease in the yield of HIF and liquefaction residue. Moreover, addition of ionic liquid [Bmim]Cl also increased the oil yield when the initial nitrogen pressure increased from 2 MPa to 3 MPa. This result was just similar to that depicted in Figure 1.
3.3 Hydrothermal liquefaction of wheat straw with ionic liquid at different reaction time
Figure 3 shows the distribution of liquefaction products obtained at different reaction time. It can be seen from Figure 3(a) that during hydrothermal liquefaction of wheat straw in the presence of sub-critical water, when the reaction time increased from 15 min to 30 min, the gas yield and the total conversion all increased, while the yields of HIF and THFSF all deceased along with a slight change in the oil yield. When the reaction time was extended from 30 min to 45 min, the oil and HIF yields and the total conversion all increased along with a decrease in the gas and THFSF yields. It can be speculated that extension of reaction time could cause repolymerization of unsaturated products[30]. It can be seen from Figure 3(b) that addition of [Bmim]Cl during hydrothermal liquefaction of wheat straw could increase the oil yield and the total conversion. Moreover, one also could find out that when the reaction time increased from 15 min to 30 min, the oil yield, the gas yield and the total conversion all increased, while the yields of HIF and THFSF all decreased; upon extending the reaction time from 30 min to 45 min, the oil yield and the HIF yield increased, while the gas yield, the THFSF yield and the total conversion all decreased. This result suggested that the ionic liquid [Bmim]Cl had an obvious effect on product distribution during the liquefaction process. When the reaction time was greater than 30 min, addition of [Bmim]Cl might also contribute to the formation of new and longer polymers, so the total conversion would decrease when the reaction time was greater than 30 min[30].
3.4 Hydrothermal liquefaction of wheat straw with ionic liquid in different solvents

Figure 3 Distribution of products from liquefaction of wheat straw in the presence of sub-critical water at different reaction duration■—15 min;■—30 min;■—45 min
Figure 4 shows the distribution of products obtained from liquefaction of wheat straw in the presence of sub-critical water and sub-critical water/ethanol (at a ratio of 1:1, v/v). It can be seen from Figure 4 that during hydrothermal liquefaction of wheat straw, when the solvent was the sub-critical water, the oil yield was 13.2%; and when the solvent was a water/ethanol mixture (at a ratio of 1:1, v/v), the oil yield was much higher to reach 33.5%, and at the same time, the totalconversion was also higher along with a reduction in the gas yield and the residue yield. The total conversion was 78.6% when the sub-critical water was used as the solvent, and it was 84.8% when the solvent was sub-critical water/ethanol (at a ratio of 1:1, v/v). The reason might be that the water/ethanol (at a ratio of 1:1, v/v) mixture could reduce the surface tension of the liquid products to improve the diffusion of solvent into the cellulose, hemicellulose, and lignin matrix. Moreover, ethanol was also expected to readily dissolve the relatively high-molecular-weight liquid products/intermediates derived from the polymeric units because of its lower dielectric constant[30]. Besides that, one also can see that when the solvent was sub-critical water, addition of the ionic liquid [Bmim]Cl could increase the oil yield; and when the solvent was water/ethanol (at a ratio of 1:1, v/v), addition of the ionic liquid [Bmim]Cl reduced the oil yield. Furthermore, it can be found out from Figure 4 that addition of [Bmim]Cl increased the gas yield and total conversion, and at the same time decreased the residue yield irrespective of whether the sub-critical water or water/ethanol (at a ratio of 1:1, v/v) were used as the solvent. This phenomenon might be attributed to the catalytic effect of the ionic liquid [Bmim]Cl imposed on the liquefaction process.
3.5 1H-NMR and FT-IR analysis of the oil from liquefaction of wheat straw
Figure 5 shows the FT-IR spectra of the liquid oil obtained from liquefaction of wheat straw at a temperature of 320 ℃, a pressure of 2 MPa and a reaction time of 30 minutes. It can be seen from Figure 5 that the split peaks appeared at 1 705 cm-1when the solvent was sub-critical water/ethanol, which was identified as the absorption peak of carbonyl radical. Moreover, one also can see that there existed an obvious peak at 1 068 cm-1only belonging to curve c (liquid oil from liquefaction of wheat straw in sub-critical water/ethanol without ionic liquid [Bmim]Cl), which might be the absorption peak of C-N bond. Figure 6 shows the1H-NMR spectrum of the liquid oil obtained from liquefaction (at 320 ℃, 2 MPa, 30 min), and Table 2 is the H distribution of the oil obtained from liquefaction under different conditions according to the result depicted in Figure 6. It can be found from Figure 6 and Table 2 that the aliphatic hydrogen content of the oil obtained from liquefaction of wheat straw in water/ethanol (at a ratio of 1:1, v/v) washigher than that of the oil obtained from liquefaction in sub-critical water, and the aliphatic hydrogen content was always higher than the aromatic hydrogen content irrespective of whether the solvent was water/ethanol or sub-critical water. Moreover, the addition of [Bmim]Cl could increase the aliphatic hydrogen content irrespective of whether the solvent was water/ethanol or sub-critical water, denoting that the addition of [Bmim]Cl during liquefaction could increase the contents of aliphatic hydrogen and phenols in liquid oil, which implied that addition of [Bmim]Cl could obtain an oil with higher quality.
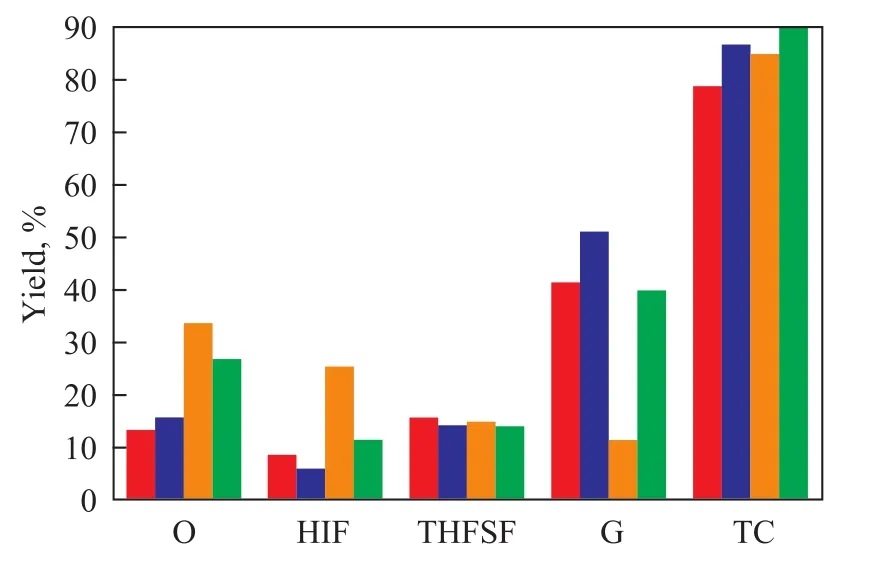
Figure 4 Distribution of products from liquefaction of wheat straw in different solvents (at 320℃, 2 MPa, 30 min)■—Without IL+water;■—4g [Bmin]Cl+water;■—Without IL+water/ethanol;■—4g [Bmim]Cl+water/ethanol

Figure 5 FT-IR spectra of oil from liquefaction of wheat straw (at 320℃, 2 MPa, 30 min)a—sub-critical water; b—sub-critical water with ionic liquid; c—subcritical water/ethanol; d—sub-critical water/ethanol with ionic liquid

Table 2 Hydrogen distribution of the oil obtained from liquefaction of wheat straw under different conditions
3.6 SEM micrographs of wheat straw and residue of liquefaction process
Figure 7 (a-d) shows the SEM micrographs of wheat straw and residue obtained from liquefaction at a temperature of 320 ℃, a pressure of 2 MPa and a reaction time of 30 min. It can be seen from Figure 7 that the pattern of wheat straw was of the long rod shape with a homogeneous and compact microstructure and smooth surface, while the pattern of the residue from liquefaction of wheat straw in sub-critical water was of the fine granular shape with a rough surface. Addition of the ionic liquid [Bmim]Cl changed slightly the structure of the residue obtained from liquefaction in sub-critical water, while adding [Bmim]Cl made the structure of the residue obtained from liquefaction of straw in water/ethanol become more loose. It occurred because more volatiles were vented during liquefaction of wheat straw in the presence of water/ethanol, resulting in more loose structure of residue. The result just coincided with the outcome of total conversion.
3.7 FT-IR analysis of the residue from liquefaction of wheat straw
The FT-IR analysis was conducted to further identify the functional groups of the residue. Figure 8 presents the FTIR curve of the residue obtained under different conditions. It can be seen from Figure 8 that organic functional groups of residue obtained from liquefaction of wheat straw in the presence of water/ethanol (at a ratio of 1:1, v/v) were more complicated, and addition of the ionic liquid [Bmim]Cl changed slightly the functional groups irrespective of whether liquefaction of straw was conducted in the presence of sub-critical water or water/ethanol (at a ratio of 1:1, v/v). This result indicated that adding [Bmim]Cl could not change the structure of the residueobtained from liquefaction of wheat straw.

Figure 6 1H-NMR spectra of oil obtained from liquefaction of wheat straw (at 320℃, 2 MPa, 30 min)

Figure 7 SEM micrographs of wheat straw and liquefaction residue (at 320℃, 2 MPa, 30 min)

Figure 8 FT-IR spectra of the residuesa—residue (sub-critical water); b—residue (sub-critical water with ionic liquid; c—residue(sub-critical water/ethanol; d—residue (subcritical water/ethanol with ionic liquid)
4 Conclusions
Hydrothermal liquefaction of wheat straw with ionic liquid was investigated. It was found out that under the same conditions, the oil yield from liquefaction of wheat straw in the presence of water/ethanol mixture was higher than that obtained in the presence of sub-critical water. The result also showed that adding [Bmim]Cl could achieve a higher total conversion and gas yield from liquefaction of wheat straw, while the yield of the hexane insoluble fraction and the tetrahydrofuran soluble fraction was reduced. Moreover, addition of [Bmim]Cl during liquefaction could increase the contents of the aliphatic hydrogen and phenols in liquid oil along with improved oil quality.
Acknowledgements:Financial support to this work by the Research Fund for the Doctoral Program of Higher Education for new teachers of China (20091404120002), the Shanxi Province Science Foundation for Youths of China (2011021008-1) and the Soft Science Program of Shanxi Province (2011041015-01) are gratefully acknowledged.
[1] Durak H, Aysu T. Effects of catalysts and solvents on liquefaction of Onopordum heteracanthum for production of biooils[J]. Bioresour Technol, 2014,166: 309-317
[2] Xu Chunbao, Etcheverry T. Hydro-liquefaction of woody biomass in sub- and super-critical ethanol with iron-based catalysts[J]. Fuel, 2008,87 (3): 335-345
[3] Chen Wanting, Zhang Yuanhui, Zhang Jixiang, et al. Co-liquefaction of swine manure and mixed-culture algal biomass from a wastewater treatment system to produce bio-crudeoil[J]. Appl Energ, 2014, 128: 209-216
[4] Zhang Hairong, Yang Huijuan, Guo Haijun, et al. Kinetic study on the liquefaction of wood and its three cell wall component in polyhydric alcohols[J]. Appl Energ., 2014, 113: 1596-1600
[5] Tekin K, Karagoz S, Bektas S. Hydrothermal liquefaction of beech wood using a natural calcium borate mineral[J]. The Journal of Supercritical Fluid, 2012, 72: 134-139
[6] Chen Yigang, Yang Fan, Wu Libin, et al. Co-deoxy-liquefaction of biomass and vegetable oil to hydrocarbon oil: Influence of temperature, residence time, and catalyst[J]. Bioresour Technol, 2011, 102 (2): 1933-1941
[7] Zhao Yunpeng, Zhu Weiwei, Wei Xianyong, et al. Synergic effect of methanol and water on pine liquefaction[J]. Bioresour Technol, 2013, 142: 504-509
[8] Ramsurn H, Gupta R B. Deoxy-liquefaction of switchgrass in supercritical water with calcium formate as an in-situ hydrogen donor[J]. Bioresour Technol, 2013,143: 575-583
[9] Li Hongyi, Hu Jiao, Zhang Zhijian, et al. Insight into the effect of hydrogenation on efficiency of hydrothermal liquefaction and physico-chemical properties of biocrude oil[J]. Bioresour Technol, 2014,163: 143-151
[10] Yona A M C, Budija F, Kricejˇ B, et al. Production of biomaterials from cork: Liquefaction in polyhydric alcohols at moderate temperatures[J]. Ind Crop Prod, 2014, 54: 296-301
[11] Sun Peiqin, Heng Mingxin, Sun Shaohui, et al. Analysis of liquid and solid products from liquefaction of paulownia in hot-compressed water[J]. Energ Convers Manage, 2011, 52: 924-933
[12] Qian Yejian, Zuo Chengji, Tan Jian, et al. Structural analysis of bio-oils from sub- and supercritical water liquefaction of woody biomass[J]. Energy, 2007, 32 (3): 196-202
[13] Toor S S, Rosendahl L, Rudolf A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies[J]. Energy, 2011, 36: 2328-2342
[14] Yuan Xingzhong, Cao Hongtao, Li, Hui, et al. Quantitative and qualitative analysis of products formed during coliquefaction of biomass and synthetic polymer mixtures in sub- and supercritical water[J]. Fuel Process Technol, 2009, 90 (3): 428-434
[15] Huang Huajun, Yuan, Xingzhong., Zhu, Huina, et al. Comparative studies of thermochemical liquefaction characteristics of microalgae, lignocellulosic biomass and sewage sludge[J]. Energy, 2013, 56: 52-60
[16] Brand, S., Hardi, F., Kim, J., et al. Effect of heating rate on biomass liquefaction: Differences between subcritical water and supercritical ethanol[J]. Energy, 2014, 68: 420-427
[17] Liu Yan, Yuan Xingzhong, Huang Huajun, et al. Thermochemical liquefaction of rice husk for bio-oil production in mixed solvent (ethanol–water) [J]. Fuel Process Technol, 2013, 112: 93-99
[18] Liu Dong, Song Linhua, Wu Pingping, et al. Direct hydroliquefaction of sawdust in petroleum ether and comprehensive bio-oil products analysis[J]. Bioresour Technol, 2014, 155: 152-160
[19] Meryemog lu, B., Hasanog˘lu, A., Irmak,S., Erbatur, S., 2014. Biofuel production by liquefaction of kenaf (Hibiscus cannabinus L.) biomass[J]. Bioresour Technol, 2014, 151: 278-283
[20] Maldas D, Shiraishi N. Liquefaction of biomass in the presence of phenol and H2O using alkalies and salts as the catalyst[J]. Biomass Bioenerg, 1997, 12: 273-279
[21] Wang Yun, Wang Hui, Lin Hongfei, et al. Effects of solvents and catalysts in liquefaction of pinewood sawdust for the production of bio-oils[J]. Biomass Bioenerg, 2013, 59: 158-167
[22] Ramosa L, Frollini E, Heinze Th. Carboxymethylation of cellulose in the new solvent dimethyl sulfoxide/tetrabutyl- ammonium fluoride[J]. Carbohydrate Polymers, 2005, 60: 259-267
[23] Vancov T, Alston AS, Brown T, et al. Use of ionic liquids in converting lignocellulosic material to biofuels[J]. Renew Energy, 2012, 45: 1-6
[24] Long J X, Li X H, Guo B, et al. Catalytic delignification of sugarcane bagasse in the presence of acidic ionic liquids[J]. Catalysis Today, 2013, 200: 99-105
[25] Shah K, Atkin R, Stanger R, et al. Interactions between vitrinite and inertinite-rich coals and the ionic liquid - [bmim] Cl [J]. Fuel, 2014, 119: 214-218
[26] Painter P, Pulati N, Cetiner R, et al. Dissolution and dispersion of coal in ionic liquids[J]. Energy Fuels, 2010, 24(3): 1848-1853
[27] Han Shaohua, Yan Xiaomin, Wang Baofeng, et al. Co-liquefaction of lignite and biomass in sub-critical water with ionic liquid[J]. CIESC Journal, 2015, 66(4): 1476-1483
[28] Wang Baofeng, Huang Yaru, Zhang Jinjun. Hydrothermal liquefaction of lignite, wheat straw and plastic waste in sub-critical water for oil: product distribution[J]. J Anal Appl Pyrol, 2014, 110: 382-389
[29] Patil P, Armbruster U, Martin A. Hydrothermal liquefaction of wheat straw in hot compressed water and subcritical water–alcohol mixtures[J]. The Journal of Supercritical Fluid, 2014, 93: 121-129
[30] Long Jinxing, Guo Bin, Teng Junjiang, et al. SO3H-functionalized ionic liquid: Efficient catalyst for bagasse liquefaction[J]. Bioresour Technol, 2011, 102: 10114 -10123
date: 2015-05-10; Accepted date: 2015-10-12.
Wang Baofeng, Telephone: + 86-357-2051192; E-mail: wangbaofeng1234@sina.com.
- 中国炼油与石油化工的其它文章
- Computational Fluid Dynamics Simulation of Liquid-Phase FCC Diesel Hydrotreating in Tubular Reactor
- Quantitative Analysis Using Fourier Transform Ion Cyclotron Resonance Mass Spectrometry and Correlation between Mass Spectrometry Data and Sulfur Content of Crude Oils
- Microbial Characterization of Denitrifying Sulfide Removal Sludge Using High-Throughput Amplicon Sequencing Method
- Promotional Effect of CoO(OH) on Selective Hydrogenation of Maleic Anhydride to γ-Butyrolactone over Supported Ruthenium Catalyst
- Synthesis and Separation Performance of Y-type Zeolite Membranes by Pre-Seeding Using Electrophoresis Deposition Method
- Design and Control of Self-Heat Recuperative Distillation Process for Separation of Close-Boiling Mixtures: n-Butanol and iso-Butanol

