Larvicidalefficacy ofm onoterpenes against the larvae of Anopheles gambiae
Eliningaya J.Kweka,Tam ires Cardoso Lima,Chrian M.Marciale,Dam ião Pergentino de Sousa,Division of Livestock and Human Diseases Vector Control,Tropical Pesticides Research Institute,P.O.Box 0,Arusha, TanzaniaDepartmentofMedical Parasitology and Entomology,Catholic University of Health and Allied Sciences,P.O.Box 6, Mwanza,TanzaniaDepartment of Pharmacy,Federal University of Sergipe,CEP 900-000,São Crist´ovão,Sergipe,BrazilDepartmentof Pharmaceutical Sciences,Federal University of Paraíba,CEP 5805-970,João Pessoa,Paraíba,Brazil
Larvicidalefficacy ofm onoterpenes against the larvae of Anopheles gambiae
Eliningaya J.Kweka1,2*,Tam ires Cardoso Lima3,Chrian M.Marciale1,Dam ião Pergentino de Sousa3,41Division of Livestock and Human Diseases Vector Control,Tropical Pesticides Research Institute,P.O.Box 3024,Arusha, Tanzania
2DepartmentofMedical Parasitology and Entomology,Catholic University of Health and Allied Sciences,P.O.Box 1464, Mwanza,Tanzania
3Department of Pharmacy,Federal University of Sergipe,CEP 49100-000,São Crist´ovão,Sergipe,Brazil
4Departmentof Pharmaceutical Sciences,Federal University of Paraíba,CEP 58051-970,João Pessoa,Paraíba,Brazil
Original article http://dx.doi.org/10.1016/j.apjtb.2016.03.001
ARTICLE INFO
Article history:
Received in revised form 29Oct,2nd
revised form 25 Dec 2015
Accepted 12 Jan 2016
Availableonline10Mar2016
Larvicidal activity
Malaria
Anopheles gambiae s.s.
Essential oils
Monoterpenes
Natural products
Mosquito
ABSTRACT
Ob jective:To evaluate the larvicidal ef fi cacy of eight volatile components of essential oils against3rd instar larvae of Anopheles gambiae s.s.
M ethods:Larvicidal effects of each compound were evaluated in both laboratory and semi-fi eld trials.Stock solution was prepared and serial dilutions were made in six concentrations for each compound.A total of 20 larvae were exposed to larvicides for each replicate and monitored at intervals of 12,24,48 and 72 h.Larvaemonitoring was done on basis of dead and live larvae in all intervals.
Resu lts:A ll assayed compoundswere larvicides and presented varying degrees of larval toxicity,w ith LC50values ranging from 1.28 to 1938.92 mg/L depending on the treatment time(12,24,48 or 72 h).(−)-Perillyl alcohol presented the strongest larvicidal activity towards Anopheles gambiae larvae,w ith LC50values of 73.60,18.36,1.72 and 1.28mg/L after 12,24,48 and 72 h of exposure,respectively.The next strongestwere (−)-isopulegol(LC50=135.10,49.39,34.39 and 20.22mg/L)and(−)-carvone epoxide (LC50=168.86,124.74,80.84 and 23.46 mg/L).A fter 12,24 and 48 h of treatment, hydroxydihydrocarvone was the least toxic compound,w ith LC50values of 1938.92, 1172.18 and 401.03mg/L,respectively.
Conclusions:The data obtained in this study suggest that all evaluated monoterpenes, especially(−)-perillyl alcohol,have remarkable larvicidal effects and may be considered as potential sources for the development of suitable natural larvicides for mosquito management programs.Further small-scale fi eld trials should be conducted.
1.Introduction
Mosquitoes constitute an important group of arthropods for public health.They transm it a w ide range of human diseases such as fi lariasis,malaria,dengue,yellow fever and Japanese encephalitis,causing m illions of deaths worldw ide each year [1,2].Global patterns of climate change and urbanization have increased the threat of humans contracting arthropod-borne viral infections[3].
Malaria is among themost important vector-borne diseases, being endem ic to more than 100 countries worldw ide,particularly in tropicaland subtropical regions[4].The disease is caused by one-celled parasites that are transm itted to humans via the bite of infected anopheline mosquitoes such as Anopheles gambiae s.s.Giles(An.gambiae s.s.),Anopheles arabiensis Patton and Anopheles stephensi Liston[5].In the last 30 years, malaria incidence has increased,due mainly to the emergence of drug and insecticide resistance in parasites and vectors, respectively,aswell as poor socioeconom ic conditions[6].Butin the recent past,malaria vector and parasite populations have declined drastically due to increased investments in intervention,diagnosis and treatment[7,8].
Plants are a rich resource of alternative synthetic compounds for the control ofmosquito larvae.They possess aw ide range of bioactive phytochem icals that are selective,biodegradable,and havem inor or no adverse effects on non-target organisms and the environment,making them potentially appropriate for use in integrated pest management programs.Approximately 2000 species of terrestrial plants have been described for their insecticidal properties[9–11].
Various studies have focused on the use of natural products, especially plant-derived essential oils,as suitable bioactive agentsagainst the larvaeof An.gambiae s.s.and othermosquito species[12–15].Essential oils are complex natural m ixtures of volatile organic compounds,principally mono-and sesquiterpenes,which are considered to be among the best alternatives for the control of disease vectors[16,17].
The present study investigated the larvicidal effects of eight monoterpenes found in volatile oils against themalaria vector mosquito An.gambiae s.s.
2.M aterials and m ethods
2.1.Mosquito larvae
The An.gambiae s.s.larvaeused in laboratory and sem i-fi eld assays were obtained from the insectary of the Tropical Pesticides Research Institute.Only 3rd instar larvae were used,according to World Health Organization protocol[18].Larval rearing in the insectary was carried out according to the protocol developed by Balestrino et al.[19].Larvae were reared at(27.0±2.0)°C,a photoperiod of 12:12 h(light: dark),and(78±2)%relative hum idity.Larvae were fed a diet of TetraM in fi sh food.
2.2.Larval assays in the laboratory
The assayed compounds(−)-perillyl alcohol,(−)-isopulegol, (+)-limonene epoxide,(+)-limonene,terpinen-4-ol,and terpinolene were acquired from Sigma–A ldrich,USA.The(−)-carvone epoxide[20]and(−)-hydroxydihydrocarvone[21]were prepared as previously described.
Larvicidal bioassayswere conducted as described by Mdoe et al.[12,13].A stock solution w as prepared for each test compound by dissolving the com pound in 98 m L normal laboratory larval rearing water and 2 m L dimethylsulfoxide (DMSO)in a 100 m L plastic container.The solution was thoroughly m ixed to get a homogeneous m ixture,and serial dilutions of 200,100,50,25 and 12.5 mg/L were prepared. Each experiment was replicated at least six times w ith two controls:one containing normal laboratory larval rearing water,the other containing an aqueous solution of 1% DMSO to evaluate the effect of the solvent on the larvae.For the larvicidal experiments,each replicate and each control received 20 live 3rd instar larvae.No nutritional supplements were added during the assays.Larval mortality was registered after 12,24,48 and 72 h of exposure. The larvae were considered dead if they did not present movement.
2.3.Larval assays in the semi-fi eld
Sem i-fi eld larvae bioassays were conducted using the same concentrations used in the laboratory assays.Sem i-fi eld environmentstructures used in thisstudy were designed according to previous studies[12,22]and follow ing World Health Organization recommendations[18].Each experiment was carried out in six replicates w ith two controls,one having an aqueous solution of 0.5%DMSO and the other having normal laboratory larval rearing water.For the larvicidal assay,20 live 3rd instar larvae were placed in each assay replicate and in each control.
2.4.Statistical analysis
Scheff´e'smultiple comparison procedure was used to determ ine the statistical signi fi cance of the larvicidal activity of the tested compounds,w ith results expressed as mean±SE.Statistical analysis was performed using SAS.Assessments of surviving larvae were recorded after 12,24,48 and 72 h of exposure.Mortalitywas reported as LC50,the concentration that produced 50%mortality.The 95%con fi dence intervals(CI)for LC50were also recorded.
3.Resu lts
In this study,the larvicidal toxicity of a series of eight monoterpenes(Figure 1)present in volatile oils was evaluated against 3rd stage larval instars of An.gambiae s.s.,one of the most anthropophilic vectors of malaria.Larval mortality rates were registered after 12,24,48 and 72 h of treatment in varying concentrations of the test solutions.The result of each bioassay was reported as the lethal concentration estimated to kill 50%of the treated larvae(LC50),expressed inmg/L.The LC50values foreach compound and treatment time,along w ith 95%CI,were given in Table 1.
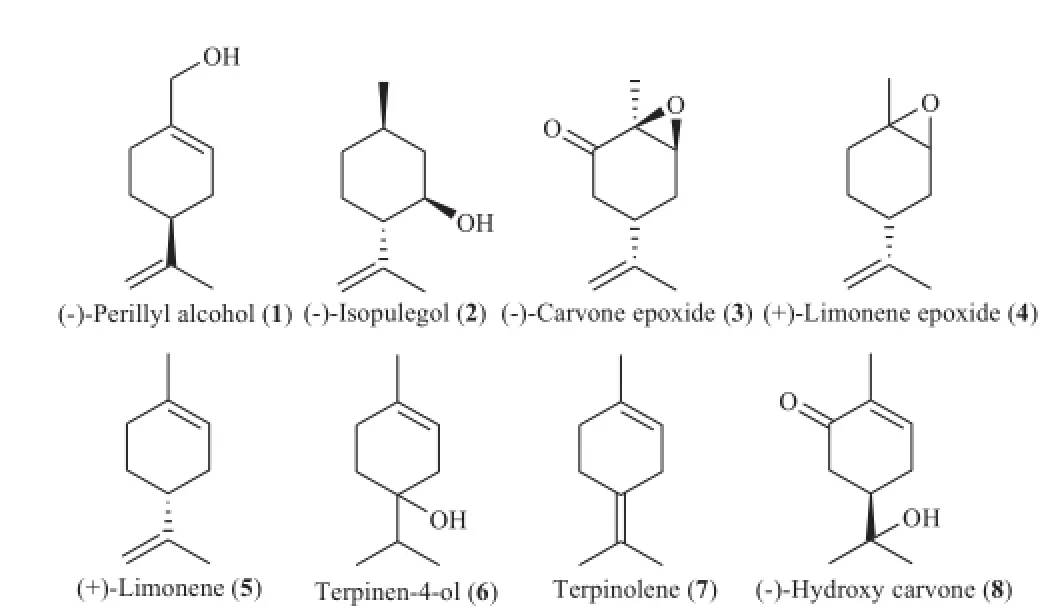
Figure 1.Chemical structuresof the evaluated compounds.
A ll the assayed compounds had larvicidal effects and exhibited different degrees of larval toxicity,w ith LC50values varying between 1.28 and 1938.92 mg/L depending on the treatment time(12,24,48 or 72 h).Among the eightmonoterpenes,(−)-perillyl alcohol(1)showed the strongest larvicidal activity towards An.gambiae larvae,w ith LC50valuesof 73.60, 18.36,1.72 and 1.28mg/L after12,24,48 and 72 h of exposure, respectively.The next strongest were(−)-isopulegol(2) (LC50=135.10,49.39,34.39 and 20.22mg/L)and(−)-carvoneepoxide(3)(LC50=168.86,124.74,80.84 and 23.46 mg/L). A fter 12,24 and 48 h of treatment,(−)-hydroxydihydrocarvone (8)was the least toxic compound,w ith LC50values of 1938.92, 1172.18 and 401.03 mg/L,respectively.Terpinolene(7) exhibited the lowest toxicity at 72 h post-treatment,w ith an LC50value of 259.40mg/L.
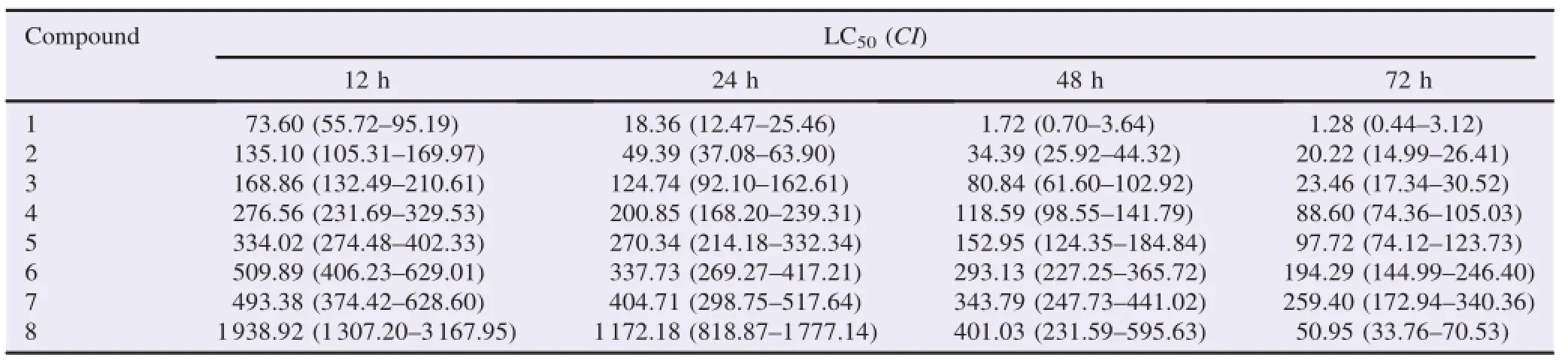
Table1 LC50(mg/L)and 95%CI of the compounds 1–8.
Larvalmortality rateswere found to be directly proportional to monoterpene concentrations.Sim ilarly,larval mortality increased w ith increasing exposure time,as all assayed compounds showed the highestmortality rates after 72 h of treatment.The most remarkable result was seen w ith (−)-hydroxydihydrocarvone(8),which was 38-fold more bioactive at 72 h post-treatment(LC50=50.95 mg/L)than at 12 h(LC50=1938.92 mg/L).To better understand the relationship between themolecular structure of the assayed monoterpenes and their larval toxicity,speci fi c structural and functional group variations were identi fi ed as possibly contributing to larvicidal activity.In general,the oxygenated monoterpenes exhibited stronger larvicidal effects than the monoterpene hydrocarbon(+)-limonene(5).The position of the carbon–carbon double bond in the p-menthane skeleton appeared to in fl uence larvicidal potency;after 48 and 72 h of treatment time,(+)-limonene(5)(LC50=152.95 and 97.72, respectively)was more bioactive than terpinolene(7) (LC50=343.79 and 259.40,respectively).Sim ilarly,the position of the hydroxyl group(endo-or exocyclic)also altered the toxicity,as seen in themonoterpenoids(−)-perillyl alcohol(1), (−)-isopulegol(2)and terpinen-4-ol(6),which each exhibited different degrees of larval toxicity.Furthermore,replacementof a C–C double bond by an epoxide group did not signi fi cantly affect larvicidal potency,as(+)-limonene epoxide(4)and (+)-limonene(5)showed sim ilar activity.However,the addition of a ketone group in the cyclohexane ring seemed to contribute to larvicidal ef fi cacy,as seen in the relatively high bioactivity of (−)-carvone epoxide(3)compared to(+)-limonene epoxide(4) and(+)-limonene(5).
4.Discussion
The fi ndings of this study have shown that,com poundsw ith different orientations of the active groups in primary structure in fl uence the outcome of larvae mortality differently.These results are interesting,since the evaluated compounds are highly volatile.Several studies in the literature have described the larvicidalactivity ofmonoterpenes against different species of mosquitoes[17,23–27].Perumalsam y et al.reported the larvicidal potential of themonoterpenes camphene,fenchone, terpinolene,γ-terpinene,(+)-and(−)-β-pinene,(+)-and (−)-α-pinene,α-terpineol,myrcene,terpinen-4-ol,(+)-limonene,Δ3-carene,borneol,1,8-cineole,linalool,verbenone and α-phellandrene against three mosquito species,Culex pipiens pallens,Aedes aegypti and Ochlerotatus togoi[28].In another study,Tabanca et al.found that(−)-perillyl alcohol, (−)-perilla aldehyde,(−)-perillic acid and(−)-limonene exhibited high toxicity against 3rd instar larvae of Aedes aegypti,w ith LC50values of 39.1,35.3,56.5 and 29.1mg/L, respectively[29].Liu et al.showed that the monoterpenes (+)-limonene and geraniol,both isolated from the essential oil from the roots of Toddalia asiatica(L.)Lam.,displayed an interesting larvicidal activity against 3rd instar larvae of Aedes albopictus,w ith LC50values of 19.84 and 30.13μg/ m L,respectively[16].
The fi ndings of the current study suggest possibilities for further research on the larvicidalactivity of plant-derived essential oils and their chem ical components.Future studies should focus on developing more stable and effective formulations, investigating themode of the constituents'actions,decreasing costs,and exam ining the effects of these compounds on nontarget organisms and the environment[6,30,31].The compounds are found in essential oils of plants,such as Conyza newii (perillyl alcohol and limonene)[32],Eucalyptus citriodora (isopulegol)[33],Carum carvi(carvone epoxide)[34],Artemisia nilagirica var.septentrionalis(terpinen-4-ol)[35],lemon (limonene-1,2-epoxide)[36],Mangifera indica L.(terpinolene) [37],and Nicotiana tabacum(hydroxydihydrocarvone)[38]. These monoterpenes toxicity against mosquito larvae have shown the prospect of replacing synthetic larvicides which are losing ef fi cacy or been incorporated in integrated vector control management programme.
A ll of the tested monoterpenes exhibited larvicidal activity against An.gambiae s.s.,w ith(−)-perillylalcohol themost toxic after 12,24,48 and 72 h of treatment.These results underscore the importance of evaluating plant essential oils and their chem ical components as effective natural larvicides for controlling Anopheles larvae,especially in areaswhere vectorshave developed resistance or dim inished susceptibility to conventional synthetic insecticides.
Con fl ict of interest statement
Acknow ledgm ents
The authors would like to thank the Tropical Pesticides Research Institute for providing infrastructure and resources toconduct the trials;Conselho Nacional de Desenvolvimento Científi co e Tecnol´ogico,Coordenação de Aperfeiçoamento de Pessoal de Nível Superior,and Fundação de Apoio`a Pesquisa e Inovação Tecnol´ogica do Estado de Sergipe for providing fi nancial support(Grant#475520/2012-2).
References
[1]Das NG,Goswam i D,Rabha B.Preliminary evaluation of mosquito larvicidalef fi cacy of plantextracts.JVector Borne Dis2007; 44:145-8.
[2]Pugazhvendan SR,Elumali K.Larvicidal activity of selected plant essential oil against im portant vectormosquitoes:dengue vector, Aedes aegypti(L.),malarial vector,Anopheles stephensi(Liston) and fi larial vector,Culex quinquefasciatus(Say)(Diptera:Culicidae).M iddle East JSciRes 2013;18(1):91-5.
[3]Intirach J,Junkum A,Tuetun B,Choochote W,Chaithong U, Jitpakdi A,et al.Chem ical constituents and combined larvicidal effects of selected essential oils against Anopheles cracens (Diptera:Culicidae).Psyche 2012;2012:591616.
[4]Rajkumar S,Jebanesan A,Nagarajan R.Effectof leaf essentialoil of Coccinia indica on egg hatchability and different larval instars ofmalarialmosquito Anopheles stephensi.Asian Pac J Trop Med 2011;4:948-51.
[5]Wachira SW,Omar S,Jacob JW,Wahome M,Alborn HT, Spring DR,et al.Toxicity of six plantextracts and two pyridone alkaloids from Ricinus communis against the malaria vector Anopheles gambiae.Parasit Vectors 2014;7:312.
为了分析IGBT的开路对电路造成的影响我们假设图2中的In方向为电流的正向,首先分析T1的开路故障(如图2所示)。当电流In为正方向时,T1发生了断路故障,为了保证Ln的电流连续性,电流In只能通过D1流向直流侧。那么由以上的分析可知在电流In为正向极性时,如果T1发生了断路故障并不会影响In。然而,当电流In的极性变成负极性时,电流In不再能够通过D1进行续流,那么必然会对In产生影响。综合单相PWM的开关指令,判断如下3种开关指令:(1010),(1001),(1000)符合上面的分析。
[6]Vatandoost H,Dehakia M,D javadia E,Abai MR,Duchson S. Comparative study on the ef fi cacy of lambdacyhalothrin and bifenthrin on torn nets against themalaria vector,Anopheles stephensi asassessed by tunnel testmethod.JVector Borne Dis2006; 43:133-5.
[7]Meyrow itsch DW,Pedersen EM,A lifrangis M,Scheike TH, Malecela MN,M agesa SM,et al.Is the current decline inmalaria burden in sub-Saharan A frica due to a decrease in vector population?Malar J 2011;10:188.
[8]Mmbando BP,Vestergaard LS,Kitua AY,Lemnge MM, Theander TG,Lusingu JP.A progressive declining in the burden of malaria in North-eastern Tanzania.Malar J 2010;9:216.
[9]Nanyonga SK,Opoku A,Lewu FB,Oyedeji AO.Chem ical composition and larvicidal activity of the essential oil of Tarchonanthus camphoratus against Anopheles arabiensis mosquito larvae.JEssentOil Bear Plants 2012;15:288-95.
[10]Cheng SS,Lin CY,Chung MJ,Liu YH,Huang CG,Chang ST. Larvicidal activities of wood and leaf essential oils and ethanolic extracts from Cunninghamia konishii Hayata against the dengue mosquitoes.Ind Crops Prod 2013;47:310-5.
[11]ShivakumarMS,Srinivasan R,Natarajan D.Larvicidalpotentialof some Indian medicinal plant extracts against Aedes aegypti(L.). Asian J Pharm Clin Res 2013;6:77-80.
[12]Mdoe FP,Cheng SS,Lyaruu L,Nkwengulila G,Chang ST, Kweka EJ.Larvicidal ef fi cacy of Cryptomeria japonica leaf essentialoils against Anopheles gambiae.Parasit Vectors 2014;7: 426.
[13]Mdoe FP,Cheng SS,Msangi S,Nkwengulila G,Chang ST, Kweka EJ.Activity of Cinnamomum osmophloeum leaf essential oil against Anopheles gambiae s.s.Parasit Vectors 2014; 7:209.
[14]Pavela R,Kaffkova K,Kumsta M.Chemical composition and larvicidal activity of essential oils from different Mentha L.and Pulegium species against Culex quinquefasciatus Say(Diptera: Culicidae).Plant Prot Sci2014;50:36-42.
[15]Ali A,Tabanca N,Dem irci B,Blythe EK,Ali Z,Baser KH,etal. Chem ical com position and biological activity of four salvia essential oils and individual compounds against two species of mosquitoes.JAgric Food Chem 2015;63:447-56.
[16]Liu XC,Dong HW,Zhou L,Du SS,Liu ZL.Essential oil composition and larvicidal activity of Toddalia asiatica roots against themosquito Aedes albopictus(Diptera:Culicidae).Parasitol Res 2013;112:1197-203.
[17]Dias CN,M oraes DF.Essential oils and their compounds as Aedes aegypti L.(Diptera:Culicidae)larvicides:review.Parasitol Res 2014;113:565-92.
[18]World Health Organization.Guidelines for laboratory and fi eld testing ofmosquito larvicides.Geneva:World Health Organization; 2005.[Online]Available from:http://apps.who.int/iris/bitstream/ 10665/69101/1/WHO_CDS_WHOPES_GCDPP_2005.13.pdf [Accessed on 20th September,2015]
[19]Balestrino F,BenedictMQ,Gilles JR.A new larval tray and rack system for im provedmosquitomass rearing.JMed Entomol2012; 49:595-605.
[20]Salgado PR,da Fonsˆeca DV,Braga RM,de Melo CG, Andrade LN,de A lmeida RN,et al.Comparative anticonvulsant study of epoxycarvone stereoisomers.Molecules2015;20:19660-73.
[21]Buechi G,Wueest H.New synthesis of beta agarofuran and of dihydroagarofuran.JOrg Chem 1979;44:546-9.
[22]Kweka EJ,Nyindo M,Mosha F,Silva AG.Insecticidal activity of the essential oil from fruits and seeds of Schinus terebinthifolia Raddi against African malaria vectors.Parasit Vectors 2011;4: 129.
[23]Evergetis E,M ichaelakis A,Kioulos E,Koliopoulos G, Haroutounian SA.Chem ical com position and larvicidal activity of essential oils from six Apiaceae fam ily taxa against the West Nile virus vector Culex pipiens.Parasitol Res 2009; 105:117-24.
[24]Zhu L,Tian YJ.Chem ical composition and larvicidal effects of essential oil of Blumea martiniana against Anopheles anthropophagus.Asian Pac J Trop Med 2011;4:371-4.
[25]Tripathi AK,Prajapati V,Ahmad A,Aggarwal KK,Khanuja SPS. Piperitenone oxide as toxic,repellent,and reproduction retardant toward malarial vector Anopheles stephensi(Diptera:Anophelinae).JMed Entomol 2004;41:691-8.
[26]Lucia A,Zerba E,Masuh H.Knockdown and larvicidalactivity of six monoterpenes against Aedes aegypti(Diptera:Culicidae)and their structure-activity relationships.Parasitol Res 2013;112: 4267-72.
[27]M ichaelakis A,Vidali VP,Papachristos DP,Pitsinos EN, Koliopoulos G,Couladouros EA,et al.Bioef fi cacy of acyclic monoterpenes and their saturated derivatives against theWestNile vector Culex pipiens.Chemosphere 2014;96:74-80.
[28]Perumalsamy H,Kim NJ,Ahn YJ.Larvicidal activity of compounds isolated from Asarum heterotropoides against Culex pipiens pallens,Aedes aegypti,and Ochlerotatus togoi(Diptera: Culicidae).JMed Entomol 2009;46:1420-3.
[29]Tabanca N,Dem irci B,A li A,Ali Z,Blythe EK,Khan IA. Essential oils of green and red Perilla frutescens as potential sources of com pounds formosquitomanagement.Ind Crops Prod 2015;65:36-44.
[30]Cheng SS,Huang CG,Chen YJ,Yu JJ,Chen WJ,Chang ST. Chemical compositions and larvicidal activities of leaf essential oils from two eucalyptus species.Bioresour Technol 2009;100: 452-6.
[31]Liu XC,Liu Q,Zhou L,Liu ZL.Evaluation of larvicidalactivity of the essential oil of Allium macrostemon Bunge and its selected major constituent compounds against Aedes albopictus(Diptera: Culicidae).Parasit Vectors 2014;7:184.
[32]Mayeku WP,Omollo NI,Odalo OJ,Hassanali A.Chemical composition and mosquito repellency of essential oil of Conyza new ii propagated in differentgeographical locationsof Kenya.Med Vet Entomol 2014;28(3):253-6.
[33]Olivero-Verbel J,Nerio LS,Stashenko EE.Bioactivity against Tribolium castaneum Herbst(Coleoptera:Tenebrionidae)of Cymbopogon citratus and Eucalyptus citriodora essential oils grown in Colombia.PestManag Sci2010;66(6):664-8.
[34]Iacobellis NS,Lo Cantore P,Capasso F,Senatore F.Antibacterial activity of Cuminum cyminum L.and Carum carvi L.essentialoils. JAgric Food Chem 2005;53:57-61.
[35]Haider F,Kumar N,Naqvi AA,Bagchi GD.Oil constituents of Artemisia nilagirica var.septentrionalis grow ing at different altitudes.Nat Prod Commun 2010;5(12):1959-60.
[36]Blanch GP,Nicholson GJ.Determination of the enantiomeric composition of limonene and limonene-1,2-epoxide in lemon peel by multidimensional gas chromatography w ith fl ame-ionization detection and selected ion monitoring mass spectrometry. JChromatogr Sci1998;36:37-43.
[37]Ramos EH,M oraes MM,Nerys LL,Nascimento SC, M ilitão GC,de Figueiredo RC,et al.Chemical composition, leishmanicidal and cytotoxic activities of the essential oils from Mangifera indica L.var.Rosa and Espada.Biomed Res Int 2014;2014:734946.
[38]Demole E,Berthet D.Chem ical study of Burley tobacco fl avor (Nicotiana tabacum L.).I.Volatile to medium-volatile constituents.Helv Chim Acta 1972;55(6):1866-82.
29 Sep 2015
*Corresponding author:Eliningaya J.Kweka,Division of Livestock and Human Diseases Vector Control,Tropical Pesticides Research Institute,P.O.Box 3024, Arusha,Tanzania.
Tel:+255 787745555
E-mail:pat.kw eka@gm ail.com
Foundation Project:Supported by Conselho Nacional de Desenvolvimento Científi co e Tecnol´ogico,Coordenação de Aperfeiçoamento de Pessoal de Nível Superior,and Fundação de Apoio`a Pesquisa e Inovação Tecnol´ogica do Estado de Sergipe(Grant#475520/2012-2).
Peer review under responsibility of Hainan M edical University.The journal implements double-blind peer review practiced by specially invited international editorial boardmembers.
2221-1691/Copyright©2016 Hainan Medical University.Production and hosting by Elsevier B.V.This is an open accessarticle under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Pacific Journal of Tropical Biomedicine2016年4期
Asian Pacific Journal of Tropical Biomedicine2016年4期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Sudden death in a captive mee rkat(Suricata surica tta)w ith arterial m edial and m yocardial calcification
- Characteristics o f obese or overweight dogs visiting private Japanese vete rinary c linics
- Risk factors from HBV infection among blood donors:A system atic review
- Com putational in telligence in tropicalm edicine
- The African Moringa is to change the lives ofm illions in Ethiopia and far beyond
- Pediculosis capitis among p rimary and m idd le school children in Asadabad,Iran:An epidem iological study
