Evaluation of norgestomet Crestar® on oestrus synchronization and reproductive performance of dairy cows in Algeria
Abdelhanine Ayad, Mourad Salaheddine, Kamal Touati, Mokrane Iguer-Ouada, Hama Benbarek
1Department of Environment and Biological Sciences, Faculty of Life and Nature Sciences, University A. Mira, Bejaia 06000, Algeria
2Department of Agricultural Sciences, Faculty of Life and Nature Sciences, University M. Istambouli, 29000, Mascara, Algeria
3Laboratory of Research on Local Animal Products, Veterinary Institute, Ibn Khaldoun University, 14000, Tiaret, Algeria
4Pharming Technologies B.V., P.O. Box 451, 2300 AL, Leiden, The Netherlands
5Surgery for Large Animals, Faculty of Veterinary Medicine, University of Liege, 4000, Liege, Belgium
Evaluation of norgestomet Crestar® on oestrus synchronization and reproductive performance of dairy cows in Algeria
Abdelhanine Ayad1,3*, Mourad Salaheddine4, Kamal Touati5, Mokrane Iguer-Ouada1, Hama Benbarek2,3
1Department of Environment and Biological Sciences, Faculty of Life and Nature Sciences, University A. Mira, Bejaia 06000, Algeria
2Department of Agricultural Sciences, Faculty of Life and Nature Sciences, University M. Istambouli, 29000, Mascara, Algeria
3Laboratory of Research on Local Animal Products, Veterinary Institute, Ibn Khaldoun University, 14000, Tiaret, Algeria
4Pharming Technologies B.V., P.O. Box 451, 2300 AL, Leiden, The Netherlands
5Surgery for Large Animals, Faculty of Veterinary Medicine, University of Liege, 4000, Liege, Belgium
ARTICLE INFO
Article history:
Received 17 October 2014
Received in revised form 14 December 2014
Accepted 18 December 2014
Available online 20 March 2015
Norgestomet Crestar®
Timing of AI
Dairy cattle
Pregnancy
Objective: To evaluate the effectiveness of norgestomet Crestar®for synchronization of oestrus and its effect on pregnancy rate in cows. Methods: after a waiting period (~ 90 days postpartum), Holstein-Frisian females kept in different dairy farms were inseminated for the detection of estrous behavour (n=133). Females were allocated randomly to treated (n=69) or control (n=64) groups. The treated animals received the Crestar®treatment during 9-10 days. After implant removal, the treated animals were artificially inseminated (AI) for the detection of standing estrous behaviour. Blood samples were collected on Days 0, 21 and 30 after service. Plasma progesterone (P4) and pregnancy-associated glycoprotein (PAG) levels were determinate by radioimmunoassay. Results: the study showed a higher rate of heat synchronization in the treated group (94%) compared to the control group (86%). Out of 133 cows inseminated, thirteen (4 treated and 9 control) had a plasma progesterone concentrations higher than 1.0 ng/mL at service. The plasma PAG levels at Day 30 after AI were significantly lower in the treated group (7.13 ± 2.29) compared to the control group (8.37 ± 2.30). Pregnancy rate was 28% (16/57) and 42% (23/54) for treated and untreated group, respectively. There were no significant difference®s in the pregnancy rates of different parity in both groups. Conclusion: The norgestomet Crestar treatment carried out during 9-10 days did not improve reproductive performance in dairy cattle. However, these data demonstrate that this hormonal protocol can be used to obtain high oestrus cow rates to initiate correct timing of insemination.
1. Introduction
Infertility is one of the pathological conditions qualified as disease of production. It is wide-spread in modern farming. Infertility affects the herd reproductive performance thereby negatively influencing productivity and return on investment of the farming business. The effectiveness of reproduction constitutes a crucial factor of productivity and profitability in the exploitation[1]. According to international standards, the interval calving-calving (IVV) of 12-13 month is considered economically optimal. The level of oestrus detection and the conception rate are essential components affecting the IVV. Hormone treatments to synchronize oestrus can reduce the calving to conception interval[2] and facilitate the use of the appropriate time insemination[3,4].
Management of reproduction is an important economic component in the success of a dairy operation. Good detection of oestrus is critically important in dairy husbandry. Incorrect detection of oestrus is related to loss of profit due to extended calving intervals, milk loss,veterinary costs, etc[5]. Synchronization of oestrus has been developed to help farmers manage reproduction more efficiently.
The hormones used pharmacologically to control the estrous cycle are identical to (or analogous to) the reproductive endogenous hormones secreted by the hypothalamus (GnRH), the pituitary (LH, FSH), the ovary (estradiol and progesterone), and the uterus (PGF2α) of female mammals. The biological activity of exogenous hormones is similar to the biological activity of endogenous hormones in the normal cow. In the review paper, the earliest oestrus synchronization method (developed in the 1960s) succeeded to block ovulation by administering exogenous progestogens[6].
Oestrus synchronization systems that use progestagens have three primary advantages over systems using prostaglandins alone. First, maintaining the blood progesterone concentration at a level greater than 1 ng/mL suppresses the LH surge and oestrus behavior. Thus, progesterone alone is a highly effective synchronization agent in cyclic cows that have regressed their corpora lutea or functional corpus luteum. Treatment with progesterone for 14 or 21 days resulted in a high oestrus response within 3 days of progesterone removal[7]. Hormonal treatments involving the use progesterone or progestagens (PRID®or Crestar®) to synchronize oestrus and ovulation in buffalo cow are interesting. Ovulation occurs 40-117 h following progestagen withdrawal[8], oestrus has been observed in 80%-93% of treated animals[9] and pregnancy rate following treatment varies from 20%[10] to 50%[11]. In the same way, eCG at norgestomet implant removal increased the rate of ovulation and pregnancy rates in cattle[12]. Crestar is a silicon implant containing 3 mg norgestomet. Treatment protocol with ear implant include an injection of 5 mg estradiol valerate and 3 mg norgestomet at the time of implant insertion to stimulate uterine-induced luteolysis[13], with implant removal after 9 day followed by oestrus detection and AI[14].
The rate of detection of returning oestrus would be substantially improved if returning oestrus could be synchronized on a period of 20 to 25 days after AI[15]. To our knowledge, the impact of incorrect timing of insemination on conception rate has not been evaluated using plasma progesterone concentration and plasma pregnancy associated glycoprotein in the field following norgestomet Crestar®treatment during 9 or 10 days. The objective of the present investigation was to evaluate the effect of norgestomet Crestar®on oestrus detection and reproductive performance in Holstein-Frisian dairy cows using the implant device during 9-10 days. P4 radioimmunoassay was used to retrospectively assess the stage of the cycle (day of heat, day 0) and to determine the number of cows returning in heat (day 21). Rectal palpation and glycoprotein associated pregnancy were used to calculate the conception rate. In addition, we aimed at determining the effect of parity on pregnancy rates in females following the use of norgestomet Crestar®synchronization protocol.
2. Materials and methods
2.1. Animals and treatment
The experiment was conducted during the period from February to April 2004 in Bass Kabylie, Algeria (36°43’N, 5°04’W). A total of 133 lactating Holstein Frisian cows from different dairy herds were used in this study. After a waiting period (~90 days postpartum), whole females (2-10 years old, 0-8 parity) were inseminated after detecting oestrus. Cows were allocated randomly to treated (n=69) or control (n=64) groups. Body condition scores (BCS) of cows were recorded before the initiation of the study. Scores were given by the same researcher based on a 1 (thin) to 5 (obese) scales using a quarter-point system[16]. Females with BCS score between 2.5 and 3.5 were included in the experiment. Note that the thin females (under 2.5) have not been considered.
The treated animals received an intramuscular administration of 3 mg norgestomet (17α-acetoxy-11β -methyl-19-norpreg-4-en-3, 20-dione) and 3.8 mg of estradiol-valerate followed by the subcutaneous insertion in the ear of an implant (Crestar®, Intervet S.A., 49071 Beaucouzé, France) containing 3 mg norgestomet. The implants were placed and kept during 9-10 days using implant device in the middle section behind the ear. The artificial inseminations (AI) were performed once at 56-60 hours post withdrawal of implant. A late pregnancy diagnosis was determined by rectal palpation between 80-90 days after AI.
2.2. Collection of blood samples
Blood samples were withdrawn from the coccegeal vessels into a tube containing EDTA (Sarstedt®, Numbrecht, Germany) on days 0, 21 and 30 after service. Subsequently, the plasma was rapidly separated (1 500 x g for 15 min)and stored at -20 ℃ until assayed[17]. Note that day 0 was considered the day AI.
2.3. Hormone assays
Progesterone (Sigma Chemical Co., St. Louis, MO) was used as standard. The first antibody in rabbit immunized was raised against Progesterone-11-hemisuccinate-BSA injected in multiple sites according to the Vaitukaitis method[18]. Progesterone concentrations were determined in the plasma by using a direct solid-phase125I radioimmunoassay method (without extraction) performed in duplicate, as described and validated previously[19]. Plasma progesterone estimation was used to identify cases of insemination outside the non-luteal phase. Intra- and inter-assay coefficients of variation were 8.5% and 9.4%, respectively.
The measurement of plasma PAG concentrations was performed by two distinct double-antibody radioimmunoassay differing on their antisera as previously described and validated[20]. Antiserum R#497 was raised against a pure bovine PAG67kDa preparation[21]. A mixture of different (bovine, caprine and ovine) constituted the antiserum named pool[20]. A threshold of pregnancy diagnosis measured by RIA-497 and RIA-Pool was 0.8 ng/mL in all pregnant females[22]. Whereas, intra- and inter-assay coefficients of variation were 3.5% and 6.8% for RIA-497 and 4.5% and 17.3% for RIA-Pool. Assay progesterone and pregnancy associated glycoprotein concentrations were performed in laboratory of endocrinology and animal reproduction at University of Liege in Belgium.
As regards to the ethical aspects, the experimental protocol was approved by the ethical committee of the University, Abderahmane Mira (Bejaia, Algeria). Blood sampling of the Friesian Holstein females was carried out following the rules of good veterinary practice under farm conditions.
2.4. Data analysis
Statistical analyses were carried out in STATVIEW (Version 4.55). Statistical analysis was performed using t-test to compare treated and control females. The data were expressed as mean ± SD, and P<0.05 was considered significant.
3. Results
The pregnancy rates as determined by plasma PAG measurement on day 30 after AI in the treatment and control groups were 28% (16/57) and 42% (23/54), respectively (Table 1). There were not significant differences between both groups. The results of this experiment showed a higher rate of heat synchronization in the treated group (94%) in comparison to control group (86%).

Table 1 Effect of norgestomet Crestar®implant during day 9-10 post AI on reproductive performance of dairy cows.
Out of 133 inseminated cows and heifers, thirteen were inseminated at a time when plasma progesterone concentration was much higher than 1.0 ng/mL (mean=4.6 ng/mL). In 4 out of 69 treated animals and in 9 out of 64 control animals the plasma progesterone was (4.53 ± 1.15) and (4.68 ± 2.82), respectively. As shown in Table 2, based on the progesterone profiles, the return rate on day 21 after AI was 60% in the treatment group and 42% in the control group.
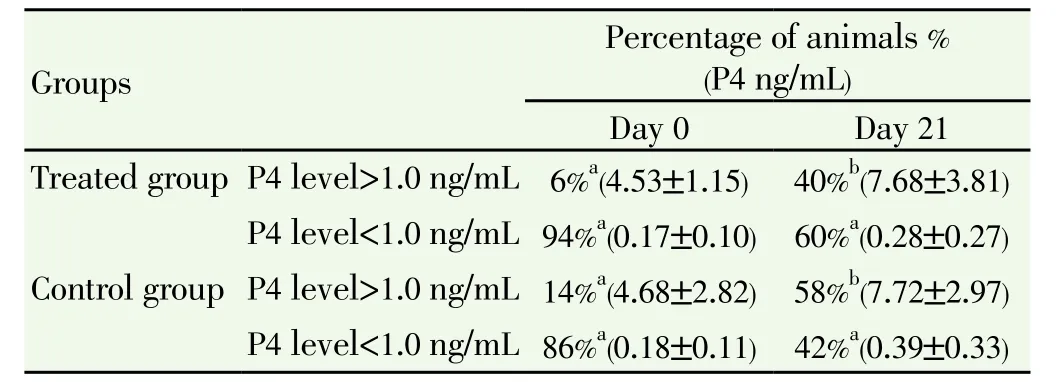
Table 2 Response to norgestomet Crestar®based on progesterone profiles (mean ± SD) at day 0 and 21 after implant removal in dairy cows.
The mean pregnancy rates in different category of parity are illustrated in Figure 1. Note that nulliparous females from the implant and natural heat group exhibit rates of 25% (1/4) and 36% (4/11), respectively. No significant differences are depicted between the two groups (P=0.95). In contrast, the mean pregnancy rates of treated primiparous cows were lower than those of the control groups; 23% (8/37) compared to 40% (6/15), respectively. In the multiparous cows, mean pregnancy rates were somewhat lower in the treated (7/24) than in the control group (12/29) (29% and 41%, respectively). For this case also, no significant differences between the groups are recorded (P=0.95).
Table 3 illustrates the mean (± SD) pregnancy-associated glycoprotein (PAG-497 and PAG-Pool) and progesterone concentrations measured in the treated and the control group. The plasma pregnancy-associated glycoprotein levels measured by RIA-497 at day 30 after AI were practically similar in both groups. On the other hand, the mean PAG determined with RIA-Pool system were slightly higher in the treated group than in the control group, however this difference was not statistically significant. Concerning the P4 concentrations, the values obtained were significantly lower in the treated group than in the control group (7.13 ± 2.29 and 8.37 ± 2.30, respectively).

Table 3 Plasma PAG concentration (ng/mL) and P4 (ng/mL) at day 30 in treated and control pregnant females (Mean±SD).
4. Discussion
Farms need to achieve good zootchnical performance and positive financial results to become profitable as quickly as possible[23]. Sá Filho and co-authors[4] reported that several factors, such as age, body condition score, stage of the oestrus at the beginning of the protocol, parity, breed, body condition score and semen quality, influence the timed of insemination artificial. Synchronization of oestrus has been developed to help farmers manage reproduction more efficiently[24]. Efficient and accurate detection of oestrus is vital to good reproductive performance in dairy herd where artificial insemination is used[25]. Sá Filho et al.[12] reported that cows that displayed oestrus were 3.3 times more likely to become pregnant than those without a display of oestrus. Numerous researchers have evaluated the potential of using a norgestomet-estradiol as a tool of synchronization oestrus in cyclic and anoestrus cattle[26-28]. However, incorrect timing of AI and the percentage of heat returning in females have not been well documented. Nevertheless, it is clearly known that these factors can significantly affect fertility. The present study was conducted to evaluate the effect of norgestomet Crestar®on pregnancy rate, taking into account the time of AI, and the ratio of oestrus returning at day 21.
A good oestrus response, with approximately 90% of animals showing oestrus soon after treatment has been achieved with PRID or Crestar®[29]. The results of this experiment showed a higher rate of heat synchronization in treated group (94%) in comparison with control group (86%). These results are in agreement with results reported in literatures which show that a rate of heat synchronization was high enough (98%) in Ndama living in different ecological zones[30]. Likewise, the work presented by Garcia and Salaheddine[31] indicates that different treatments used, including Crestar implant, were effective in inducing synchronous behavioral oestrus and ovulation.
In cattle, the detection of pregnancy-associated glycoprotein in maternal circulation is currently used as an accurate method for pregnancy diagnosis at approximately day 30 after breeding or artificial insemination[32,33]. The mean pregnancy-associated glycoprotein concentrations measured (day 30) by the two RIA systems in the treated and control groups were in agreement with those reported by Ayad and collaborators[34]. These values were not significantly different in both groups. However, PAG concentrations obtained by RIA-Pool were significantly higher than RIA-497, because Pool is a mixture of different antisera[19]. According to the PAG concentration at day 30 post-AI, our data showed differences in fertility between the implant-treated (16/57) and control group (23/54) when insemination was carried out after 90 days post calving. Note that the PAG concentrations decrease steadily in thepostpartum period reaching undetectable levels only by day 100 postpartum[35]. These results confirmed the work performed by Williams et al.[36] in which norgestomet treatment revealed lower conception in cows compared with Syncho-Mate-B and Ovsynch.
Plasma progesterone concentration on day 0 is a measure of the accuracy of oestrus detection. Table 1 shows interesting results for progesterone concentrations classified as either low (<1.0 ng/mL) or high (>1.0 ng/mL). Incorrect timing of AI based on P4 concentrations at day 0 was practically half in the treated compared to untreated group (6% and 14%, respectively). In another study, the percentage of cows in the luteal phase on the day of insemination (i.e. false oestrus detection) was similar in the treated group[37,38]. It is well known that 90% of the cows standing to be mounted are in oestrus[39]. This is the most accurate indicator of oestrus but not the most sensitive[40,41]. In this study, the proportion of females inseminated in non opportune time could be explained by the overestimation of oestrus signs as pretended by farmers. Indeed, the sampling for progesterone assays on the day of artificial insemination might have a positive effect on reproductive performance of dairy cattle, in particular for the oestrus detection. Poor oestrus expression and a prolonged inter-calving interval compromise the reproductive efficiency of female buffaloes[42].
In 2011, Sá Filho and co-authors[43] concluded that administration of estradiol increased the occurrence of oestrus and pregnancy per AI in suckeld Bos indicus beef cow submitted to an estradio/progesterone-based synchronization protocol timed AI. Moreover, Mann and Lamming[44] detected a positive effect of estradiol production during proestrus on progesterone production in the subsequent luteal phase and on embryonic survival. In addition, recent study concludes that the beneficial effects of estradiol supplementation on conception rate were likely caused by post-fertilization processes[45]. It appeared that, on the basis of the variation in blood progesterone levels at day 21, the return rate to oestrus of females treated by implant were higher than in untreated group (60% compared to 42%, respectively). The higher return rate of females to oestrus could be justified by a fertilization failure or an early embryonic death[46,47]. But, it is very difficult to confirm it, because there is no availability of biological or immunological methods that could be used to measure pregnancy associated glycoprotein concentrations during the early stage.
Significant differences in the pregnancy rates in nulliparous, primiparous and multiparous cows were recorded in both groups. The results obtained with treated and untreated groups in primiparous and multiparous were similar to the findings of Cavalieri and Macmillan[48]; and Bülbül and Atman[48], respectively. Ponsart et al.[49] obtained higher pregnancy rate in multiparous cows than primiparous cows when synchronization protocols used progesterone implant. In Buffalo cattle, parity shows an effect on synchronization treatment efficacy with multiparous animals responding better than primiparous animals[51]. In our work, the pregnancy rates were similar to previous research. These differences suggest that parity could affect the efficiency of a synchronization protocol[49]. In contrast, nulliparous females exhibited higher pregnancy rates than primiparous cows (25% vs. 23%). These slight differences could be due to reproductive problems after calving. These divergence between nulliparous and primiparous may be also explained by the nutritional status, body score and time of initiation of the protocol[52]. In conditions sub-tropical, the pregnancy rate in heifers was very higher than in cows[53]. Additionally, other experiment has reported an effect of eCG on pregnancy outcome only in primiparous cows[54]. Ansari-Lari and collaborators[55] concluded that high milk production is risk factor for decreasing fertility. The reproductive tract of lactating dairy cows may provide a less favorable environment for very early embryo development than that of the heifers[56].
In conclusion, the norgestomet Crestar®treatment during 9-10 days did not improve reproductive performance in dairy cows. However, these data demonstrate that this hormonal protocol can be used to obtain satisfactory cyclic cow rates and less incidence of incorrect timing of insemination. Furthermore, there were no differences in overall rates pregnancy between nulliparous, primiparous and multiparous when using implant protocol.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Acknowledgements
Dr Ayad A. gratefully acknowledges Professor Beckers J.F. (Laboratory of Physiology of Animal Reproduction, Faculty of Veterinary Medicine, ULg, Belgium) for furnishing of reagents, technical assistance and reception in his laboratory. The authors thank M. Harrats Ch. and Ouail O. for the English correction.
[1] Hamudikuwanda H, Erb HN, Smith RD. Effects of sixty-day milk yield on postpartum breeding performance in holstein cows. J Dairy Sci 1987; 70(11): 2355-2365.
[2] Ryan DP, Snijders S, Yaakub H, O’Farrell KJ. An evaluation of estrus synchronization programs in reproductive management of dairy herds. J Anim Sci 1995; 73: 3687-3695.
[3] Duffy P, Crowe MA, Austin EJ, Mihm M, Boland MP, Roche JF. The effect of eCG or estradiol at or after norgestomet removal on follicular dynamics, estrus and ovulation in early post-partum beef cows nursing calves. Theriogenology 2004; 61: 725-734.
[4] Sá Filho MF, Crespilho AM, Santos JEP, Perry GA, Baruselli PS. Ovarian follicle diameter at timed insemination and estrous response influence likelihood of ovulation and pregnancy after estrous synchronization with progesterone or progestin-based protocols in suckled Bos indicus cow. Anim Reprod Sci 2010b; 120: 23-30.
[5] Roelofs J, López-Gatius F, Hunter RHF, Van Eerdenburg FJCM, Hanzen Ch. When is a cow in estrus? Clinical and practical aspects. Theriogenology 2010; 74(3): 327-344.
[6] Lucy MC, McDougall S, Nation DP. The use of hormonal treatments to improve the reproductive performance of lactating dairy cows in feedlot or pasture-based management systems. Anim Reprod Sci 2004; 82-83: 495-512.
[7] Macmillan KL, Peterson AJ. A new intravaginal progesterone releasing device for cattle (CIDR-B) for oestrous synchronisation, increasing pregnancy rates and the treatment of post-partum anoestrus. Anim Reprod Sci 1993; 33: l-25.
[8] Bartolomeu CC, Del Rey JA, Madureira EH, Carvalho NAT, Bernabe RC, Baruselli PS. Sincronizacao do cielo estral e da ovulacao com utilizacao de CIDR-B e Cresta em bubalinos. Arquivos da Faculdade de Veterinária UFRGS 1999; 25: 8.
[9] Baruselli PS, Control of follicular development applied to reproduction biotechnologies in buffalo. In: Proceeding of the I congress Nazionale sull’allevamento del buffalo. Book of the congress. 2001,p.128-46.
[10] Hattab SA, Kadoom AK, Palme R, Bamberg E. Effect of CRESTAR on estrus synchronization and the relationship between fecal and plasma concentrations of progestagens in buffalo cows. Theriogenology 2000; 54: 1007-1017.
[11] Sá Filho MF, Ayres H, Ferreira RM, Marques MO, Reis EL, Silva RC, et al. Equine chorionic gonadotropin and gonadotropinreleasing hormone enhance fertility in a norgestomet-besed, timed artificial insemination protocol in suckled Nelore (Bos indicus) cows. Theriogenology 2010a; 73: 651-658.
[12] Kastelic JP, Olson WO, Martinez M, Cook RB, Mapletoft RJ. Synchronization of estrus in beef cattle with norgestomet and estradiol valerate. Can Vet J 1999; 40: 173-178.
[13] Edmonson AJ, Lean IJ, Weaver LD, Farver T, Webster G. A body condition scoring chart for Holstein dairy cows. J Dairy Sci 1989; 72: 68-78.
[14] Delahaut Ph, Beckers JF, Ectors F. Effet de l’azide de sodium sur la dégradation de la progestérone dans les échantillons de sang total chez les bovins. Ann Méd Vét 1979; 123: 567-572.
[15] Vaitukaitis J, Robbins JB, Nieschlag E, Ross GT. A method for producing specific antisera with small doses of immunogen. J Clin Endorcrinol Metabol 1971; 33: 988-991.
[16] Lopez-Gatius F, Garbayo JM, Santolaria P, Yaniz J, Ayad A, Sousa NM, et al. Milk production correlates negatively with plasma levels of pregnancy-associated glycoprotein (PAG) during the early fetal period in high producing dairy cows with live fetuses. Domest Anim Endocrinol 2007; 32: 29-42.
[17] Ayad A, Sousa NM, Sulon J, Iguer-Ouada M, Beckers JF. Comparison of five radioimmunoassay systems for PAG measurement: ability to detect early pregnancy in cows. Reprod Domest Anim 2007; 42: 433-440
[18] Zoli AP, Beckers JF, Wouters-Ballman P, Closset J, Falmagne P, Ectors F. Purification and characterization of a bovine pregnancyassociated glycoprotein. Biol Reprod 1991; 45; 1-10.
[19] Szenci O, Beckers JF, Humblot P, Sulon J, Sasser RG, Taverne MAM, et al. Comparison of ultrasonography, bovine pregnancyspecific protein B, and bovine pregnancy-associated glycoprotein 1 tests for pregnancy detection in dairy cows. Theriogenology 1998; 50: 77-88.
[20] Silveira EF, Kozicki LE, Segui MS, Weiss RR, Santos IW, Bertol MAF. Comparison of long-term progesterone-based protocol on reproductive performance of prepubertal and pubertal beef. Arch Vet Sci 2014; 19(1): 1-16.
[21] Larson LL, Ball PJ. Regulation of estrous cycles in dairy cattle: A review. Theriogenology 1992; 38: 255-267.
[22] Kinsel ML, Etherington WG. Factors affecting reproductive performance in Ontario dairy herds. Theriogenology 1998; 50: 1221-1238.
[23] Gümen A, Yilmazbaş Mecitoğlu G, Keskin A, Karakaya E, Alkan A, Taşdemir U, et al. The effect of intrauterine cephapirin treatment after insemination on conception rate in repeat breeder dairy cows subjected to the progesterone-based Ovsynch protocol. Turk J Vet Anim Sci 2012; 36(6): 622-627.
[24] Long ST, Nakao T, Wakayake S, Okakoi M. Effect of CIDR 12 to 19 days after AI on detection of returning estrus and conception rate in dairy cows. J Reprod Develop 2010; 56(2): 251-255.
[25] Pancarci SM, Lehimcioğlu NC, Çağin Ari U, Güngör Ö, Akbulut Ö. Efficacy of hCG and GnRH with respect to follicular size and presence of the corpus luteum in cosynch protocol integrated with norgestomet in lactating cows. Bull Vet Instit Pulawy 2013; 57: 61-64.
[26] Barile VL, Galasso A, Marchiori E, Pacelli C, montemurro N, Borghese A. effect of PRID treatment on conception rate in Mediterranean buffalo heifers. Liv Prod Sci 2001; 68: 283-287.
[27] Vivek Nayak, Agrawal RG, Srivastav OP, Thakur MS. Induction of estrus in true anestrus buffaloes using Crestar implants alone andin combination with PMSG. Buffalo Bull 2009; 28(2): 51-54.
[28] Tregaskes LD, Broadbent PJ, Dolman DF, Grimmer SP, Franklin MF. Evalutaion of Crestar, a synthetic progestogen regim, for synchronizing oestrus in maiden heifers used as recipients for embryo transfert. Vet Record 1994; 134: 92-94.
[29] Diop PE, Faye L, Fall R, Ly OK, Mbaye M, Boye C. Control of reproduction in the female Ndama cow by Norgestomet (CRESTAR). Dakar Medical 1994; 39(2): 129-134.
[30] Garcia A, Salaheddine M. Effect of oestrous synchronization with estradiol 17β and progesterone on follicular wave dynamics in dairy heifers. Reprod Domest Anim 2001; 36: 301-307.
[31] Friedrich M, Holtz W. Establishment of an ELISA for measuring bovine pregnancy-associated glycoprotein in serum or milk and its application for early pregnancy detection. Reprod Domest Anim 2010; 45: 142-146.
[32] Green JC, Volkmann DH, Poock SE, McGrath MF, Ehrhardt M, Moseley AE, et al. Technical note: A rapid enzyme-linked immunosorbent assay blood test for pregnancy in dairy and beef cattle. J Dairy Sci 2099; 92: 3819-3824.
[33] Ayad A, Sousa NM, Sulon J, Iguer-Ouada M, Beckers JF, Correlation of five radioimmunoassay systems for measurement of bovine plasma pregnancy-associated glycoprotein concentrations at early pregnancy period. Res Vet Sci 2009; 86(3): 377-382.
[34] Zoli AP, Guilbaut LA, Delahaut P, Benitez Ortiz W, Beckers JF. Radioimmunoassay of a bovine pregnancy-associated glycoprotein in serum: its application for pregnancy diagnosis. Biol Reprod 1992; 46: 83-92.
[35] Williams SW, Stanko RL, Amstalden M, Williams GL. Comparison of three approaches for synchronization of ovulation for timed artificial insemination in Bos indicus-influenced cattle managed on the texas gulf coast. J Anim Sci 2002; 80: 1173-1178.
[36] Grimard B, Freret S, Chevallier A, Pinto A, Ponsart C, Humblot P. Genetic and environmental factors influencing first service conception rate and late embryonic/foetal mortality in low fertility dairy herds. Anim Reprod Sci 2006; 91: 31-44.
[37] Stevenson JS. A review of oestrous behaviour and detection in dairy cows. In: Animal Science 1, Occasional Publication, 2001; 26: 43-62.
[38]Michel A, Ponsart C, Freret S, Humblot P. Influence de la conduite de la reproduction sur les résultats à l’insémination en période de pâturage. Rencontre Recherche Ruminants 2003; 10: 131.
[39] Van Eerdenburg FJ, Loeffler HS, Van Vliet JH. Detection of oestrous in dairy cows: a new approach to an old problem. Veterinary Q 1996; 18: 52-54.
[40] Van Eerdenburg FJ. Oestrous detection in dairy cattle: how to beat the bull. Reprod Domest Anim 2003; 38: 322.
[41] De Rensis F, Lopez-Gatius F. Protocols for synchronizing estrus and ovulation in buffalo (Bubalus bubalis): A review. Theriogenology 2007; 67: 209-216.
[42] Sá Filho MF, Santos JEP, Ferreira RM, Sales JNS, Baruselli PS. Importance of estrus on pregnancy per insemination in suckled Bos indicus cows submitted to estradiol/progesterone-based timed insemination protocols. Theriogenology 2011; 76: 455-463.
[43] Mann GE, Lamming GE. The role of sub-optimal preovulatory oestardiol secretion in the aetiology of premature luteolysis during the short oestrous cycle in the cow. Anim Reprod Sci 2000; 64: 171-180.
[44] Cerri RL, Rutigliano HM, Chebel RC, Santos JE. Period of dominance of the ovulatory follicle influences embryo quality in lactating dairy cows. Reproduction 2009; 137: 813-23.
[45] Breukelman SP, Szenci O, Beckers JF, Kindahl H, Jonker FH, Van Der Weijden GC, et al. Ultrasonographic appearance of the conceptus, fetal heart rate and profiles of pregnancy-associated glycoproteins (PAG) and prostaglandin F2a-metabolite (PGF2ametabolite) after induction of fetal death with aglepristone during early gestation in cattle. Theriogenology 2005; 64: 917-33.
[46] Celi P, Merlo M, Da Dalt L, Stefani A, Barbato O, Gabai G. Relationshipbetween late embryonic mortality and the increase in plasma advanced oxidised protein products (AOPP) in dairy cows. Reprod Fertil Develop 2011; 23: 527-533.
[47] Cavalieri J, Macmillan KL. Synchronisation of oestrus and reproductive performance of dairy cows following administration of oestradiol benzoate or gonadotrophin releasing hormone during a synchronized pro-oestrus. Aust Vet J 2002; 80: 486-493.
[48] Bülbül B, Ataman MB. Effect of parity on oestrus synchronization success in cows. Rev Méd Vét 2006; 157(3): 158-162.
[49] Ponsart C, Sana M, Humblot P, Grimard B, Jeanguyot N, Ponter AA, et al. Variation factors of pregnancy rates after oestrus synchronization treatment in French charolais beef cows. Vet Res 1996; 27: 227-239.
[50] Baruselli PS, Madureira EH, Visintin JA, Barnabe RC, Amaral R. Timed insemination unsing synchronization of ovulation in buffalo. Rev Bras Reprod Anim 1999; 23: 360-2.
[51] El-Wishy AB. Fertility of Holstein cattle in a subtropical climate of Egypte. Iranian J Appl Anim Sci 2013; 3(1): 45-51.
[52] Grohn YT, Rajala-Schultz PJ. Epidemiology of reproductive performance in dairy cows. Anim Reprod Sci 2000; 60-61: 605-614. [53] Small JA, Colazo MG, Kastelic JP, Mapletoft RJ. Effects of progesterone presynchronization and eCG on pregnancy rates to GnRH-based, timed-AI in beef cattle. Effects of progesterone presynchronization and eCG on pregnancy rates to GnRH-based, timed-AI in beef cattle. Theriogenology 2009; 71: 698-706.
[54] Ansari-Lari M, Kafi M, Sokhtanlo M, Nategh Ahmadi H. Reproductive performance of Holstein dairy cows in Iran. Trop Anim Health Prod 2010; 42: 1277-1283.
[55] Rizos D, Carter F, Besenfelder U, Havlicek V, Lonergan P. Contribution of the female reproductive tract to low fertility in postpartum lactating dairy cows. J Dairy Sci 2010; 93: 1022-1029.
*Corresponding author: Abdelhanine Ayad, Department of Environment and Biological Sciences, Faculty of Life and Nature Sciences, University A. Mira, Bejaia 06000, Algeria.
Tel: +213.7727.722.595.
E-mail: hanine06@gmail.com; abdelhanine.ayad@univ-bejaia.dz
 Asian Pacific Journal of Reproduction2015年1期
Asian Pacific Journal of Reproduction2015年1期
- Asian Pacific Journal of Reproduction的其它文章
- Molecular dysregulation of renal development: Congenital anomalies of the kidney and urinary tract
- Effect of seasons on semen production, effect of melatonin on the liquid storage (5 ℃) with correlated study of birth rate in mithun (Bos frontalis)
- Variations in semen characteristics rams of Ouled Djellal breed have received an important dietary supplement after regular and intensive collection
- Effects of long term storage of semen in liquid nitrogen on the viability, motility and abnormality of frozen thawed Frisian Holstein bull spermatozoa
- Effect of different thawing procedures on the quality and fertility of the bull spermatozoa
- Naloxone affects reproductive system in a rat model with polycystic features
