Acoustic Characteristics of Advertisement Calls in Babina adenopleura
Xiaobin FANG, Xia QIU Yilin ZHOU Luyi YANG Yi ZHAO Weihong ZHENGand Jinsong LIU*
1School of Life and Environmental Sciences, Wenzhou University, Wenzhou 325035, China
2School of Nature Conservation, Beijing Forestry University, Beijing 100083, China
Acoustic Characteristics of Advertisement Calls in Babina adenopleura
Xiaobin FANG1,2, Xia QIU1, Yilin ZHOU1, Luyi YANG1, Yi ZHAO1, Weihong ZHENG1and Jinsong LIU1*
1School of Life and Environmental Sciences, Wenzhou University, Wenzhou 325035, China
2School of Nature Conservation, Beijing Forestry University, Beijing 100083, China
Acoustic communication is the most important form of communication in anuran amphibians. To understand the acoustic characteristics of male Babina adenopleura, we recorded advertisement calls and analyzed their acoustic parameters during the breeding season. Male B. adenopleura produced calls with a variable number of notes (1–5), and each note contained harmonics. Although 6% of call notes did not exhibit frequency modulation (FM), two call note FM patterns were observed: (1) upward FM; (2) upward–downward FM. With the exception of 1- and 5- note calls, the duration of successive notes decreased monotonically. With the exception of 1 note calls, the fundamental frequency of the first note was lowest, then increased; the greatest change in the fundamental frequency was always between notes 1 and 2. The dominant frequency varied between calls. For example for the first call note the dominant frequency occurred in some cases in the first harmonic (located in the 605.320 ± 64.533 Hz frequency band), the second harmonic(918 ± 9 Hz band), the fourth harmonic (1712 ± 333 Hz band), the sixth harmonic (the 2165 ± 152 Hz band), the seventh harmonic (the 2269 ± 140 Hz band), the eighth harmonic (the 2466 ± 15 Hz band) or the ninth harmonic (the 2636 ± 21 Hz band). Although male B. adenopleura advertisement calls have a distinctive structure, they have similar characteristics to the calls of the music frog, B. daunchina.
anuran amphibians, advertisement calls, Babina adenopleura, call structure
1. Introduction
Vocalizations are one of the most important means of animal communication, playing a very important role in species identification and female mate choice in frogs and toads (Cunningham and Birkhead, 1998; Brenowitz and Rose, 1999). On the basis of their function and environmental context, four types of anuran calls have been categorized; reciprocation calls, release calls, distress calls and advertisement calls (courtship, territorial, and encounter calls) (Duellman and Trueb, 1986). Of these, advertisement calls have been the most extensively studied due to their unique species-specific characteristics (Gerhardt, 1994). During the breedingseason, advertisement calls have a direct impact on individual reproductive success (Lingau and Bastos, 2007), and are regarded as one of the key characters responsible for reproductive isolation and speciation in anurans (Wells, 1977; Cocroft and Ryan, 1995; Pough et al., 2004; Hernandez et al., 2010; Bilate and Lack, 2011). In some species, such as Duttaphrynus melanostictus (synonym: Bufo melanostictus) (Wei et al., 2012), male calls are relatively simple, consisting of a series of identical repeated notes, whereas in others, such as Babina daunchina, males can produce dozens of complex calls (Chen et al., 2012). Due to this high level of species-specific variability, advertisement calls have been used to clarify the taxonomic relationships between closely related anuran species (Abrunhosa et al., 2005; Bickford et al., 2007; Nunes et al., 2007; Xu et al., 2005; Yu and Zheng, 2009). This kind of analysis must take into account variation in call parameters caused by factorssuch as air temperature (Chen et al., 2012; Giacoma et al., 1997; Navas and Bevier, 2001), male size and weight (Robertson, 1986; Giacoma et al., 1997), and social interactions among individuals within the reproductive aggregation (Wells, 1988).
So far, the advertisement calls of more than 30 Chinese anurans have been reported, including Microhyla ornata (Jiang et al., 1995; Wei et al., 2013), Rhacophorus dennysi (Wang et al., 2012), D. melanostictus (Wei et al., 2012), B. daunchina (Chen et al., 2011; Cui et al., 2010, 2011, 2012), Hoplobatrachus rugulosus (Wei et al., 2011), Amolops wuyiensis (Feng et al.,2006;Shen et al., 2008) and Paa spinosa (Chen et al., 2012; Yu and Zheng, 2009). Previous research on Chinese anuran vocal behavior has used different methods to describe the acoustic structure of advertisement calls. For example, Jiang et al. (1995) used pulse duration, pulse rate, call duration and dominant frequency to describe the call characteristics of three frog species, but Yu and Zheng (2009) measured other parameters, including note number, note rate, note duration, number of formants (harmonics), and dominant frequency, in their analysis of the advertisement call of P. spinosa.
Babina adenopleura is a small-to medium-sized frog with a body length of 53–60 mm, and distributed in central and southern China. This species was first designated as Rana adenopleura by Boulenger (1909), but renamed as B. adenopleura according to the currently accepted taxonomy (Frost et al., 2006). Its skin is bluegreen or gray-brown with a pattern of dots on the dorsal surface (Boulenger, 1909). The species inhabits paddy fields, marshes, ditches, ponds, and hydrophytes-filled lakes at altitudes between 30 and 1 800 m. The breeding season of these frogs is prolonged and females begin to lay eggs in the second half of April; tadpoles of B. adenopleura in different development stage can be found between April to August; tadpoles during metamorphosis and young frogs after metamorphosis can be found in August; few tadpoles born too late begin metamorphosis after winter (Fei et al., 2009).
B. adenopleura and B. daunchina are closely related and so similar in appearance that they were formerly classified as the same species; B. daunchina was considered to be the synonym of B. adenopleura and they were named R. adenopleura (Pope and Boring, 1940). These two related species all have paired internal pharyngeal vocal sacs which are diverticula of the mouth cavity and may serve as resonators, sound couplers, or acoustic radiators (Narins et al., 2007). The most obvious differences between these two species are their spawning habits and calls (Fei et al., 2009). Before pairing occurs, the male B. daunchina builds a mud-based burrow for mating and egg incubating (Liu, 1950; Ye et al., 1993), and produce calls of varying lengths consisting of a series of “musical” note from inside or outside the burrows(Cui et al., 2010; Chen et al., 2011). But B. adenopleura does not have this unique behavior. Moreover, their calls can be easily distinguished by human ear. The acoustic structure of male B. daunchina calls and their correlation with air temperature and humidity has been described by Cui et al. (2010, 2011) and Chen et al. (2012). Males of this species use calls to advertise that they possess a nest to potential mates, thereby facilitating mate selection by females (Cui et al., 2012). However, little is known about the call properties of B. adenopleura and previous research did not analyze its components in detail (Zhou et al., 2014). The aims of this study were to describe the components of the advertisement call of B. adenopleura, determine the extent to which identifiable call features vary between calls and individuals, and compare the calls of this species with those of B. daunchina.
2. Materials and Methods
2.1 Recording of calls Male B. adenopleura advertisement calls were recorded in the Gutian Mountain National Nature Reserve in Quzhou City, Zhejiang Province, China (29.14° N, 118.05° E) during June and July 2012 and July 2013. Calls were recorded between 19:00 to 22:00 using Sony IC recorders (ICD-FX8) with an internal microphone. To avoid repeated recording, we found and identified subjects according to calls. Having identified a subject, the microphone was placed at about 50 cm from the subject. The distance was kept as constant as possible such that variation in microphone position. Calls of different males were recorded for 5–30 min. All calls were recorded from rice field with water, and frogs stayed along the ridge of the fields. After recording, six males (July 2013) were captured and body mass measured to the nearest 0.1g with an electric balance (Mettler Toledo International Inc., PL2001-L, Switzerland). Body size (snout-vent length) was measured to the nearest 0.01 mm using a digital caliper (Guilin Measuring and Cutting Tool Co. Ltd., China). Then the males were released into the site from which they were captured.
We used a Humidity Temperature Meter (Center Technology Group Company, CENTER 311, Taiwan, China) to measure air temperature (21–26 °C) and humidity (83%–94%). The value of temperature and humidity showed litter variation, so we did not analysewhether these variables affected male calls.
2.2 Acoustic parameters Recording files were transferred with Cool Edit Pro2.0 (Syntrillium Software Corporation, USA) and saved as wav format. The calls of individual male frogs recorded in the field were usually contaminated acoustically by the calls of other males or other environmental sounds. Therefore, the calls of individual males were first edited from the original recordings using Cool Edit Pro2.0 software so that selected clean, uncontaminated, calls could be analyzed. More than ten calls in each call sequence within a sound file were analyzed. We successfully recorded the advertisement calls of 16 males and analyzed a total of 190 calls.
All sound files were digitized and analyzed using Praat (Boersma and Weeninkk, Version 5.3.32, University of Amsterdam) at a sampling frequency resolution of 44100 Hz and 16 bit resolution. Call parameters were defined and illustrated based on Pröhl (2003), Yu and Zheng (2009) and Chen et al. (2012). To describe clearly the acoustic structure of calls, the frequency band containing the most energy, or the single greatest amplitude, was defined as the dominant frequency (DF/Hz) (Figure 1C). The value of the fundamental frequency (Hz) was obtained by calculating the reciprocal of the period of the longest cycle (Chen et al., 2011; Simmons and Ferragamo, 1993). Six other acoustic properties were measured: call duration (s), note number, note duration (s), note rate (notes/s), note interval (s) and number of harmonics. Yu and Zheng (2009) calculated the note rate with the equation: note rate = (note number – 1) / call duration. Since some calls have only one note in our study, we calculated note rate with the modified equation: note rate = note number / call duration. All spectral components were measured at the midpoint of notes. To provide a normative picture of frequency changes within and between the notes of advertisement calls, all harmonics (i.e., frequency components) of each note within all calls recorded for individual males were measured. Temporal and spectral parameters were measured for each of the first call notes because every call contained at least one note.
Subharmonics with durations shorter than the duration of the note could be identified in some call spectrograms(Figure 1B), and are refected by the smaller peaks in the power spectrum (Figure 1C). Although the subharmonic bands were not always obvious and existed in only some calls, these features of B. adenopleura calls were considered pertinent to measure. Thus the number of subharmonics in note 1 and the duration of subharmonics in note 1 were measured.
To determine if the maximum value for the fundamental of call notes is related to the body mass and body size for individual males, we determined the highest fundamental frequency for all notes of the same call.
2.3 Analysis and statistics B. adenopleura calls consist of variable numbers of notes. To describe call characteristics, calls consisting of same numbers of notes were classified together for analysis. Differences in note duration and fundamental frequency between different notes were also analyzed with a one-way ANOVA. In the case of calls with just two notes, an Independent-Samples t-test was used to evaluate differences in note duration and fundamental frequency between notes. In the case of calls that consisted of variable numbers of notes, a oneway repeated measures ANOVA was used to evaluate the significance of differences in call duration, note rate, note interval, note duration, frequency bands and dominant frequency. Pearson’s correlation analysis was used to detect whether the fundamental is related to the body mass and size of individual males.
Data were expressed as mean ± SD, and P < 0.05 was considered statistically significant. Statistical analyses were performed with SPSS 13.0 for windows. All data were tested for normality (Kolmogorov-Smirnov test) and homogeneity of variances (F-max test) prior to statistical analyses.
3. Results
3.1 Structure of B. adenopleura advertisement calls Male B. adenopleura advertisement calls have a distinctive structure. Advertisement calls (190 calls from 16 males) contained 1-5 notes (2.74 ± 0.92), each of approximately 0.2–0.9 s in duration. Three-note calls were the most common (70/190; 36.84%), followed by two-note calls (66/190; 34.74%), four-note calls (36/190; 18.95%), one-note calls (14/190; 7.37%), and five-note calls (4/190; 2.11%). There were silent intervals between the notes of a call (Figure 1A).
The mean note amplitude showed little variation within the calls of individual males (Figure 1A). To evaluate differences in note interval and note duration between different calls with variable numbers of notes, we measured and analyzed the mean values of these parameters. As shown in Table 1, call duration, note rate and note duration were significantly different between different calls with variable numbers of notes. Call duration was correlated with note number, while note interval and note duration were negatively correlatedwith the note number. For calls with the same number of notes, except for 1 and 5 note calls, the first note in each call was significantly longer than the succeeding notes, whereas the succeeding notes differed little, if at all, in length (Table 2).
Each call note consisted of several frequency components that were not integer multiples of the lowest frequency band, indicating that the lowest frequency band (F1) in each note was not the fundamental frequency which has been suppressed (Figure 1B, 1C). All frequency components of nine 3-note calls recorded from an individual male are shown in Table 3; frequency components were nearly integer multiples of 300 Hz. Fifth frequency bands (F5) differ significantly between the first, second and third notes(F2,24= 3.450, P < 0.05, Table 3). The number of subharmonics in note 1 is 3.088 ± 2.465 and the duration of submarmonics in note 1 is 0.053 ± 0.023 s.
The mean male body mass and size of B. adenopleura were 11.3 ± 1.0 g and 53.2 ± 3.1 mm, respectively (n = 6). Pearson’s correlation analysis showed that the highest fundamental frequency was not positively correlated with either body mass (r = 0.799, P > 0.05, n = 6) or size (r = 0.371, P > 0.05, n = 6).
3.2 Frequency modulation patterns of calls Three different frequency modulation harmonic band (FM) patterns were observed. Six percent (6%) of call notes did not exhibit frequency modulation (Figure 2A). That is to say, their harmonics can be represented as horizontalbands. However, two FM patterns were observed in other notes: (1) An upward FM pattern with ΔHz/ms of 0.882 ± 0.020 (Figure 2B); (2) A downward–upward FM pattern with ΔHz/ms of 0.537 ± 0.020 (Figure 2C). The upward FM pattern was a simple upward curve, whereas the downward–upward FM pattern was concave in shape; an upward curve followed by downward modulation to the baseline. Moreover, the first notes in multi-note calls were usually the note most likely to show the downward–upward FM pattern. Frequency modulation patterns varied for harmonic bands within single calls, and in some cases, varied from call to call.
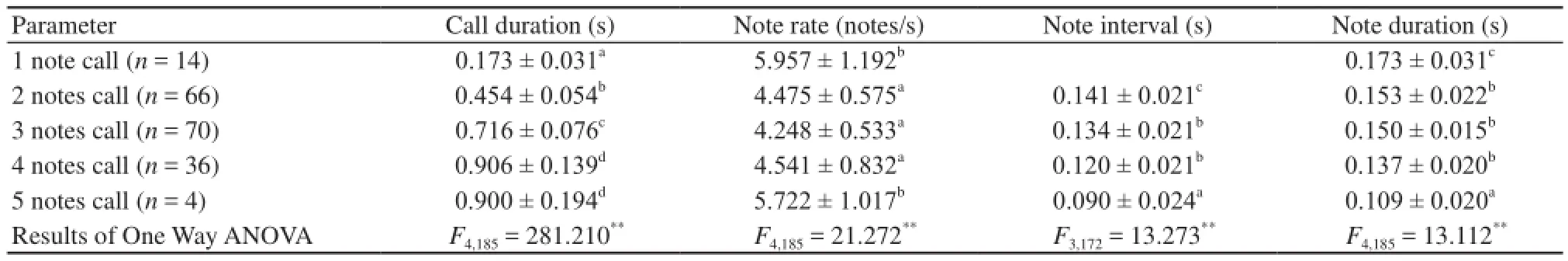
Table 1 Acoustic characteristics of male B. adenopleura advertisement calls.
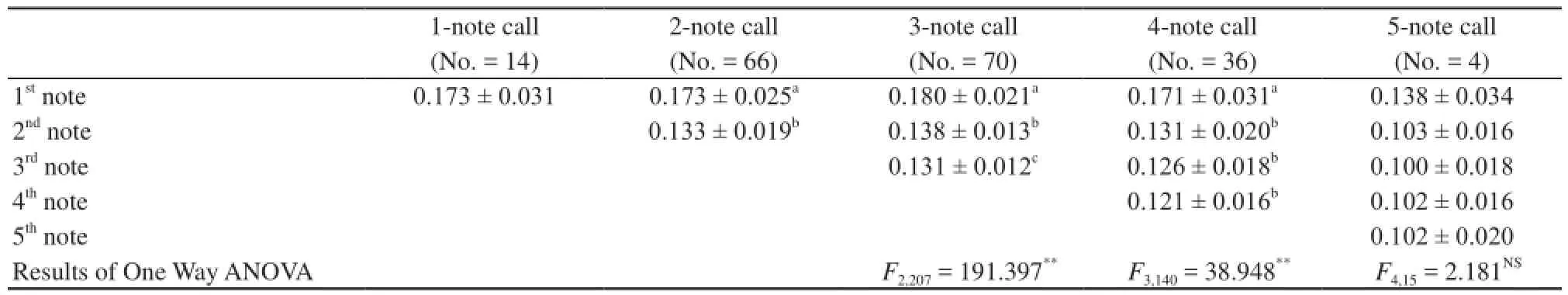
Table 2 Durations of successive notes in male B. adenopleura advertisement calls.
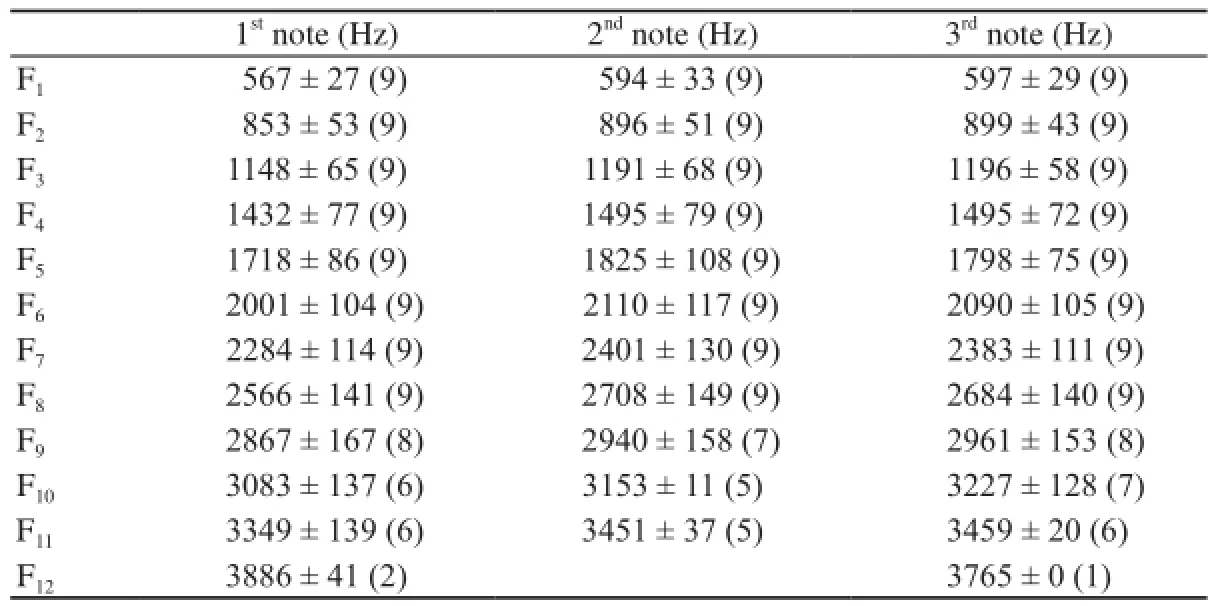
Table 3 Mean frequencies of the first 11 partials for 3-note calls produced by a single male B. adenopleura.
3.3 Fundamental frequency increments between notes The fundamental frequency of successive notes varied between notes. This can also be seen in the results of the analysis of each note of 9 three note calls of an individual male (Table 3). To investigate this phenomenon further, we analyzed calls with the same number of notes (Table 4). The fundamental frequency of the first note within all multi-note calls was lowest, and increased substantially in the second notes. In 3 note (F2,207= 8, P < 0.001) and 4 note (F3,140= 4, P < 0.05) calls, the fundamental frequency of different notes was significantly different. The highest fundamental frequency of all multi-note calls (except 2 note calls) was usually found in the middle note. The first notes’ fundamental frequency was lower than the last notes fundamental frequency in successive calls. This suggests that there an upper limit to fundamental frequency.
3.4 Dominant frequency variation The dominant frequency of calls was highly variable. For some calls, the lowest frequency band contained the highest energy, that is, the lowest frequency was the dominant frequency (Figure 3A), whereas in others calls the dominant frequency was much higher than the lowest harmonic. Analysis of the dominant frequency of first notes indicates that there were seven patterns of dominant frequency. The sixth harmonic was most common (located in 2165 ± 152 Hz section) (Figure 3D), followed by the eighth harmonic (located in 2466 ± 16 Hz section) (Figure 1C),fourth harmonic (located in 1712 ± 333 Hz section)(Figure 3C), seventh harmonic (located in 2269 ± 140 Hz section) (Figure 3E), first harmonic (located in 605 ± 65 Hz section) (Figure 3A), second harmonic (located in 919 ± 10 Hz section) (Figure 3B) and ninth harmonic(located in 2637 ± 21 Hz section) (Figure 3F). The value is significantly different (F6,182= 321.088; P < 0.001).
4. Discussion

Table 4 Mean fundamental frequencies of male B. adenopleura advertisement calls of different length.
The advertisement calls of male anurans have two functions: attraction of conspecific females and male territorial advertisement (Wells, 1977; Tárano, 2001). Female anurans use species-specific male advertisement calls to identify conspecific males (McClelland et al., 1996; Lea et al., 2002; Krishna and Bosch, 2007), and often to decide which male to mate with (Gerhardt, 1994; Pröhl, 2003; Yu and Zheng, 2009; Cui et al., 2012).
We found that the advertisement calls of male B. adenopleura were typically comprised of 1–5 notes, the mean amplitude of which did not vary. Each note consisted of a stack of frequency bands and there were silent intervals between notes (Figure 1A). Except for 1- and 5-note calls, the first note in each call was significantly longer than the succeeding notes, and note duration subsequently decreased sequentially (Table 2). Note interval was negatively correlated with note number (Table 1). Male anurans typically spend a significant amount of time calling, for example, Yu et al. (2008) found that calling comprised 20% of the time budgets of individual male P. spinosa during the breeding season. It’s possible, therefore, that male B. adenopleura mostcommonly give three-note calls because these are relatively energy efficient to produce. This is because, for multi-note calls, the longer the inter-note interval, the smaller the energy required to produce the call (Jiang et al., 2002). Yu et al. (2008) speculated that the absence of silent intervals between notes in the calls of P. spinosa may reduce energy consumption.
The fundamental frequency is a missing spectral component of B. adenopleura advertisement calls; a similar phenomenon occurs in the green frog (R. clamitans) (Bee et al., 2001). Among anuran amphibians (frogs and toads), fundamental frequency depends on the shape and mass of the laryngeal apparatus, which is related to overall body size (Martin, 1972). Although the body mass and size of individual subjects was not positively correlated with the highest fundamental frequency in this study, there is much evidence suggesting this relation which supports the idea that the call characters of anuran males can provide information about body size (Robertson, 1986; Wang et al., 2012). Therefore, the female can evaluate the potential male’s quality on the basis of the acoustic features of male communication signals (Morris and Yoon, 1989). Furthermore, the fundamental frequency increased substantially in the second notes, but changed little in the following notes. This also indicates that increases in the fundamental frequency are limited by the size of the vocal sac in B. adenopleura as is the case for the túngara frog (Physalaemus pustulosus) (Ryan, 1983). More study is needed to resolve this question.
The dominant frequency of each note varied to some degree and was not associated with any particular harmonic band, i.e., the harmonic with the highest energy varied. We found seven patterns of dominant frequency in the first note of calls; these were in the first, second, fourth, sixth, seventh, eighth and ninth harmonic, respectively. This is a very interesting phenomenon. As is the case for the fundamental frequency, the dominant frequency of the call is negatively correlated with size in many species (Bee et al., 2001; Sullivan and Wagner 1988; Wang et al., 2012), due to morphological constraints on the sound producing apparatus (Martin, 1972). Because of this relationship, males of many species use the dominant frequency of the call to assess the size of an opponent (Davies and Halliday 1978; Arak 1983; Robertson 1986). However, there is also empirical evidence that the dominant frequency of frog calls may be plastic (Grafe, 1995; Lopez et al., 1988; Wagner, 1992), especially during social interactions among competing males. For example, male white-lipped frogs (Leptodactylus albilabris) increased or decreased the dominant frequency of their calls to match that of a nearby male (Lopez et al., 1988). At the time our recordings were made, a large number of male B. adenopleura called in the same rice field. Furthermore, the dominant frequency of the call from the same frog was also changed. So males may vary the dominant frequency of their calls for competing with other males (Wei et al., 2011). Playback tests are needed to explore this phenomenon further.
In summary, male B. adenopleura calls are spectrally and temporally complex. Most call notes exhibited frequency modulation; fundamental frequency changes between notes and variation in the dominant frequencyof each note. This may be to reduce energy consumption or because of anatomical constraints. The acoustic characteristics we identified are useful for distinguishing B. adenopleura from other species such as the morphologically very similar B. daunchina.
Acknowledgements We thank Dr. Jianguo Cui of the Chengdu Institute of Biology, Chinese Academy of Sciences, and Prof. Dingzhen Liu of Beijing Normal University for directing this study and giving suggestions for improving this manuscript. We also thank the anonymous reviewers for their helpful comments. This study was financially supported by the National Science and Technology Project (2008BAC39B02–11), the National Undergraduate Innovation and Entrepreneurship Training Program (201310351015) and the Zhejiang Province “Xinmiao” Project (2012R 424021).
Abrunhosa P. A., Pimenta B. V. S., Cruz C. A. G., Haddad C. F. B. 2005. Advertisement calls of species of the Hyla albosignata group (Amphibia, Anura, Hylidae). Arq Mus Nac: Rio de Janeiro, 62: 275–282
Arak A. 1983. Sexual selection by male-male competition in natterjack toad choruses. Nature, 306: 261–262
Bee M. A., Gerhardt H. C. 2001. Neighbour–stranger discrimination by territorial male bullfrogs (Rana catesbeiana), I, Acoustic basis. Anim Behav, 62: 1129–1140
Bee M. A., Kozich C. E., K. J. Blackwell K., Gerhardt H. C. 2001. Individual variation in advertisement calls of territorial male Green Frogs, Rana clamitans: Implications for individual discrimination. Ethology, 107: 65–84
SQLite拥有一个简洁的、模块化的体系结构,并引进了一些独特的方法进行关系型数据库的管理。它由3个子系统中的8个独立的模块组成,如图1所示。这个模块将查询过程划分为几个独立的任务。在体系结构栈的顶部编译查询语句,在中部执行,在底部处理存储并与操作系统交互。
Bickford D., Lohman D. J., Sohdi N. S., Ng P. K. L., Meier R., Winker K., Ingram K. K., Das I. 2007. Cryptic species as a window on diversity and conservation. Trends Ecol Evol, 22(3): 148–155
Bilate M., Lack E. 2011. The advertisement call of Scinax similis(Cochran, 1952) (Amphibia, Anura). S Am J Herpetol, 6(1): 54–58
Boulenger, G. A. 1909. Descriptions of four new frogs and a new snake discovered by Mr. H. Sauter in Formosa. Ann Mag Nat Hist, 4: 492–495
Brenowitz E. A., Rose G. J. 1999. Female choice and plasticity of male calling behaviour in the Pacific treefrog. Anim Behav,57(6): 1337–1342
Chen Q., Cui J. G., Fang G. Z., Brauth S. E., Tang Y. Z. 2011. Acoustic analysis of the advertisement calls of the music frog, Babina daunchina. J Herpetol, 45(4): 406–416
Chen P., Zheng R. Q., Huang H., Yu X. Y., Xu Z. W. 2012. Mating call of Paa spinosa is related to temperature but not to body size. Sichuan Journal of Zoology, 31(4): 513–517(In Chinese with English abstract)
Cocroft R. B., Ryan M. J. 1995. Patterns of advertisement call evolution toads and chorus frogs. Anim Behav, 49: 283–303
Cui J. G., Song X. Y., Fang G. Z., Xu F., Brauth. S. E., Tang Y. Z. 2011. Circadian Rhythm of Calling Behavior in the Emei Music Frog (Babina daunchina) is Associated with Habitat Temperature and Relative Humidity. Asian Herpetol Res, 2(3): 149–154
Cui J. G., Tang Y. Z., Narins P. M. 2012. Real estate ads in Emei music frog vocalizations: Female preference for calls emanating from burrows. Biol Lett, 8(3): 337–340
Cui J. G., Wang Y. S., Brauth S., Tang Y. Z. 2010. A novel female call incites male-female interaction and male-male competition in the Emei music frog, Babina daunchina. Anim Behav, 80: 181–187
Cunningham E. J. A., Birkhead T. R. 1998. Sex roles and sexual selection. Anim Behav, 56(6): 1311–1321
Davies N. B., Halliday T. R. 1978. Deep croaks and fighting assessment in toads Bufo bufo. Nature, 274:683–585
Duellman W. E., Trueb L. 1986. Biology of Amphibians. New York: McGraw-Hill.
Feng A. S, Narins P. M., Xu C. H., Lin W. Y., Yu Z. L., Qiu Q., Xu Z. M., Shen J. X. 2006. Ultrasonic communication in frogs. Nature, 440(7082): 333–336
Fei L., Hu S. Q., Ye C. Y., Huang Y. Z. 2009. Fauna Sinica Amphibia, Vol. 2, Anura. Beijing: Science Press (In Chinese)
Frost, D. R., Grant T., Faivovich J., Bain R., Haas A., Haddad C. F. B., De Sá R. O., Donnellan S. C., Raxworthy C. J., Wilkinson M., Channing A., Campbell J. A., Blotto B. L., Moler P., Drewes R. C., Nussbaum R. A., Lynch J. D., Green D., Wheeler W. C. 2006. The amphibian tree of life. Bull Am Mus Natl Hist, 297: 1–370
Gerhardt H. C. 1994. The evolution of vocalization in frogs and toads. Annu Rev Ecol S, 25: 293–324
Giacoma C., Zugolaro C., Beani L. 1997. The advertisement calls of the green toad Bufo viridis: Variability and role in mate choice. Herpetologica, 53: 454–464
Grafe T. U. 1995. Graded aggressive calls in the African painted reed frog Hyperolius marmoratus (Hyperoliidae). Ethology, 101: 67–81
Hernández M., Alonso R., Rodriguez A. 2010. Advertisement call of Peltophryne Florentinoi (Anura: Bufonidae), an endemic toad from Zapata Swamp, Cuba. Amphibia-Reptilia, 31: 265–272
Jiang J. P, Xie F., Fei L., Ye C. Y., Zheng M. 2002. Mating calls of six forms of pleobatid in Wa Wu Mountain National Forest Park, Sichuan, China (Anura: Pelobatidae). Zool Res, 23: 89–94
Jiang S. R., Ding P., Zhuge Y. 1995. The comparative study on the characteristics of calling songs of three frog species. Zool Res, 16(1): 75–81 (In Chinese)
Krishna S. N., Bosch J. 2007. The breeding behaviour and advertisement calls of the tree-hole breeding frog Ramanella Montana (Microhylidae) in the Western Ghats, S. India. Acta Zool Sin, 53: 575–578
Lea J., Dyson M., Halliday T. 2002. Phonotaxis to advertisement calls by midwife toads (Alytes muletensis) is not necessarily related to mating. Amphibia-Reptilia, 23(2): 151–159
Lingau R., Bastos R. P. 2007. Vocalizations of the Brazilian torrent frog Hylodes heyeri (Anura: Hylodidae): Repertoire and influence of air temperature on advertisement call variation. J Nat Hist, 41: 1227–1235
Liu C. 1950. Amphibians of Western China. Chicago: ChicagoNatural History Museum Press
Lopz P. T., Narins P. M., Lewis E. R., Moore S. W. 1988. Acoustically induced call modification in the white-lipped frog,Leptodactylus albilabris. Anim Behav, 36: 1295–1308
Martin W. F. 1972. Evolution of vocalization in the genus Bufo. In Blair W. F. (Ed.), Evolution in the genus Bufo. Austin: University of Texas Press, 279–309
McClelland B. E, Wilczynski W., Ryan M. J. 1996. Correlations between call characteristics and morphology in male cricket frogs (Acris crepitans). J Exp Biol, 99(9): 1907–1919
Morris M. R., Yoon S. L. 1989. A mechanism for female choice for large males in the treefrog Hyla chrysoscelis. Behav Ecol Sociobiol, 25: 65–71
Narins P. M., Feng A. S., Fay R. R., Popper A. N. 2006. Hearing and Sound Communication in Amphibians. New York: Springer, 88
Navas C. A., Bevier C. R. 2001. Thermal dependency of calling performance in the eurythermic frog Colostethus subpunctatus. Herpetologica, 57: 384–395
Nunes I., Sampaio R. S., Junca F. A. 2007. Advertisement calls of four Hylid frogs from the state of Bahia, Northeastern Brazil (Amphibia, Anura, Hylidae). S Am J Herpetol, 2: 89–96
Pope C. H., Boring A. M. 1940. A survey of Chinese Amphibia. Pek Nat Hist Bull, 15(1): 13–86
Pough F. H., Andrews R. M., Cadle J. E., Crump M. L., Savltzky A. H., Wells K. D. 2004. Herpetology, 3rdEd. London: Pearson Education
Pröhl H. 2003. Variation in male calling behaviour and relation to male mating success in the Strawberry Poison Frog (Dendrobates pumilio). Ethology, 109: 273–290
Robertson J. G. M. 1986. Female choice, male strategies and the role of vocalizations in the Australian frog Uperoleia rugosa. Anim Behav, 34: 773–784
Ryan, M. J. 1983. Sexual selection and communication in a Neotropical frog, Physalaemus pustulosus. Evolution, 39: 261–272
Shen J. X., Feng A. S., Xu Z. M., Yu Z. L., Arch V. S., Yu X. J., Narins P. M. 2008. Ultrasonic frogs show hyperacute phonotaxis to female courtship calls. Nature, 453(7197): 914–916
Simmons A. M., Ferragamo M. 1993. Periodicity extraction in the anuran auditory nerve, I, “Pitch-shift” effects. J Comp Physiol A, 172: 57–69
Sulfvan B. K., Wagner W. E. Jr. 1988. Variation in advertisement and release calls, and social infuences on calling behavior in the Gulf Coast toad (Bufo valliceps). Copeia, 1016–1022
Tárano Z. 2001. Variation in male advertisement calls in the neotropcial frog Physalaemus enesefae. Copeia, 4: 1064–1072
Wagner W. E. 1992. Deceptive or honest sigalling of fighting ability? A test of alternative hypotheses for the function of changes in call dominant frequency by male cricket frogs. Anim Behav, 44: 449–462
Wang J. C., Cui J. G., Shi H. T., Brauth S. E., Tang Y. Z. 2012. Effects of Body Size and Environmental Factors on the Acoustic Structure and Temporal Rhythm of Calls in Rhacophorus dennysi. Asian Herpetol Res, 3(3): 205–212
Wei L., Lin Z. H., Ma X. M., Zhao L. H., Ma X. H. 2011. Acoustic characteristics of the tiger frog, Hoplobatrachus rugulosus, during the breeding season. Zool Res, 32(4): 456–460
Wei L., Shao W. W., Lin Z. H. 2013. Characteristics of courtship calls of Microhyla ornata (Anuran: Microhylidae). Zool Res, 34(1): 1–7 (In Chinese with English abstract)
Wei L., Zhao L. H., Ma X. H., Fan X. L., Ma X. M., Lin Z. H. 2012. Advertisement call variability in the black-spined toad Bufo melanostictus (Anura: Bufonidae) during the breeding season in Lishui, Zhejiang, China. Asian Herpetol Res, 3(2): 157–162
Wells K. D. 1977. The social behavior of anuran amphibians. Anim Behav, 25: 666–693
Wells K. D. 1988. The effect of social interactions on anuran vocal behavior. In Fritzsch B., Ryan M. J., Wilczynski W., Hetherington T. E., Walkowiak W. (Eds.), The evolution of the amphibian auditory system. New York: John Wiley and Sons, 433–454
Ye C. Y., Fei L., Hu S. Q. 1993. Rare and economic amphibians of China. Chengdu: Sichuan Publication House of Science and Technology, 239–241
Yu B. G., Ye R. H., Zheng R. Q., Zhou Y., Liu C. T. Chen X. 2008. The ethogram and activity rhythm of captive Paa spinosa during breeding period. Acta Ecol Sin, 28(12): 6371–6378
Yu B. G., Zheng R. Q. 2009. The advertisement call of the giant spiny frog Paa spinosa. Curr Zool, 55: 411–415
Xu J. X., Xie F., Jiang J. P., Mo Y. M., Zheng Z. H. 2005. The acoustic features of the mating call of 12 anuran species. Chn J Zool, 40: 12–19 (In Chinese)
Zhou Y. L., Qiu X., Fang X. B., Yang L. Y., Zhao Y., Fang T., Zheng W. H., Liu J. S. 2014. Acoustic characteristics of eight common Chinese anurans during the breeding season. Zool Res, 35(1): 42–50
Dr. Jinsong LIU, from School of Life and Environmental Sciences, Wenzhou University, Zhejiang, China, with his research focusing on energy metabolism of birds.
E-mail: ljs@wzu.edu.cn
5 December 2014 Accepted: 29 June 2015
——以大别山区9县(市)为例
 Asian Herpetological Research2015年3期
Asian Herpetological Research2015年3期
- Asian Herpetological Research的其它文章
- A New Species of Japalura (Squamata: Sauria: Agamidae) from Upper Lancang (Mekong) Valley of Eastern Tibet, China
- Seasonal Dynamics of Male and Female Reproductive Systems in the Siberian Salamander, Salamandrella keyserlingii (Caudata, Hynobiidae)
- Oviposition Site Selection in the Malayan Giant Frog (Limnonectes blythii) in Singapore: Conservation Implications
- Comparative Studies on Sperm Ultrastructure of Three Gecko Species, Gekko japonicus, Gekko chinensis and Hemidactylus bowrigii (Reptilia, Squamata, Gekkonidae)
- Genetic and Morphological Variations within Laudakia microlepis (Blanford, 1874) (Sauria: Agamidae) Populations in Southeastern Iran with Description of a New Subspecies
- Potential Distribution Modeling and Morphology of Pelias barani (Böhme and Joger, 1983) in Turkey
