Effects of 1,7-Bromine Substitution at Bay Area on Self-assembly Behavior and Photo Physical Properties of Perylene Diimide
ZENG Ting(曾 婷),WU Pei-yue(吴佩跃),ZHANG Xin-lin(张新林),WANGATIA Lordrick Makokha (王 洛),SUN Bin(孙 宾),ZHU Mei-fang(朱美芳)
State Key Laboratory for Modification of Chemical Fibers and Polymer Materials,College of Materials Science and Engineering,Donghua University,Shanghai 200051,China
Effects of 1,7-Bromine Substitution at Bay Area on Self-assembly Behavior and Photo Physical Properties of Perylene Diimide
ZENG Ting(曾 婷),WU Pei-yue(吴佩跃),ZHANG Xin-lin(张新林),WANGATIA Lordrick Makokha (王 洛),SUN Bin(孙 宾)*,ZHU Mei-fang(朱美芳)
State Key Laboratory for Modification of Chemical Fibers and Polymer Materials,College of Materials Science and Engineering,Donghua University,Shanghai 200051,China
N,N'-bis(n-dodecyl)-3,4:9,10-tetracarboxyl-diimide(1,7-H-PDI-C12)and its bay position 1,7-Br substituted derivative have been synthesized and characterized by1H-NMR,13C-NMR,FT-IR,and X-ray diffraction(XRD).A comparison of the two samples by measuring their photo physicalproperties using UV visible absorption and fluorescence emission spectra revealed that bay substitutions of bromine do not have significant effect on the perylene diimide(PDI)photo physical properties in solution.However,the solid state fluorescence properties were enhanced by brominating at bay areas.The solid fluorescence quantum yield of 1,7-Br-PDI-C12was 2.83%(Фf=2.83%)and 1,7-H-PDI-C12was only 0.02%(Фf=0.02%).This behavior was also reflected in the steady-state fluorescence spectra.This work shows that solid state photo physical properties of PDI could be improved without changing the other properties by simply using bromine substitution at bay areas.These types of materials are useful intermediate for further synthesis of PDI with tunnable optoelectronic properties.
disubstituted perylene diimide(PDI); self-assembly; absorption;fluorescence
Introduction
Photoelectric devices are basically made from semiconducting materials which can convert light into electricity.Silicon has traditionally been used as a main raw material for developing photovoltaic devices.However,the cost of silicon devices is very high[1].This is due to the complex lithography processing involved in silicon device production.In recent years,organic semiconductors have been attracting considerable attention due to the fact that most organic materials are cheap and the processing is quite simple comprising of only one step like spin casting.Various organic materials have been studied,and their applications in electronic devices including organic solar cells(OSC)[2-3],organic light emitting diodes (OLED)[4]and organic field emission transistors(OFET)[5-6]and photovoltaic cells[7-8]have been reported previously.
The process of transforming exciton to electron in organic semiconductors require both p-type and n-type material.The excitons are generated in the p-type material and disassociated to form electrons at the interface which can be used for generation of electricity.This implies that two materials n-type and p-type are required for any successfuldevelopmentof organic semiconductors.Most of all reported organic semiconductors are usually p-type.Therefore,n-type organic semiconducting materials are highly demanded for realization of various organic electronic applications.Perylene diimide(PDI)is one of the very few n-type organic materials,which originally was used as a dyeing material.In the recent years,PDI has been intensively studied as an organic semiconductor.It also has high fluorescence,photo-chemical stabilities,and its optoelectronic properties can be tuned to a wide range by simple modification processes at the side and bay areas.
PDI is highly conjugated perylene core and can be exploited to engineer excellent charge transfer.Due to the strong π-system in the dye,one dimensional structures can be easily developed through its self-assembly behavior[9].This well ordered structures have high charge transport properties.Linear or branched long chain can be symmetrically or asymmetrically[10]functionalized at theN-positionsto alterthesolubility and crystalpacking arrangement of the dye without significant alteration in electronic properties for nodes at the N-imides,which limit the electronic interactions between PDI and corresponding substituent[11].
However,various substitutions at PDI core positions results into alteration of electronic properties including the redox values of the dye.This gives an option to vary the electronic properties of the dye to a wide range.Since bay area substitution comes with distortion of the planarity of PDI,little work has been done to study the ability of bay area substituted PDI to form one dimensional structures.In an effort to illuminate this aspect,we have synthesized bromine bay area functionalized PDI(1,7-Br-PDI-C12)and compared its optoelectronic properties both in solution and solid to similar PDI without the substitution on the bay area(1,7-H-PDI-C12).The two structures are shown in Scheme 1.

Scheme 1 Chemical structures of the compounds
1 Experimental
All the reagents were commercially obtained and used without further purification.The1H-NMR and13C-NMR spectra were recorded on a Bruker-MHz 400 spectrometer in deuterated chloroform(CDCl3).All chemical shifts were quoted in ppm relative to tetramethylsilane(TMS).The FT-IR spectra were recorded on Nicolet 5700 FT-IR in which KBr pellets were used for powder sample.UV visible(UV-vis)absorption spectra were measured by a Perkin ElmerLambda35 UV-vis spectrometer and fluorescence spectra were recorded on a JASCO FP-6600 spectrometer. Cyclic voltammetry was recorded with Zahner-Zennium electron workstation using a normal three electrode cell,platinum disk electrode as working electrode,a platinum wire electrode as the counter electrode,Ag/AgCl as a reference electrode in saturated KCl.Compounds 1,7-H-PDI-C12and 1,7-Br-PDI-C12were synthesized according to the previous report[12].
2 Results and Discussion
2.1 Synthesis and purification of 1,7-Br-PDI-C12
The mixture of 1,7-Br-PDI-C12and 1,6-Br-PDI-C12was obtained as one component with the same Rfvalue(the ratio between migration distance of solute and eluent in chromatography)by column chromatography and it can not be separated by column chromatography.Fortunately,the major pure regioisomer 1,7-Br-PDI-C12could be obtained by repetitive recrystallization from CHCl3/MeOH(1∶1)as reported[13].The pure 1,7-Br-PDI-C12products were ensured by1H-NMR(A Bruker-MHz 400 spectrometer, Fig.1) after three recrystallizations.As the1H-NMR spectra revealed,three sets of signals were observed in the region of 8.5-9.6 ppm,
including two doublets(protons:a,b) and one singlet
(protons:c),indicating that our product is pure 1,7-Br-PDIC12or 1,6-Br-PDI-C12[14].There is only one triplet appeared ca.4.2 ppm,which further confirmed the product's purity[15].

Fig.1 1H-NMR spectra of pure 1,7-Br-PDI-C12after three recrystallizations
2.2 Self-assembly in solution
PDI solutions in monomeric form usually havethree characteristic absorption peaks,which are observed between 550 and 400 nm.The normalized absorption spectra of 1,7-H-PDIC12and 1,7-Br-PDI-C12were measured in CHCl3and the results are represented in Fig.2(a).From the results,it could be observed that 1,7-H-PDI-C12had three pronounced peaks at 525,488,and 458 nm,while the absorption peaks of 1,7-Br-PDI-C12were observed at 523,487,and 456 nm.From the long wavelength to the short,the three peaks correspond to 0-0,0-1,and 0-2 transition,respectively.Comparing the two spectra we found that there was a slight blue shifting(from 525 nm to 523 nm) of all the three absorption peaks when bromine was introduced on the PDI core.Corresponding fluorescence emission spectra were observed at 542,575,and 625 nm for 1,7-H-PDIC12,while 1,7-Br-PDI-C12fluorescence bands were observed at 549,576,and 628 nm as shown in Fig.2(b).Similar to theabsorption spectra,a small shift was observed in the fluorescence peaks but it was red shifted(from 542 nm to 549 nm).The presence of the characteristic absorption peak in both the two samples reveals the strong π-π effect of the PDI core.The blue shifting in the absorption indicates that the planarity of PDI has been altered thus leading to higher molecule to molecule spacing (d-spacing),and these can also be confirmed by the fact that the stroke shift 1,7-Br-PDI-C12(26 nm)is higher than that of 1,7-HPDI-C12(17 nm),which is an indication of weaker π-π interaction in the former[16].

Fig.2 Normalized UV-vis absorption spectra and fluorescence emission spectra in CHCl3at 1e-5mol·L-1solution of compound(a) 1,7-H-PDI-C12and(b)1,7-Br-PDI-C12at room temperature
Organic π-system chromophores have strong intermolecular π-π interaction,which lead to regular molecular packing under a process called self-assembly.These interaction forces are maximum in planar PDI and tend to be decreased when PDI core is reduced.The self-assembly process of 1,7-H-PDI-C12and 1,7-Br-PDI-C12was monitored by concentration dependent UV-vis absorption spectra and fluorescence spectra in 1∶1 chloroform:methanol(C:M)solvent.The results are shown in Figs.3 and 4.From the results,the absorption peaks intensity of 1,7-H-PDI-C12uniformly increased with concentration increase and a new peak appeared at 570 nm.The new peak became steady as the concentration increased.This new peak is usually used as an indicator of molecular aggregation.It was further observed in the corresponding fluorescence spectra(Fig.3(b)) whereby increase in concentration resulted into reduction in maximum peak intensity as the minimum peak became steady. Contrary to thisobservation, the concentration dependent absorption spectra of 1,7-Br-PDI-C12showed a different trend.Within the same concentration range as the previous sample,the absorption peaks of 1,7-Br-PDI-C12similarly increased with increase in concentration in Fig.4(a).However at very dilute concentration,the absorption peaks were not well pronounced,the peak maxima was at 488 nm which formed a broad shapeless peak.As the concentration increased,the peak maxima gradually reversed to 525 nm and the usual PDI characteristic peak shape appeared.A weak peak was observed at a higher wavelength (580 nm),which did not seem to steadily increase as it was in the case of 1,7-H-PDI-C12.The corresponding fluorescence spectra as shown in Fig.4(b)were not sensitive to concentration increase except that the emission peak at 548 nm showed a red shift trend due to its self-absorption.These results clearly suggested that 1,7-H-PDI-C12had higher tendency to aggregate than 1,7-Br-PDI-C12.Since the two samples only differed by the substitution at the bay area,it could be stated that the reduction in aggregation wasbeing broughtaboutby the bromine substitution at the bay areas.Quantum chemical calculations of the bromine-substituted PDI derivatives were performed bydensity functionaltheory (DFT) with the Gaussian 03 package[17].To optimize geometry of the molecular structure,B3LYP method with 6-31G**basic set[18-19]via energy minimization has been used.The perylene core is highly twisted with dihedral angle of 22°associated with the bay area carbon atoms(C1-C2-C3-C4)(Fig.5).Therefore,it indicated that the reduction in aggregation of 1,7-Br-PDI-C12was because bromine was able to twist the PDI core and making it nonplanar,which resulted into reduced π-π interactions.
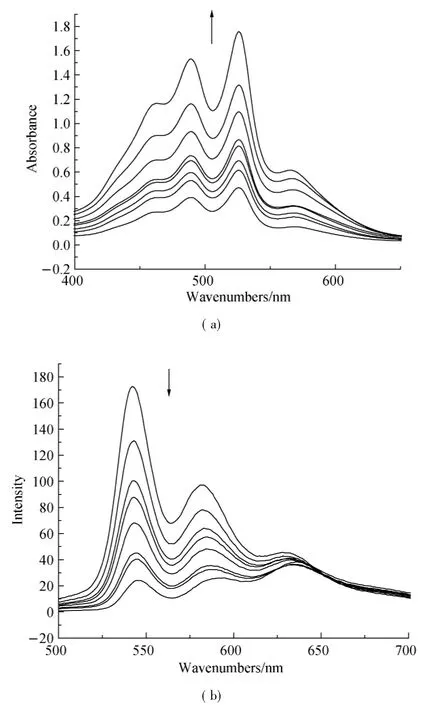
Fig.3 Concentration-dependent(a)UV-vis absorption and(b) fluorescence emission spectra(λex=470 nm)of compound 1,7-H-PDI-C12in CHCl3/MeOH(1∶1)(Arrows indicate the direction of change with increasing concentration,and the concentration amounts to 4.2,4.9,5.9,6.6,7.7,8.7,9.2,15 e-5mol·L-1respectively.)
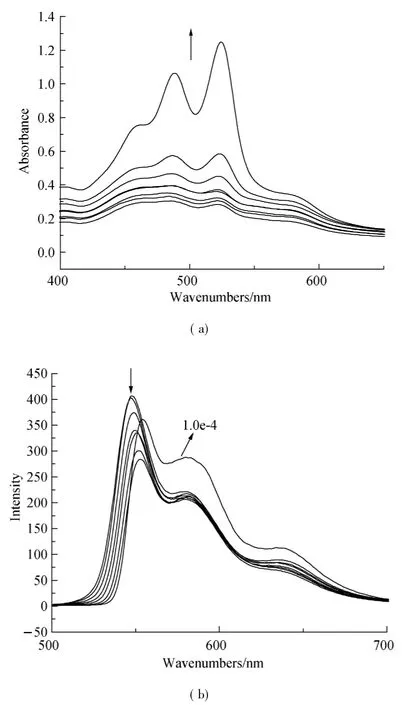
Fig.4 Concentration-dependent(a)UV-vis absorption and(b) fluorescence emission spectra (λex=470 nm) of compound 1,7-Br-PDI-C12in CHCl3/MeOH(1∶1) (arrows indicate the direction of change with increasing concentration,and the concentration amounts to 3.7,4.3,4.9,5.8,6.7,7.6,8.7,10 e-5mol·L-1,respectively.)
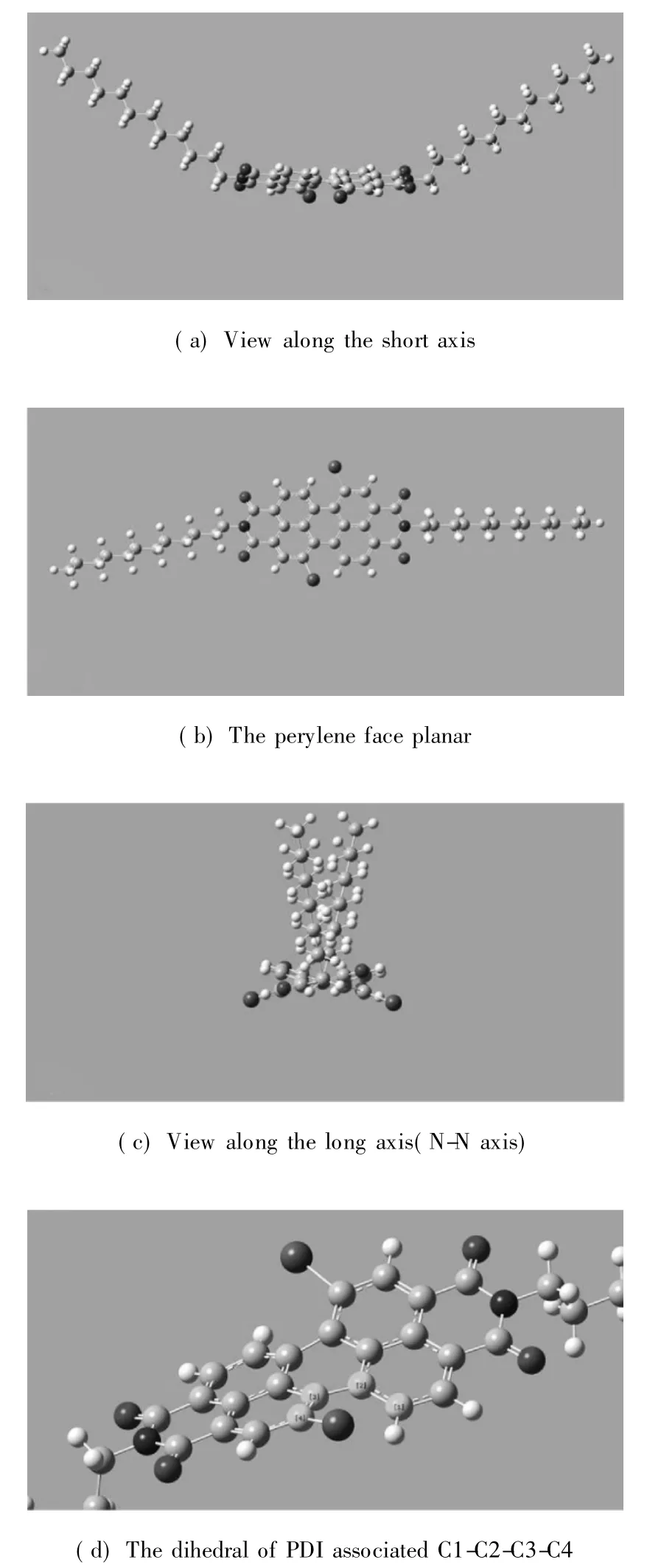
Fig.5 Different views of PDI structure obtained at B3LYP/6-31G**level
Solvent polarity can also be used to monitor the aggregation process in a self-assembling π-system.Chloroform(C)is a good solvent to both 1,7-H-PDI-C12and 1,7-Br-PDI-C12,while methanol(M)is a poor solvent.Mixing the two solvents in varying ratios provides a wide range of solvent polarities,which altersthe solvation effecton the π-π interaction. The fluorescence spectra of 1,7-H-PDI-C12and 1,7-Br-PDI-C12were tested in varying ratios of chloroform/methanol and the results were illustrated in Fig.6.From the results of 1,7-H-PDI-C12(Fig.6(a))it could be observed that when the amount of methanol was very small,i.e.,Chloroform:methanol=9∶1,the fluorescence peaks resembled the usually mirror image spectra of PDI.However,a small increase in methanol from 9∶1 to 7∶1 resulted into red shifting of the maximum emission peaks from 540 nm to 548 nm.It was important to note that only the maximum peak red shifted while the other shoulder peaks did not shift.There was no further red shifting with increase of methanol up to 1∶1 ratio.However,the proportion of chloroform and metharol between 7∶1 and 1∶1 resulted in maximum peak reversal.Further increase in methanol resulted in peak quenching.This was due to the π-π electronic coupling which splits the π orbital of PTCDI(perylene-3,4,9,10-tetracarboxylic)into two bands with the higher-energy band possessing most of the oscillator strength[11].Comparing theseresults to the fluorescence spectra of 1,7-Br-PDI-C12,it was realized that the later had totally different results.Unlike 1,7-HPDI-C12sample which had peak intensity increase accompanied by peak reversal with increase in methanol volume from 9∶1 to 1∶1,1,7-Br-PDI-C12peaks intensity reduced with increase in methanol volume.This was accompanied by maximum peak reversal(see Fig.6(b)).The shoulder peak transformed to be the maximum peak which was at 560 nm slowly.At 1∶1 ratio,there was a sudden blue shift of the maximum from 560 to 542 nm.Further increase of methanol beyond 1∶1 ratio resulted into complete quenching ofthefluorescence bands.The less complicated peaks change including reversal and shifting in 1,7-Br-PDI-C12indicates that the sample has weaker aggregation compared with 1,7-H-PDI-C12.

Fig.6 Solvent ratio-dependent emission spectra(λex=470 nm) of compound(a)1,7-H-PDI-C12and(b)1,7-Br-PDI-C12in a mixed solvent(CHCl3/MeOH)with a concentration of 5e-4mol·L-1at room temperature(the absorption curve can not be accepted to base line shift above induced by suspension of aggregate,but the fluorescence emission spectra can give some)
Temperature would also influence the self-assembly behavior because solubility of molecules was dependent on temperature.The two samples were dissolved in a poor solvent MCH(methyl cyclohexane) at 10-4mol· L-1.Their absorption spectra were recorded in varying temperature and the results were shown in Fig.7.From the results it could be observed that when 1,7-H-PDI-C12was heated from 20℃ to 70℃,there was blue shifting of the peaks(Fig.7(a)).This was due to the fact that at low temperature the molecules of 1,7-HPDI-C12were immobile and had strong π-π interaction to aggregate.As the temperature was increased,the molecules gained energy to break the aggregation and became free to move as individual thus resulting into blue shifting.For 1,7-Br-PDIC12there was no peak shifting observed(Fig.7(b)),this was because 1,7-Br-PDI-C12had weakened aggregation force and the sample already was in monomeric state and no aggregation existed which could be broken by heating at 10-4mol·L-1concentration in MCH.

Fig.7 Temperature-dependentUV-visabsorption spectra of compound(a)1,7-H-PDI-C12and(b)1,7-Br-PDI-C12in MCH at 1e-4mol·L-1(temperature increases from 20℃ to 70℃,and 10℃ temperature interval for every curve)
2.2 Electro chemical properties
Bay area substitutions on PDI have a direct effect of altering the electrochemicalproperties ofthe dye. The electrochemical properties of 1,7-Br-PDI-C12alongside solution maximum absorption and fluorescence peaks were tested and compared with that of unsubstituted PDI(1,7-H-PDI-C12).The results were summarized in Table 1.From the table it can be observed that bay substitution of PDI did not adversely change both the photo physical and electrochemical properties of PDI.A small blue shifting of 2 nm was observed in the maximum absorption peak of 1,7-Br-PDI-C12as compared to 1,7-H-PDI-C12both in chloroform and C∶M 1∶1.The corresponding fluorescent red shift was about 7 nm,due to sensitivity of fluorescence spectra.There was a slight negative shifting in both LUMO and HOMO when PDI was baysubstitutedwith bromine.This was because bromine has electron withdrawing effect,which did not greatly alter the HOMO and LUMO.Thus the band gaps of the two samples were relatively similar.

Table 1 Electro chemical properties of 1,7-H-PDI-C12and 1,7-Br-PDI-C12in CHCl3or C∶M(1∶1)at 1e-4mol·L-1
2.3 Photo physical properties in solid state
The packing of PDI structures in solid state strongly depends on the type of the substituents at the N-imides and bay positions.Bay area substitution has the effect of twisting the PDI core,thus giving it a different structure shape which has reduced π-π interaction compared to planar PDI.This result strongly affects the packing of the molecules in solid form.
Crystal structure of the two samples were made through dissolving the molecules in one part of chloroform and slowly laying four parts of methanol on top,leaving it alone to crystallize for around two weeks.Both 1,7-H-PDI-C12and 1,7-Br-PDI-C12were able to form solid crystals as shown in Table 2.Fluorescence microscope(excited by 550-580 nm)images of 1,7-H-PDI-C12and 1,7-Br-PDI-C12revealed that both crystals had strong red fluorescence[20].The crystals of 1,7-H-PDI-C12grow along the one dimensional direction in shape but not long,whereas the crystals formed from 1,7-Br-PDI-C12were welldefined ultralong nanobelts.

Table 2 Optical properties of 1,7-H-PDI-C12and 1,7-Br-PDI-C12
Solid absorption and fluorescence spectra of 1,7-H-PDI-C12and 1,7-Br-PDI-C12were tested and the results were presented in Fig.8.From the results,the characteristic PDI absorption peaks almost disappeared in 1,7-H-PDI-C12thus resulting into one broad featureless band while the absorption spectra of 1,7- Br-PDI-C12still retained the characteristic peaks in their normal intensity ratio.The corresponding fluorescence of the two samples was similar with a small red shifting of 4 nm for 1,7-Br-PDI-C12in reference to 1,7-H-PDI-C12.This result helps us to draw a conclusion that the molecular packing in the 1,7-Br-PDI-C12was not tightly packed as in 1,7-H-PDI-C12.A comparison of the two samples'Фfagreed well with this conclusion,and the Фfof 1,7-Br-PDI-C12(2.83%)was much higher than that of 1,7-H-PDI-C12(0.02%)clearly confirming that the 1,7-H-PDI-C12molecules are tightly packed in the crystal than the 1,7-Br-PDI-C12molecules(Table 2).

Fig.8 Normalized UV-vis absorbance(solid line)and fluorescence spectra(dashed line)of(a)1,7-H-PDI-C12and(b)1,7-Br-PDI-C12solid crystal
The X-ray diffraction(XRD)measurements of 1,7-Br-PDI-C12showed three strong peaks at 2θ=10.03,20.13,22.79,with several smaller peaks at 12.75,16.97,25.91,26.5730.39,33.02,36.70,46.55°(Fig.9),and the d values were determined to be 0.88,0.44,0.39,0.69,0.52,0.34,0.33,0.29,0.27,0.25,0.20 nm of these peaks,which indicated that the building blocks formed a well-ordered crystalline arrangement.The XRD data of 1,7-H-PDI-C12also indicated it could form crystal.The π-π stacking distance of 1,7-Br-PDI-C12was calculated from the wide angle region as 0.39 nm,which was larger than that of 1,7-H-PDI-C12(0.38 nm).This was because of the nonplanar PDI core of the former.

Fig.9 XRD patterns of 1,7-H-PDI-C12and 1,7-Br-PDI-C12crystal structure
3 Conclusions
We have investigated the effect of functionalizing PDI with bromine at bay areas by comparing 1,7-H-PDI-C12with 1,7-Br-PDI-C12,including solution and solid photo physical properties,self-assembly behavior,electrochemical properties,and solid structure morphology difference.The bay substitutions of PDI with bromine did not have a significant effect on the absorption and fluorescence emission spectra,the photo physical properties in solution.Also there was no significant alteration of electro chemical properties.However,the solid state properties showed enhancement of solid state fluorescence properties,i.e.,well developed structures which were of higher crystalline,highly fluorescence emission and higher Фfthan planar PDI crystals.This work showed that we could improve solid state photo physical properties of PDI without changing the other properties by simply using bromine bay area substitution.This type of molecule was demanded to be further modified and obtain more functionalized PDI materials in electronic device applications.
[1]O'regan B,Graetzel M.A Low-Cost,High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal Titanium Dioxide Films[J].Nature,1991,353(6346):737-740.
[2]Cheng Y J,Yang S H,Hsu C S.Synthesis of Conjugated Polymers for Organic Solar Cell Applications[J].Chemical Reviews,2009,109(11):5868-5923.
[3]Jiang Y,Lu L,Yang M,et al.Taking the Place of Perylene Diimide:Perylene Tetracarboxylic Tetraester as a Building Block for Polymeric Acceptors to Achieve Higher Open Circuit Voltage in All-Polymer Bulk Heterojunction Solar Cells[J].Polymer Chemistry,2013,4(23):5612-5620.
[4]Wong W Y,Ho C L.Functional Metallophosphors for Effective Charge Carrier Injection/Transport:New Robust OLED Materials with Emerging Applications[J].Journal of Materials Chemistry,2009,19(26):4457-4482.
[5]Jones B A,Facchetti A,Wasielewski M R,et al.Effects of Arylene Diimide Thin Film Growth Conditions on n-Channel OFET Performance[J].Advanced Functional Materials,2008,18(8):1329-1339.
[6] Kola S,Kim J H,Ireland R,et al.Pyromellitic Diimide-Ethynylene-Based Homopolymer Film as an N-Channel Organic Field-Effect Transistor Semiconductor[J].ACS Macro Letters,2013,2(8):664-669.
[7] Huang C,Barlow S,Marder S R.Perylene-3,4,9,10-Tetracarboxylic Acid Diimides:Synthesis,Physical Properties,and Usein OrganicElectronics[J].JournalofOrganic Chemistry,2011,76(8):2386-2407.
[8]Jiang L,Dong H L,Hu W P.Controllable Growth and Assembly of One-Dimensional Structures of Organic Functional Materials for Optoelectronic Applications [ J]. One-Dimensional Nanostructures,2013:397-414.
[9] KazmaierP M,Hoffmann R.A TheoreticalStudy of Crystallochromy.Quantum Interference Effects in the Spectra of Perylene Pigments[J].Journal of theAmerican Chemical Society,1994,116(21):9684-9691.
[10]Tuerkmen G,Erten-Ela S,Icli S.Highly Soluble Perylene Dyes: Synthesis,Photophysical and Electrochemical Characterizations[J].Dyes and Pigments,2009,83(3):297-303.
[11]Wuerthner F.Perylene Bisimide Dyes as Versatile Building Blocks for FunctionalSupramolecularArchitectures[J].Chemical Communications,2004,(14):1564-1579.
[12]Huang Y,Fu L,Zou W,et al.Ammonia Sensory Properties Based on Single-Crystalline Micro/Nanostructures of Perylenediimide Derivatives:Core-Substituted Effect[J].Journal of Physical Chemistry C,2011,115(21):10399-10404.
[13]Wurthner F,Stepanenko V,Chen Z,et al.Preparation and Characterization ofRegioisomerically Pure 1,7-Disubstituted Perylene Bisimide Dyes[J].Journal of Organic Chemistry,2004,69(23):7933-7939.
[14]Fan L,Xu Y,Tian H.1,6-Disubstituted Perylene Bisimides: Concise Synthesis and Characterization as Near-Infrared Fluorescent Dyes[J].Tetrahedron Letters,2005,46(26):4443-4447.
[15]Dey S,Efimov A,Lemmetyinen H.Diaryl-Substituted Perylene Bis(imides): Synthesis, Separation, Characterization and Comparison of Electrochemical and Optical Properties of 1,7-and 1,6-Regioisomer[J].European Journal of Organic Chemistry,2012,(12):2367-2374.
[16]Kaiser T E,Wang H,Stepanenko V,et al.Supramolecular Construction of Fluorescent J-Aggregates Based on Hydrogen-Bonded Perylene Dyes[J].Angewandte Chemie,International Edition,2007,46(29):5541-5544.
[17]Cheeseman J R,Frisch M J,Devlin F J,et al.Hartree-Fock and Density FunctionalTheory ab Initio Calculation ofOptical Rotation Using GIAOs:Basis Set Dependence[J].Journal of Physical Chemistry A,2000,104(5):1039-1046.
[18]Ruiz Delgado M C,Kim E-G,da Silva Filho D A,et al.Tuning the Charge-Transport Parameters ofPerylene Diimide Single Crystals via End and/or Core Functionalization: A Density Functional Theory Investigation[J].Journal of the American Chemical Society,2010,132(10):3375-3387.
[19]Sivamurugan V,Kazlauskas K,Jursenas S,et al.Synthesis and Photophysical Properties of Glass-Forming Bay-Substituted Perylenediimide Derivatives[J].Journal of Physical Chemistry B,2010,114(5):1782-1789.
[20]Kaiser T E,Stepanenko V,Wurthner F.Fluorescent J-Aggregates of Core-Substituted Perylene Bisimides:Studies on Structure-Property Relationship,Nucleation-Elongation Mechanism,and Sergeants-and-Soldiers Principle[J].Journal of the American Chemical Society,2009,131(19):6719-6732.
TQ617.3
A
1672-5220(2015)03-0372-07
date:2014-01-20
s:Shanghai Natural Science Foundation,China(No.13ZR1400700);The Program for Innovative Research Team in University,China(No.IRT1221)
*Correspondence should be addressed to SUN Bin,E-mail:sunbin@dhu.edu.cn
 Journal of Donghua University(English Edition)2015年3期
Journal of Donghua University(English Edition)2015年3期
- Journal of Donghua University(English Edition)的其它文章
- Effect of Sludge Retention Time on the Fate of Proteins and Polysaccharides in AAO Process
- Compressive Strength Estimation for the Fiber-Reinforced Polymer(FRP)-Confined Concrete Columns with Different Shapes Using Artificial Neural Networks
- Force-Based Quadrilateral Plate Bending Element for Plate Using Large Increment Method
- Theoretical and Experimental Analyses of Poisson Ratios for Plain-Woven Fabrics
- Dynamic Engaging Characteristics of Wet Clutch in Automatic Transmission
- Cavitation Bubble Luminescence in Cone-Type Throttle Valve
