替米沙坦对心肌细胞脂联素受体1表达的影响及其可能机制研究
吴杰萍,郭志新
替米沙坦对心肌细胞脂联素受体1表达的影响及其可能机制研究
吴杰萍,郭志新
目的探讨替米沙坦调节糖尿病心肌细胞脂联素受体1表达的作用机制。方法取H9C2心肌细胞作为研究对象,分两个部分进行实验。第一部分检测血管紧张素Ⅱ(AngⅡ)抑制的影响:以不同浓度(0、10-8、10-7、10-6、10-5mol/L) AngⅡ作用48h以及AngⅡ(10-7mol/L)作用不同时间(0、12、24、36、48h)后检测心肌细胞内脂联素受体1 mRNA和蛋白的表达;以10-5mol/L替米沙坦孵育1h,然后用10-7mol/L AngⅡ培养24h后检测前述指标的表达。第二部分检测过氧化物酶体增殖物活化受体-γ(PPAR-γ)激活的影响:将H9C2心肌细胞分5组:①对照组(NG组);②高糖组(HG组);③高糖+替米沙坦组(HG+T组);④高糖+替米沙坦+PPAR-γ抑制剂GW9662组(HG+T+GW组);⑤低糖+甘露醇组(NG+M组)。按分组处理24h后检测心肌细胞脂联素受体1 mRNA和蛋白的表达。采用实时荧光定量聚合酶链反应法和免疫印迹法测定心肌细胞脂联素受体1 mRNA和蛋白的表达。结果在AngⅡ浓度为10-8、10-7、10-6、10-5mol/L时心肌细胞脂联素受体1 mRNA和蛋白表达均明显降低(P<0.05),尤以10-7mol/L时下降最显著(P<0.01)。10-7mol/L AngⅡ作用12、24、36、48h后心肌细胞脂联素受体1 mRNA和蛋白表达均明显降低(P<0.05),尤以24h下降最显著(P<0.01)。替米沙坦可显著上调AngⅡ作用后的心肌细胞脂联素受体1 mRNA和蛋白表达(P<0.05)。与NG组比较,HG组心肌细胞脂联素受体1 mRNA和蛋白表达显著降低(P<0.05),而NG+M组表达无显著变化(P>0.05)。与HG组比较,HG+T组心肌细胞脂联素受体1 mRNA和蛋白表达显著升高(P<0.05);与HG+T组比较,HG+T+GW组心肌细胞脂联素受体1 mRNA和蛋白表达显著降低(P<0.05)。结论高糖和AngⅡ可显著降低心肌细胞脂联素受体1的表达。替米沙坦通过激活PPAR-γ、抑制AngⅡ而上调高糖培养的心肌细胞脂联素受体1的表达。
替米沙坦;PPARγ;血管紧张素Ⅱ;肌细胞,心脏;受体,脂联素
糖尿病性心肌病是糖尿病最常见的慢性并发症之一,是导致糖尿病患者死亡的重要原因。脂联素(adiponectin,APN)是脂肪细胞分泌的一种蛋白质,通过与其受体结合发挥抗炎、抗糖尿病和抗动脉粥样硬化作用[1]。替米沙坦是一种特异性血管紧张素Ⅱ(angiotensin Ⅱ,AngⅡ)受体AT1的拮抗剂,同时能选择性调节过氧化物酶体增殖物活化受体-γ(PPAR-γ)的活性,其激活PPAR-γ的作用可达传统PPAR-γ配体的25%~30%[2-3]。本课题组前期研究表明,替米沙坦能显著上调2型糖尿病大鼠心肌脂联素受体的表达,降低心脏肥大指数,改善心功能,对心脏产生保护作用[4-5],但是其作用机制尚未阐明。本研究旨在明确替米沙坦的作用与PPAR-γ途径激活和(或)AngⅡ途径阻断的关系,以期为临床治疗糖尿病性心脏病的药物选择提供理论依据。
1 材料与方法
1.1 实验材料 H9C2细胞系(ATCC细胞库),DMEM培养基(美国Hyclone公司),胎牛血清(杭州四季清公司),0.25%胰蛋白酶(美国Hyclone公司),替米沙坦(美国Sigma公司),选择性PPAR-γ抑制剂GW9662(美国Sigma公司),AngⅡ(美国Hyclone公司),反转录-聚合酶链反应试剂盒(北京天根公司),Trizol(TaKaRa公司),细胞蛋白抽提试剂盒(武汉博士德公司),引物(上海生工生物工程有限公司),CO2培养箱(HERAcell 150),实时荧光定量聚合酶链反应仪(美国Bio-Rad公司),脂联素受体1抗体、GAPDH抗体、羊抗兔IgG-HRP(武汉博士德公司)。
1.2 检测替米沙坦与AngⅡ对H9C2心肌细胞脂联素受体1 mRNA和蛋白表达的影响
1.2.1 不同浓度AngⅡ的影响 将H9C2心肌细胞分别于含0、10-8、10-7、10-6、10-5mol/L AngⅡ的无血清DMEM培养基中培养48h(n=3)后测定脂联素受体1表达。
1.2.2 10-7mol/L AngⅡ作用不同时间的影响H9C2心肌细胞在含10-7mol/L AngⅡ的无血清DMEM培养基中培养0、12、24、36、48h(n=3)后测定脂联素受体1表达。
1.2.3 替米沙坦的阻断作用 H9C2心肌细胞在含10-5mol/L替米沙坦的无血清DMEM培养基中预孵育1h,然后加入10-7mol/L AngⅡ培养24h(n=3),测定脂联素受体1的表达。
1.3 检测替米沙坦与GW9662对H9C2心肌细胞脂联素受体1 mRNA和蛋白表达的影响 H9C2心肌细胞在含10%胎牛血清的DMEM培养液中培养至状态良好并铺满培养瓶底部,0.25%胰蛋白酶消化,以105个/ml密度接种于6孔板,置37℃ CO2孵箱(5%CO2,95%空气)中培养,24h后换为无血清DMEM培养基。
将细胞随机分为5个组(n=3):①正常对照(NG)组:以无血清低糖(5.6mmol/L)DMEM培养基培养;②高糖(HG)组:以无血清高糖(25.5mmol/L)DMEM培养基培养;③高糖+替米沙坦(HG+T)组:在HG组的基础上加入10-5mol/L替米沙坦;④高糖+替米沙坦+GW9662(HG+T+GW)组:在HG组基础上先加入10μmol/L GW9662预孵育1h,再加10-5mol/L替米沙坦培养;⑤低糖+甘露醇(NG+M)组:在NG组基础上加入19.9mmol/L甘露醇培养。按照上述分组培养24h后检测心肌细胞脂联素受体1 mRNA和蛋白的表达。
1.4 脂联素受体1表达检测
1.4.1 标本收集 用Trizol试剂盒提取细胞总RNA,用蛋白抽提液提取细胞总蛋白,将所得到的标本置-70℃冰箱保存备用。
1.4.2 mRNA表达检测 取出总RNA,用RT-PCR试剂盒行反转录制备cDNA,用实时荧光定量PCR仪进行扩增。所用引物序列如下:脂联素受体1上游5'-GCTGGCCTTTATGCTGCTCG-3',下游5'-TCTAGGCCGTAACGGAATTC-3';内参GAPDH上游5'-ATGGTGAAGGTCGGTGTG-3',下游5'-AACTTGCCGTGGGTAGAG-3'。PCR反应体系为:FastStart Universal SYBR Green Master (ROX)10μl,上、下游引物(15μmol/L)各0.5μl,cDNA 2μl,无DNase和RNase水7μl,共20μl。PCR扩增条件:95℃预变性15min,活化Tag酶;95℃10s,60℃31s,40个循环。目的基因表达阳性的标本荧光定量扩增曲线呈S形,用实时荧光定量PCR系统读取Ct值。以2-ΔΔCt表示样品中目的基因mRNA相对于内参基因的表达量。
1.5 蛋白表达检测 取总蛋白,采用Western blotting检测脂联素受体蛋白的表达。用BCA法测定蛋白浓度,将适量蛋白与5×SDS上样缓冲液混匀,煮沸5min,使其变性。每孔上样量20μg,10% SDS-聚丙烯酰胺凝胶行电泳分离,将电泳后的凝胶转移至PVDF膜上。TBST液洗膜5min×3次,5%脱脂奶粉封闭2h。一抗1:1000稀释)4℃孵育过夜。TBST液清洗5min×3次。加入二抗(1:500稀释)室温孵育1h,TBST液清洗5min×3次,ECL化学发光,凝胶自动成像仪扫描图像,采用Odyssey 3.0软件对条带行半定量分析。
1.6 统计学处理 采用SPSS 16.0软件进行统计分析。数据结果以表示,多组间比较采用方差分析,进一步两两比较采用LSD-t检验。P<0.05为差异有统计学意义。
2 结 果
2.1 不同浓度AngⅡ对H9C2心肌细胞脂联素受体1 mRNA和蛋白表达的影响 与0mol/L AngⅡ组比较,10-8、10-7、10-6、10-5mol/L AngⅡ组心肌细胞脂联素受体1 mRNA和蛋白表达均明显降低(P<0.05),尤以10-7mol/L组下降显著(P<0.01,图1)。
2.2 AngⅡ作用不同时间对H9C2心肌细胞脂联素受体1 mRNA和蛋白表达的影响 与0h比较,10-7mol/L AngⅡ作用12、24、36、48h后心肌细胞脂联素受体1 mRNA和蛋白表达均明显降低(P<0.05),尤以24h下降显著(P<0.01,图2)。

图1 不同浓度AngⅡ对H9C2心肌细胞脂联素受体1 mRNA和蛋白表达的影响Fig.1 E ff ect of di ff erent concentrations of AngⅡ on mRNA and protein expressions of adiponectin receptor 1 in H9C2 cardiomyocytes (1)P<0.05, (2)P<0.01 compared with 0mol/L AngⅡ
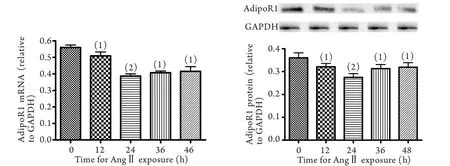
图2 AngⅡ(10-7mol/L)作用不同时间对心肌细胞脂联素受体1 mRNA和蛋白表达的影响Fig.2 Comparison of the mRNA and protein expressions of adiponectin receptor 1 in cardiomyocytes treated by AngⅡ (10–7mol/L) for di ff erent time
2.3 替米沙坦阻断AngⅡ作用后对心肌细胞脂联素受体1 mRNA和蛋白表达的影响 与10-7mol/L AngⅡ组比较,10-5mol/L替米沙坦+10-7mol/L AngⅡ组心肌细胞脂联素受体1 mRNA和蛋白的表达显著升高(P<0.05,图3)。
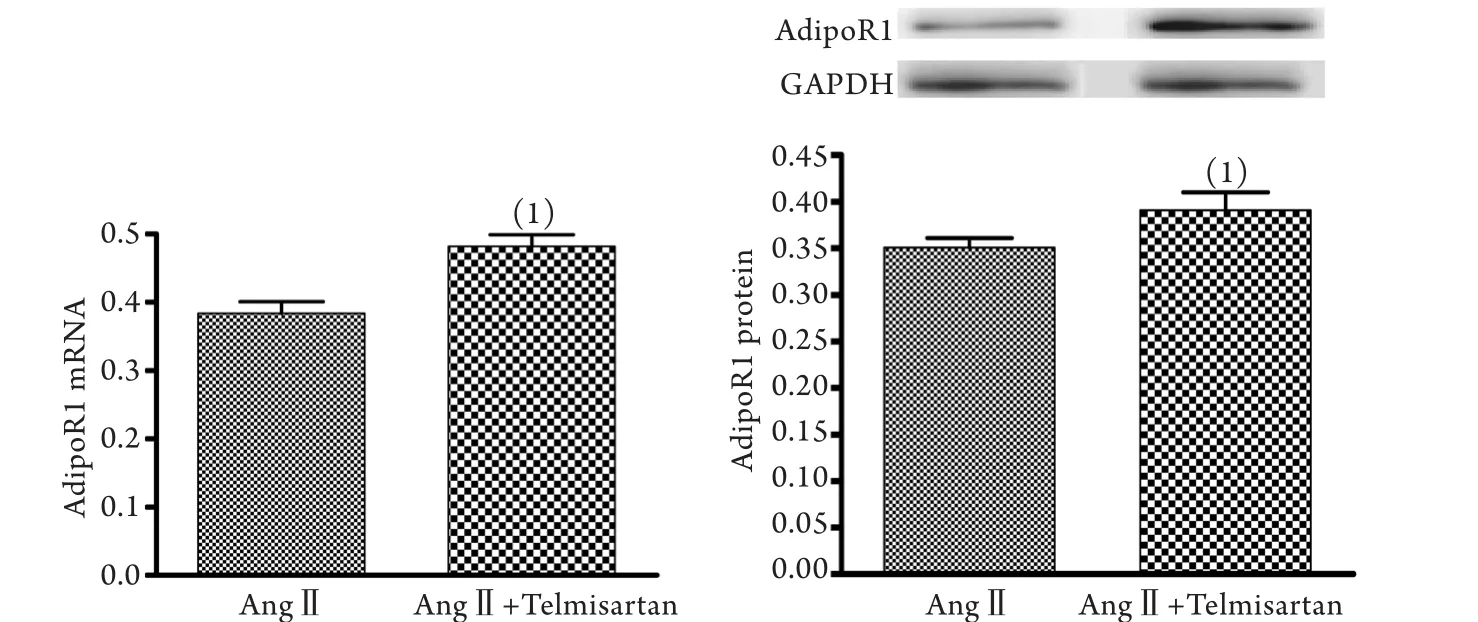
图3 替米沙坦阻断AngⅡ作用后对心肌细胞脂联素受体1 mRNA和蛋白表达的影响Fig.3 Effect of telmisartan on mRNA and protein expressions of adiponectin receptor 1 in cardiomyocytes by blocking the effect of AngⅡ
2.4 替未沙坦与GW9662对心肌细胞脂联素受体1 mRNA和蛋白表达的影响 与NG组比较,HG组及HG+T+GW组心肌细胞脂联素受体1 mRNA和蛋白表达均明显降低(P<0.05),而NG+M组及HG+T组表达无明显变化(P>0.05)。与HG组比较,HG+T组和NG+M组心肌细胞脂联素受体1 mRNA和蛋白表达明显升高(P<0.05),而HG+T+GW组表达无明显变化(P>0.05)。与HG+T组比较,HG+T+GW组心肌细胞脂联素受体1 mRNA和蛋白表达显著降低(P<0.05,图4)。
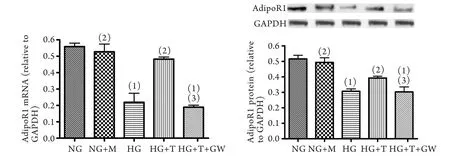
图4 替米沙坦及选择性PPAR-γ抑制剂GW9662对心肌细胞脂联素受体1 mRNA和蛋白表达的影响Fig.4 E ff ect of telmisartan and GW9662, a potent antagonist of PPAR-γ, on expression of adiponectin receptor 1 in cardiomyocytes
3 讨 论
本研究结果显示,AngⅡ呈浓度-时间依赖性下调心肌细胞脂联素受体1的表达,而替米沙坦可阻断AngⅡ的上述作用,提示替米沙坦可通过阻断AngⅡ途径上调心肌细胞脂联素受体1的表达。本研究结果还显示,高糖培养的心肌细胞脂联素受体1 mRNA和蛋白表达显著降低,替米沙坦可显著升高高糖培养心肌细胞脂联素受体1 mRNA和蛋白的表达,而选择性PPAR-γ抑制剂GW9662能显著抑制替米沙坦的上述作用,提示替米沙坦可通过激活PPAR-γ途径上调心肌细胞脂联素受体1的表达。
AngⅡ是肾素-血管紧张素系统的主要成分。激活肾素-血管紧张素系统和增加AngⅡ的形成在左室重构和心衰发展中均起重要作用。升高的AngⅡ通过与血管紧张素受体结合促进了心肌肥大、心肌间质纤维化和心肌细胞凋亡[6-7]。研究发现,AngⅡ可上调心肌细胞脂联素表达[8],而脂联素可减轻AngⅡ诱导的心肌细胞凋亡[9],并可通过脂联素受体1改善AngⅡ诱导的大鼠心房细胞肥大和纤维化[10]。本研究结果还显示,AngⅡ作用于心肌细胞使脂联素受体1的表达量明显下降,且具有浓度-时间依赖性,给予替米沙坦干预后,心肌细胞脂联素受体1的表达显著升高,提示阻断AngⅡ作用是替米沙坦调节心肌细胞脂联素受体1表达的机制之一。Li等[11]的研究结果显示,AngⅡ可呈浓度-时间依赖性下调心肌细胞脂联素受体1的表达,AngⅡ受体拮抗剂洛沙坦可显著抑制AngⅡ的上述作用,与本研究结果相似。
脂联素是脂肪细胞分泌的一种蛋白质,通过与其受体结合发挥抗炎、抗糖尿病和抗动脉粥样硬化等作用[1]。脂联素受体1主要在骨骼肌表达,脂联素受体2主要在肝脏表达[12]。除骨骼肌和肝脏外,心脏和肾脏也表达脂联素受体[12-15]。替米沙坦同时具有部分PPAR-γ激动剂的功能,其作用可达传统PPAR-γ配体最大激活作用的25%~30%[2-3],是唯一在治疗浓度下能激活PPAR-γ的AngⅡ受体拮抗剂[16]。本课题组前期研究显示,2型糖尿病大鼠心肌脂联素受体表达显著降低,替米沙坦和PPAR-γ激动剂罗格列酮可显著上调2型糖尿病大鼠心肌脂联素受体的表达[4-5,14,17]。Ding等[18]的研究结果显示,罗格列酮可显著上调体外培养的心肌细胞脂联素受体1的表达。本研究结果显示,高糖培养的心肌细胞脂联素受体1 mRNA和蛋白表达显著降低,替米沙坦可显著升高高糖培养的心肌细胞脂联素受体1 mRNA和蛋白表达,而选择性PPAR-γ抑制剂GW9662可显著抑制替米沙坦的上述作用,提示激活PPAR-γ途径是替米沙坦调节心肌细胞脂联素受体1表达的机制之一。
综上所述,高糖和AngⅡ可显著降低心肌细胞脂联素受体1的表达。替米沙坦通过激活PPAR-γ、抑制AngⅡ上调高糖培养心肌细胞脂联素受体1的表达。
[1] Furukawa S, Fujita T, Shimabukuro M,et al. Increased oxidative stress in obesity and its impact on metabolic syndrome[J]. J Clin Invest, 2004, 114(12): 1752-1761.
[2] Kurtz TW. Treating the metabolic syndrome: telmisartan as a peroxisome proliferator-activated receptor-gamma activator[J]. Acta Diabetol, 2005, 42 (Suppl 1): S9-S16.
[3] Benson SC, Pershadsingh HA, Ho CI,et al. Identification of telmisartan as a unique angiotensin Ⅱ receptor antagonist with selective PPARgamma-modulating activity[J]. Hypertension, 2004, 43(5): 993-1002.
[4] Guo Z, Zhang R, Li J,et al. Effect of telmisartan on the expression of adiponectin receptors and nicotinamide adenine dinucleotide phosphate oxidase in the heart and aorta in type 2 diabetic rats[J]. Cardiovasc Diabetol, 2012, 11: 94.
[5] Guo Z, Zhang C, Qin Z,et al. Effect of telmisartan on the expression of cardiac adiponectin and its receptor 1 in type 2 diabetic rats[J]. J Pharm Pharmacol, 2011, 63(1): 87-94.
[6] Serneri GG, Boddi M, Cecioni I,et al. Cardiac angiotensinⅡ formation in the clinical course of heart failure and its relationship with left ventricular function[J]. Circ Res, 2001, 88(9): 961-968.
[7] Bouzegrhane F, Thibault G. Is angiotensin Ⅱ a proliferative factor of cardiac fibroblasts[J]? Cardiovasc Res, 2002, 53(2): 304-312.
[8] Guo B, Li Y, Han R,et al. Angiotensin Ⅱ upregulation of cardiomyocyte adiponectin production is nitric oxide/cyclic GMP dependent[J]. Am J Med Sci, 2011, 341(5): 350-355.
[9] Guo BY, Han R, Li YJ. Intervention of adiponectin to angiotesinⅡ-mediated cardiomyocyte apoptosis[J]. Med J Chin PLA, 2010, 35(8): 933-936. [郭炳彦, 韩瑞, 李拥军. 脂联素对血管紧张素Ⅱ介导的心肌细胞凋亡的干预作用[J]. 解放军医学杂志, 2010, 35(8): 933-936.]
[10] Cao T, Gao Z, Gu L,et al. AdipoR1/APPL1 potentiates the protective effects of globular adiponectin on angiotensinⅡ-induced cardiachypertrophy and fibrosis in neonatal rat atrial myocytes and fibroblasts[J]. PLoS One, 2014, 9(8): e103793.
[11] Li L, Zhang ZG, Lei H,et al. Angiotensin Ⅱ reduces cardiac AdipoR1 expression through AT1 receptor/ROS/ERK1/2/ c-Myc pathway[J]. PLoS One, 2013, 8(1): e49915.
[12] Yamauchi T, Kamon J, Ito Y,et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects[J]. Nature, 2003, 423(6941): 762-769.
[13] Guo Z, Xia Z, Yuen VG,et al. Cardiac expression of adiponectin and its receptors in streptozotocin-induced diabetic rats[J]. Metabolism, 2007, 56(10): 1363-1371.
[14] Guo ZX, Qin ZH, Wei P,et al. Effect of rosiglitazone on protein expression of cardiac adiponectin and its receptor type 1 in rats with type 2 diabetes[J]. Med J Chin PLA, 2009, 34(7): 851-854. [郭志新, 秦志宏, 魏萍, 等. 罗格列酮对2型糖尿病大鼠心肌脂联素及其受体1蛋白表达的影响[J]. 解放军医学杂志, 2009, 34(7): 851-854.]
[15] Guo Z, Zhao Z. Effect of N-acetylcysteine on plasma adiponectin and renal adiponectin receptors in streptozotocin-induced diabetic rats[J]. Eur J Pharmacol, 2007, 558(1-3): 208-213.
[16] Kintscher U, Unger T. Vascular protection in diabetes: a pharmacological view of angiotensin Ⅱ type 1 receptor blockers[J]. Acta Diabetol, 2005, 42(Suppl 1): S26-S32.
[17] Guo Z, Qin Z, Zhang R,et al. Effect of rosiglitazone on the expression of cardiac adiponectin receptors and NADPH oxidase in type 2 diabetic rats[J]. Eur J Pharmacol, 2012, 685(1-3): 116-125.
[18] Ding G, Qin Q, He N,et al. Aiponectin and its receptors are expressed in adult ventricular cardiomyocytes and upregulated by activation of peroxisome proliferator-activated receptor gamma[J]. J Mol Cell Cardiol, 2007, 43(1): 73-84.
Effects of telmisartan on the expression of adiponectin receptor 1 in cardiomyocytes and its mechanism
WU Jie-ping, GUO Zhi-xin*
Department of Endocrinology, Second Hospital of Shanxi Medical University, Taiyuan 030001, China
*Correspondence author, E-mail: zhxguo1966@aliyun.com
This work was supported by the Science and Technology Innovation Foundation of Shanxi Medical University (01201015), Shanxi Scholarship Council of China (2011-048), Natural Science Foundation of Shanxi Province (2013011047-1), and Technology Faundation for Selected Overseas Chinese Scholar by Department of Personnel of Shanxi Province (Approved in 2012)
ObjectiveTo explore the mechanisms of telmisartan in regulating the expression of adiponectin receptor 1 (AdipoR1) in diabetic cardiomyocytes.MethodsInvestigation of the inhibitory effects of AngⅡ were performed in 3 subexperiments as follows: One detects mRNA and protein expression of AdipoR1 in different batches of H9C2 cells pre-treated with AngⅡ for 48 hours at a concentration of 0, 10-8, 10-7, 10-6, 10-5mol/L respectively, one in cells pre-treated with AngⅡ at a concentration of 10-7mol/L for 0, 12, 24, 36 and 48 hours respectively, while the last in cells pre-treated with telmisartan at 10-5mol/L for 1 hour followed by AngⅡ at 10-7mol/L for 24 hours. Cultured H9C2 cardiomyocytes were randomly divided into following five groups: normal control group (NG), high glucose group (HG), high glucose plus telmisartan group (HG+T), high glucose plus telmisartan and PPAR-γ antagonist GW9662 group (HG+T+GW), and normal glucose plus mannitol group (NG+M). Cardiomyocytes in the above-mentioned five groups were continued to be cultured for 24h, then the mRNA and proteinexpressions of AdipoR1 in cardiomyocytes were assayed. Fluorescent quantitative real-time PCR and Western blotting were used respectively to analyze the mRNA and protein expressions of AdipoR1 in cardiomyocytes.ResultsThe mRNA and protein expressions of AdipoR1 in cardiomyocytes were significantly decreased by AngⅡ at concentrations of 10-8, 10-7, 10-6and 10-5mol/L (P<0.05), reaching a maximal decrease at concentration of 10-7mol/L (P<0.01). The mRNA and protein expressions of AdipoR1 were significantly down-regulated in cardiomyocytes after being incubated with AngⅡ for 12h, 24h, 36h and 48h, reaching a maximal down-regulation at 24h (P<0.01). Telmisartan significantly up-regulated the mRNA and protein expressions of AdipoR1 in cardiomyocytes treated with AngⅡ (P<0.05). Compared with group NG, group HG showed a significant down-regulation in the mRNA and protein expressions of AdipoR1 in cardiomyocytes (P<0.05). However, no significant difference was found between group NG and group NG+M in the mRNA and protein expressions of AdipoR1 in cardiomyocytes (P>0.05). The mRNA and protein expressions of AdipoR1 in cardiomyocytes were significantly up-regulated in group HG+T compared with that in group HG (P<0.05), and significantly down-regulated in group HG+T+GW compared with that in group HG+T (P<0.05).ConclusionsThe expression of myocardial AdipoR1 may be significantly reduced by high glucose and AngⅡ. Telmisartan may up-regulate the expression of AdipoR1 in cardiomyocytes cultured in high glucose content by activating PPAR-γ and inhibiting AngⅡ.
telmisartan; PPAR gamma; angiotensin Ⅱ; myocytes, cardiac; receptors, adiponectin
R587.1
A
0577-7402(2015)01-0030-05
10.11855/j.issn.0577-7402.2015.01.07
2014-08-18;
2014-11-29)
(责任编辑:张小利)
山西医科大学科技创新基金(01201015);山西省回国留学人员科研资助项目(2011-048);山西省自然科学基金(2013011047-1);山西省留学人员科技活动择优资助项目(2012年批准)
吴杰萍,硕士研究生。主要从事糖尿病及其慢性并发症的研究
030001 太原 山西医科大学第二医院内分泌科(吴杰萍、郭志新)
]郭志新,E-mail:zhxguo1966@aliyun.com

