Oxygen Transfer Rate of Shaoxing Rice Wine Jars
Oxygen Transfer Rate of Shaoxing Rice Wine Jars
AbstractTraditional Shaoxing rice wine was stored in the pottery jar for aging because the jar are porous and allow oxygen in the air to permeate into the wine,and oxygen plays a key role in the wine aging as it affects the color,aroma and stability of the wine.In this paper we evaluated how much oxygen penetrated into the pottery jar during the rice wine storage.Iron (Ⅱ) solution as the reducing agent was stored in Shaoxing rice wine jars for 30 days.During this period some iron (Ⅱ) was oxidized into iron (Ⅲ) by the oxygen penetrated into the pottery jars.The mass concentrations of the iron (Ⅲ) were determined by a KSCN colorimetric method,thus the oxygen transfer rate (OTR) of the pottery jars was calculated.The result showed that the OTR value is 0.106 mg/L per day,and this value is an important reference for designing the large stainless container with micro-oxygeneration devices for Shaoxing rice wine.
Key wordsShaoxing rice wine; Pottery jar; Oxygen transfer rate
Received 2015-11-03Returned2016-01-02
First authorZHOU Xiaodan,female,college student of biological sciences.E-mail: 1083491629@qq.com
Shaoxing rice wine is a traditional Chinese alcoholic beverage that has been consumed in China for centuries[1].The wine is made from rice and stored in the pottery jar for a long time for aging or maturing[2].The pottery jar is an ideal container for Shaoxing rice wine because it is cheap,easy to obtain,permeable to oxygen,and befitting to form the special flavor of the rice wine[3],but the its disadvantages are also obvious: (1) result in a high labor cost and require a large wine library because it needs about 44 pottery jars to store per ton wine; (2) wine loss from 0.5% to 1.0% per year during storage; (3) it is difficult to blend and fill.Therefore,it is important to develop the large stainless steel tanks with the micro-oxygenation device to store Shaoxing rice wine,but as a prerequisite,the designer should know how much oxygen will permeat into the pottery jars during the rice wine storage.
For above purposes,we try to evaluate the oxygen transfer rate (OTR) of the pottery jars.Fe2+solution as a reductant was stored in the hermetically sealed pottery jars.During 30 days storage,some Fe2+ions were oxidized into the Fe3+by the oxygen permeated into the jars.The OTR value was calculated by the mass concentration of Fe3+which can be measured by a KSCN colorimetric method.
1Materials and Methods
1.1Apparatus
UV6001PCS spectrophotometer,100 mL beaker,10 mL colorimetric tube,1 mL cuvette,electric furnace,25 mL burette,glass pipette,and three selected 24 L Shaoxing pottery wine jars made in Zhuji city.
1.2Reagents
HCl (10 mol/L),FeCl2,H2O2(φ=30%),KSCN,iron powder reduced,and deionized water.
Iron (Ⅲ) standard stock solution (1.0 g/L): weighed 0.100 0 g iron powder reduced and put into 100 mL beaker,added 10 mL of HCl (10 mol/L) and 1 mL H2O2(φ=30%).After the iron powder was entirely dissolved,boiled the solution for 5 min to remove excess hydrogen peroxide.When the solution cooled to room temperature,placed it into 100 mL volumetric flask,diluted with deionized water to 100 mL,and mixed by inversing the flask.This standard solution contains 1.0 g iron per liter.
Iron (Ⅲ) standard work solution (10 mg/L):pipetted 1 mL iron standard stock solution into 100 mL volumetric flask,diluted with deionized water to 100 mL,and mixed,then diluted this solution with different mass concentration for making the calibration curve.
Iron (Ⅱ) work solution:poured boiling water into 3 new pottery jars which are randomly selected with a volume of 24 L,sealed with plastic sheeting,after cooled to room temperature,added 24 g FeCl2and 20 mL HCl (10 mol/L) in each jar,and sealed again.The mass concentration of the iron (Ⅱ) work solution is about 1 g/L.Placed this solution for 5 days to measure the iron (Ⅲ) mass concentration as the initial one.
KSCN Solution:dissolve 30 g KSCN (potassium thiocyanate) in deionized water and make up the solution to 100 mL with deionized water.
Calibration curve:added 1 drop (about 0.05 mL) of KSCN solution (300 g/L) in each iron standard solution,and then mixed,placed under room temperature (10-35 ℃) for 5 minutes,poured into 1 cm cuvette for detection.The mass concentration of iron (Ⅲ) standard solution are: 2 mg/L,4 mg/L,6 mg/L,8 mg/L,10 mg/L.Absorbance was measured at 500 nm with deionized water as a reference (10 mL containing a drop of KSCN solution).The calibration curve was shown in Fig.1,and the regression equation is:A=0.058 5c+0.007 9(R2= 0.999).

Fig.1 Calibration curve
1.3Preparation sample and measurements
The three selected jars labeled A,B and C were filled with boiled water,after cooled to room temperature (25 ℃) added with 3.5 g FeCl2and 20 mL HCl (10 mol/L,role as a stabilizer) per liter.Then sealed with plastic films,and stored under 25 ℃ for measurements.After the FeCl2solutions (about 1 g/L) had been made in pottery jars for 5 days,pipetted 2.0 mL sample solution from upper,middle and lower parts of the pottery jar respectively,diluted to 10 mL in the colorimetric tube (dilution factor is 5),added 1 drop (about 0.05 mL) solution of KSCN (300 g/L),and mixed,placed under room temperature (10-35 ℃) for 5 minutes,then poured into 1 cm cuvette,measured the absorbance at a wavelength of 500 nm with deionized water (10 mL containing a drop of the KSCN solution) as a reference.Calculated the iron (Ⅲ) mass concentration as the initial one,and then used the same method to measure the iron (Ⅲ) mass concentration once every five days in a month (30 days).
2Results and Analysis
Pottery jars are made of clay which are porous and allow oxygen slowly to permeat into the chamber of the jar,resulting that the iron (Ⅱ) is oxidized into the iron (Ⅲ).Therefore,by measuring the iron (Ⅲ) mass concentration we are able to calculate how much oxygen has been penetrated into the pottery jar during a given period.
Iron (Ⅲ) mass concentration were calculated with the regression equationA= 0.058 5c+0.007 9 (R2=0.999).The data was listed in Table 1 and 2.
The equivalent mass concentration of oxygen permeated into the pottery jar chambers was calculated with the following formula:
In which:
CO2:Oxygen permeated into pottery jar,mg/L.
CFe:Fe(Ⅲ) mass concentration,mg/L.
15.999:mass of molecule of oxygen.
55.84:mass of molecule of iron.
2:each oxygen atom needs to gain 2 electrons.
Variations of the iron (Ⅲ) mass concentration and the equivalent mass concentrations of oxygen in a month are listed in table 3,and the trend is illustrated in Fig.2.

Table 1 Initial Fe3+ and equivalent oxygen mass concentration
Note: A,B and C are three repetitions,sample dilution factor is 5,the same as table 2.
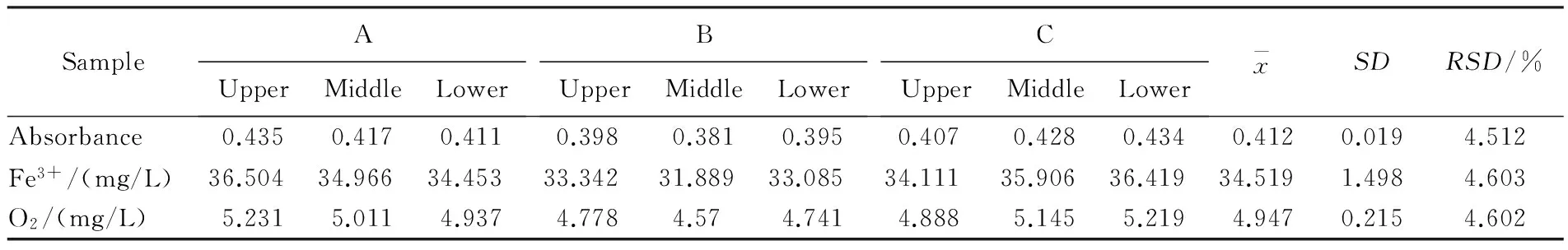
Table 2 Fe3+ and equivalent oxygen mass concentration after 30 days
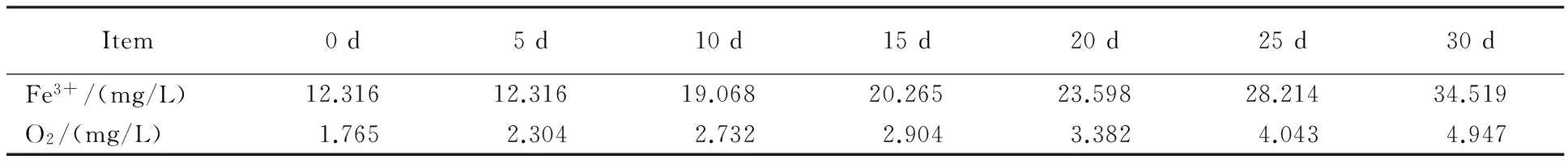
Table 3 Fe3+ and equivalent oxygen mass concentration measured once every 5 days

Fig.2 Relationship between equivalent oxygen
Since initial equivalent oxygen mass concentration is 1.765 mg/L,and the cumulative amount of oxygen permeated into pottery jar in 30 days is:(4.947-1.765) mg/L = 3.182 mg/L,the oxygen transfer rate of hermetically sealed pottery jars is 0.106 mg/L per day,or 38.69 mg/L per year.
3Discussion
Shaoxing rice wine is a unique non-distilled alcoholic drink in the world.It is characterized with the saccharificated rice with fungoids and the wine is stored in the pottery jar for aging.Nowdays many new wine making techniques and facilities have been widely used in Shaoxing rice wine breweries,such as computer monitoring and mechanical transporting,but the rice wine is still stored in the ancient jars which critically suppress the automatization of Shaoxing rice wine industries.Ever Shaoxing winemakers believe that the rice wine must be stored in pottery jars to form its unique odors and tastes,but no one knows the detail mechanisms.Coincident with the continually increasing labor costs it seems inevitable to develop the large tank devices for the rice wine storage.
It is well known that the traditional grape wine is stored in oak barrels which are porous,allow traces of oxygen continually penetrating into the barrel,benefit oxidization to occur and promote the mature of the wine.Therefore,in order to decrease the cost many wine manufacturers now have used stainless steel tanks instead of the barrels to store wines[4-7].Because the steel tanks are airtight containers,a technique named micro-oxygenation was developed in 1991 in France,which involves the controlled introduction of a low mass concentration of oxygen during wine maturation[8].It is claimed that micro-oxygenation can reproduce the benefits of barrel-aging but in a much shorter time and at a fraction of the cost.
Several researchers have evaluated the oxygen transfer rate (OTR) of oak barrels filled with wine,this value ranging from 0.7 to 45 mg/L per year in new oak barrels[9].Caietal[10]in 2014 had taken a preliminary study of the oxygen permeability of Shaoxing pottery wine jar and they concluded that the OTR of the wine jar was 0.35 mg/L,but in their paper the initial oxygen content was measured by iodometric method,which would entirely neglect the Fe3+preexisted in the FeCl2solution.For this reason we carried out this study,and calculated that the OTR of the pottery jars was 38.69 mg/L per year.This value is very similar to that of the oak barrels for the grape wine,and can be taken as an important reference for designing the container with micro-oxygeneration devices for Shaoxing rice wine.
Reference参考文献:
[1]QI P,TIAN R G,LI B B,etal.Determination of luteoskyrin in rice wine by high-performance liquid chromatography-ion trap tandem mass spectrometry [J].AnalyticalLetters,2015,48(1):9-15.
[2]谢广发,胡志明.试论绍兴黄酒工艺的成型年代[J].酿酒科技,2004,121 (1):79-80.
XIE G F,HU ZH M.Discussion on the generation age of production techniques of Shaoxing yellow rice wine [J].LiquorMakingScience&Technology,2004,121 (1):79-80(in Chinese with English abstract).
[3]杨国军.黄酒的陈化[J].酿酒科技,2006,144(6):74-76.
YANG G J.Aging of yellow rice wine[J].LiquorMakingScience&Technology,2006,144 (6):74-76(in Chinese with English abstract).
[4]兰玉倩,王丽华,薛洁,等.微氧技术在黄酒生产中的应用[J].酿酒科技,2010,196(10):65-67.
LAN Y Q,WANG L H,XUE J,etal.Application of micro-oxygenation technique in yellow rice wine production[J].Liquor-MakingScience&Technology,2010,196(10):65-67(in Chinese with English abstract).
[5]刘延琳,李华,张予林.葡萄酒的微氧酿造技术简介[J].酿酒科技,2002,113(5):54-55.
LIU Y L,LI H,ZHANG Y L.Review on micro-oxygenation in wine[J].Liquor-MakingScience&Technology,2002,113(5):54-55(in Chinese with English abstract).
[6]郭晓晖,王雨雪,王颖,等.葡萄酒发酵微氧化技术研究进展[J].食品科学,2012,33(9):291-295.
GUO X H,WANG Y X,WANG Y,etal.Research progress of micro-oxygenation (MOX) technology in wine fermentation[J].FoodSciences,2012,33(9):291-295(in Chinese with English abstract).
[7]康文怀,李华,杨雪峰,等.微氧技术在葡萄酒陈酿中的应用[J].食品与发酵工业,2006,32(5):77-80.
KANG W H,LI H,YANG X F,etal.Effect of micro-oxygenation on wine[J].FoodandFermentationIndustries,2006,32(5):77-80(in Chinese with English abstract).
[8]DEMPSEY C.Micro-oxygenation in wine:integrated tannin management[J].WineBusinessMonthly,2001,8(11):56-58.[9]DEL ALAMO-SANZA M,NEVARES I.Recent advances in the evaluation of the oxygen transfer rate in oak barrels [J].JournalofAgriculturalandFoodChemistry,2014,62(35):8892-8899.
[10]蔡静,田润刚,鲍张咪,等.绍兴黄酒陶坛透氧能力的初步研究[J].酿酒科技,2014,237(3):34-36.
CAI J,TIAN R G,BAO ZH M,etal.A preliminary study of the oxygen permeability of Shaoxing pottery wine jar [J].Liquor-MakingScience&Technology,2014,237(3):34-36(in Chinese with English abstract).
ZHOU Xiaodan,WANG Ruiqi,SHEN Qitian,ZHU Yiyao,WU Yuhan and TIAN Rungang
(School of Life Science,Shaoxing University,Shaoxing Zhejiang312000,China)
CLC numberTS261.4
Document codeAArticle ID1004-1389(2016)06-0950-05
Foundation itemShaoxing Key Scientific and Technological Project (No.2014A32002); Zhejiang Provincal New Talent Plan for College Students (No.2014R426009).
Corresponding authorTIAN Rungang,male,Ph.D,professor.Research area: brewing technology and engineering.E-mail 642175685@qq.com
网络出版日期:2016-06-01
网络出版地址:http://www.cnki.net/kcms/detail/61.1220.S.20160601.0920.046.html

