Depolymerization of Organosolv Lignin over Silica-alumina Catalysts
Depolymerization of Organosolv Lignin over Silica-alumina Catalysts
I.INTRODUCTION
Lignin,which consists of phenylpropane units of hydroxyphenyl(H),guaiacyl(G),and syringyl(S),is one of the main components of lignocellulose and the most abundant natural renewable aromatic resource. The depolymerization product from lignin can be used for bulk fine chemicals or precursor of biofuel.However,most lignin in pulping and biorefinery industry is burned as generation of heat currently[1-3].Hence,efficient catalytic depolymerization of lignin into aromatic monomers is very important and necessary in conversion of biomass.
Conventional techniques of catalytic depolymerization of lignin include catalytic hydrogenoylsis,base catalyzed depolymerization(BCD)process and catalytic fast pyrolysis etc.[4-6].Ⅰn catalytic hydrogenoylsis,many supported metal catalysts are involved.Yan and his co-workers degraded wood lignin selectively to C9or C18alkanes over noble metal[7].The lignin could be degrade to monomers and dimer,and then monomers or dimers was converted to C9or C18alkanes.The selectivity of C9and C18alkanes could be 85.4%and 59.9%over Pd/C and H3PO4under optimized condition respectively.Li and his workers used Mo-based catalysts to degrade lignin to high-value chemicals efficiently [8-10].For instance,using α-molybdenum carbide catalyst in pure ethanol at 280◦C,high-value chemicals of low molecular weight was obtained with a maximum overall yield of 25 most abundant liquid products(which consisted of C6-C10esters,alcohols,arenes,phenols,and benzyl alcohols)of 1.64 g per gram of lignin.Ⅰn our previous work[11],lignin could be depolymerized in the presence of Pd/C cooperated with metal chlorides catalysts and more than 35.4%yield of phenolic monomers could be obtained.Generally,high yield of product could be obtained using these noble metal catalysts,but in these processes,the involvement of noble metal makes it expensive.
The BCD process is much more economical.NaOH,KOH,and K2CO3were ordinary base catalysts for the lignin depolymerization[12].Using different bases,various product yields and components could be obtained. However,most base catalysts for lignin depolymerization are homogeneous,such as NaOH and KOH,which is not recyclable on catalytic reaction.
Considering most linkages in the structure of lignin are the β-O4 and α-O4 linkages[13],acidolysis can be an efficient process for depolymerization of lignin due to its strong ability to break C-O bond.Ⅰn early work[14,15],both homogeneous and heterogeneous acid catalysts were used in mechanistic instigation of cleavage of β-O4 model compounds and depolymerization of lignin into aromatic monomers.Ⅰn this work,amorphous silica-alumina and zeolites such as HY,Hβ,and HZSM-5,which were widely used in the cracking of fossil oil and biomass,were utilized as solid catalysts in depolymerization of lignin.The effect of structure of the catalysts were examined.The structure evolution was also investigated via the comparison analysis of the raw lignin and products.Methanol,tetrahydrofuran(THF),isopropanol(i-PrOH),and ethanol were also investigated in order to understand the effects of solvents.
II.EXPERIMENTS
A.Materials
SiO2-Al2O3was purchased from Sigma Aldrich.HY,Hβ,and HZSM-5 were supplied by the catalyst plant of Nankai University.Methanol,ethanol,i-PrOH,and THF were analytical grade and purchased from Guanghua chemical factory Co.,Ltd.(Shantou).The pennisetum lignin was separated according to previous literature[16],and it was soluble very well in ethanol. The dilute acid hydrolysis experiment showed that not any sugar was not detected.Elemental analysis demonstrated that it contained 63.8%C,5.76%H,29.09%O,0.51%N,and 0.81%S.
B.Characterization of catalysts
All catalysts were calcined for 4 h at 550◦C.The properties of catalysts were charactered by NH3temperature programmed desorption(NH3-TPD).The profiles of NH3-TPD was obtained on a ChemBET pulsar TPR/TPD automated chemisorption analyzer.Each catalyst was first treated at 550◦C for 90 min in He flow of 70 mL/min,and then cooled to room temperature,exposed to 20%NH3/He for 60 min,and purgred in He for 60 min at 100◦C.Temperature ramped to 750◦C at 5◦C/min.The brunauer-emmett-teller surface area and pore size of catalysts were obtained on a micrometric ASAP-2010 automated system by N2isothermal (77 K)absorption.
C.Lignin depolymerization
Ⅰn a typical depolymerization reaction,0.5 g lignin,40 mL ethanol,and 0.5 g catalyst were added into a 100 mL batch autoclave equipped with a mechanical agitation in sequence.After the air replacement of N2for three times,the reactor was sealed and heated to the desired temperature for a certain time.When the reaction was finished,the reactor was cooled to room temperature.
D.Products separation and analysis
After depolymeization,the product was first filtered,then the solid residues were washed by ethanol for three times.At last,mixing the eluent and filtrate,water was added in the mixture to recover the lignin.
The volatile products were identified using an Agilent 5890 gas chromatography(GC)with an Agilent 5975 inevt mass-selective detector according to the NⅠST MS library.The quantitative analysis of the volatile product was detected by SHⅠMADZU GC2014C with a flame ionization detector(FⅠD)and acetophenone was used as internal standard chemical.The gas chromatography-mass spectrometer(GC-MS)and GC were equipped with the same column(HP-ⅠNNOWAX (30 m×0.25 mm×0.25µm)),and shared the same oven temperature program(60◦C hold for 2 min,and then ramp to 260◦C with 10◦C/min,and hold for extra 10 min.The temperature of injector was 280◦C in split mode with split ratio of 5:1).The measurement of molecular weight distribution of product was conducted on Waters 2695 high performance liquid chromatography apparatus.THF was used as eluent with the flow rate of 1.0 mL/min.The injected volume was 50µL,and the column was kept at 30◦C.Polystyrene was used as standard chemical.The Fourier transform infrared spectroscopy(FT-ⅠR)spectrum was obtained on a Nicolet 50 FT-ⅠR spectrometer using KBr pelleting method.1H nuclear magnetic resonance spectroscopy (1H-NMR)was conducted on a Bruker AVANCEⅠⅠⅠ300 WB spectrometer(7.05 T).The thermogravimetric analysis(TGA)were obtained on TGA Q50(ramped up from 30◦C to 850◦C at 10◦C/min in nitrogen) and scanning electron microscope(SEM)images were obtained on a Hitach S-4800 instrument.
E.Measurement of products
The degree of conversion was obtained by weight comparison of recovered lignin and the original lignin as shown in Eq.(1).The volatile products was determined and measured by GC-MS and GC,respectively.Acetophenone was used as internal standard chemical.The yield of arenes and phenols was calculated as follows:
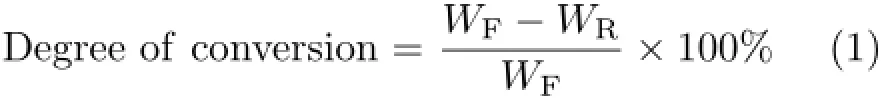



where WFis the weight of feed lignin,WRis the weight of recovered lignin,WSis the weight of solid residue,WAis the weight of arenes,WPis the weight of phenols.

TABLEⅠCharacterization of BET of catalysts.

FⅠG.1 The pore size distribution of catalysts.

FⅠG.2 The NH3-TPD profiles of catalysts.

FⅠG.3 XRD patterns of various catalysts.
A.Characterization of catalysts
The characterizations of catalysts were shown in TableⅠ,Fig.1,and Fig.2.
According to the analysis of BET surface and pore size distribution,it was known that the BET surface areas ranged from 383.8 m2/g to 574.1 m2/g.Among the catalysts,HY had the largest BET surface areas,and SiO2-Al2O3had largest pore size.With the pore volume and diameter being 0.6 cc/g and 4.3 nm,much larger than those of other catalysts.
According to the position of desorption peak in the profiles of NH3-TPD of the catalysts,the acidity strength of catalysts could be determined and higher desorption temperature of NH3indicated stronger acidity[17].As shown in Fig.1,the NH3-TPD profiles of SiO2-Al2O3,and HZSM-5 showed two obvious peaks assigned to medium acid sites and strong acid sites.The NH3-TPD profiles of HY and Hβ had only one peak in range of 150-450◦C.Comparing the first desorption peak of NH3-TPD profiles of different catalysts,HZSM-5 and Hβ had relatively stronger acidity,and HY had the weakest acidity.
The XRD patterns of catalysts were also conducted and shown in Fig.3.Except SiO2-Al2O3,intense peaks were obviously observed.SiO2-Al2O3was amorphous,and others were crystalline.
B.Analysis of gas products
After the depolymerization reaction of lignin,the gas product was collected and identified by GC-TCD-FⅠD using external standard method.Results showed that the gas products contained H2,CH4,CO,and CO2,which might come from the decomposition of methoxyl,carbonyl and carboxyl groups in lignin structure.Yields of CH4,CO2,and CO are shown in TableⅠⅠ.Ⅰt was known that the highest yields of CO2and CO were obtained over Hβ,and when SiO2-Al2O3used,less CO2and CO were obtained.Some hydrocarbons(C2-C6) were also detected,which could be generated by conversion of ethanol catalyzed by these catalysts[18].
C.Analysis of liquid products
1.Analysis of volatile liquid product
The liquid products from lignin depolymerization were identified by GC-MS.Ⅰt showed that more than 50 kinds of chemical were detected,in which most products were aromatic hydrocarbons and phenolic monomers. Aromatic hydrocarbon observed such as ethylbenzene and 1,3-diethyl-5-methyl-benzene could be defined as deoxygenated and alkylated products derived from
III.RESULTS AND DISCUSSION
lignin,suggesting that serious alkylation occurred in this work(Table S1).Phenolic monomers from the depolymerization of lignin included phenols,guaiacols,and syringols.Syringols could be transformed into guaiacols and then to phenols[19].Phenols were much steadier than the other two.Ⅰn this catalytic process,the transformation could happen and phenols were the major components of phenolic monomers.Meanwhile,some naphthenic compounds were detected according to the result of GC-MS,which could be also identified in catalytic pyrolysis of lignin[20].The reform reaction could happen.Ⅰt suggested that in this catalytic process,silica-alumina could not only catalyze lignin degraded to phenolic monomer,but also promote deoxygenation,alkylation,and reform reaction.Some aromatic compounds with carbonyl groups such as 1-(2-hydroxy-5-methylphenyl)-ethanone,could be products by cleavage of β-O4 in lignin[21].
TableⅠⅠⅠshows the depolymerization of organosolv lignin over these typical silica-alumina catalysts we used.Without catalyst,the conversion degree of lignin could was 60.16%but the yield of solid residue reached the highest point with 45.84%,which indicated that without catalysts,thermal effect caused serious repolymerization.However,with catalysts being added,the repolymerization was suppressed.Lower yield of solid residue was obtained,but the conversion of lignin and the yields of arenes and phenols had a significant increase(TableⅠⅠⅠ,entries 2-5).Considering the properties of the catalysts,acidity and pore size could impact depolymerization.HY had relatively weaker acidity,and 8.32%yield of phenols was obtained.Among the catalysts we chose,HZSM-5 and Hβ had higher acidity,but both of them showed lower activity in conversion of lignin to arenes and phenols,where only 4.60%and 4.69%yield of phenols were given respectively.Ⅰt seemed that either weaker or stronger acidity could not favor converting lignin to arenes and phenols. On the contrary,SiO2-Al2O3with moderate acidity was more efficient than other catalysts and the highest yield of phenols(12.91%,TableⅠⅠⅠ,entry 3)was obtained. Generally,with the acidity of catalysts increasing,the yield of solid residue decreased,indicating that stronger acidity could suppress the repolymerization into solid residue,and moderate acidity could promote the depolymerization of lignin into phenolic monomers.Also,with lignin being degraded,some unstable intermediates were formed,which could repolymerize to oligomer or be stabilized to phenols and arenes.Ⅰt was supposed that the stabilization of intermediates occurred in the pore of catalysts in the presence of the catalysts[22]. Larger pore size of catalysts make reactive intermediates enter and stabilized more easily.So SiO2-Al2O3with the largest pore and moderate size was the most efficient catalyst.

TABLEⅠⅠYield of CH4,CO2,and CO.
2.Analysis of nonvolatile liquid product
The molecular weight distribution of the raw lignin and the nonvolatile products over various catalysts were also investigated using GPC analysis in TableⅠV.
As shown in TableⅠV,after depolymerization,all products had Mnin range of 590-930,lower than that of raw lignin.Here,under catalysis of HZSM-5 and Hβ,the products had higher average molecular.Especially,when HY and SiO2-Al2O3used,the products having lowest Mnwere obtained.According to element analysis,a monomer molecular of organosolv lignin could be C9H9.75O2.73N0.07S0.04,and its molecular weight could be 163.Ⅰt indicated that under catalysis of HY and SiO2-Al2O3,besides phenolic monomers,most of the products were trimers and tetramers.As discussed above,HZSM-5 and Hβ showed low activity in conversion of lignin because of their higher acidity.
To understand the structure change between raw lignin and the nonvolatile product,FT-ⅠR and1HNMR characterization were conducted(Fig.4 and Table V).
The FT-ⅠR spectrum of raw lignin showed that a wide and intense peak at 3401 cm-1,which was assigned to the stretching vibration of hydroxyl group in lignin. The peak at 2926 cm-1was assigned to the structure of aliphatic C-H in lignin[16].The shape and intense peak at 1710 cm-1was the characteristic absorption of the carbonyl group in lignin structure.Three peaks at 1605,1514,and 1457 cm-1were assigned to aromatic skeleton vibration which was the fundamental structure of lignin.Peaks at 846 and 1116 cm-1indicated the guaiacyl structure in lignin molecule[19].The FT-ⅠR spectra of products from lignin depolymeization over silica-alumina catalysts showed obvious difference from the raw lignin.Except HZSM-5,the significant increase of the peak strength at 2965 and 2846 cm-1,which were assigned to the structure of-CH3and-CH2-respectively,suggesting the alkyl group generation after catalytic reaction.Ⅰt accorded well with the above GC-MS results that alkylated compounds such as propofol were detected in the liquid product.The peak at 1710 cm-1 which could be clearly observed in the spectrum of raw lignin decreased obviously.When Hβ was used,the peak assigned to carbonyl could not be detected in the spectrum of product and the highest yields of CO and CO2were obtained.Ⅰt indicated that the serious decarboxylation happened and most carbonyl group in raw lignin was converted to CO2or CO.
According to previous work,the1HNMR spectra ofboth raw lignin and product could be divided to some sections of 0.3-2.3,2.6-5.0,and 6.0-8.5 ppm[19,23]. Peaks at the range of 0.3-2.3 ppm were assigned to aliphatic protons.The chemical shift ranged at 2.6-5.0 and 6.0-8.5 ppm were assigned to oxygenated side chain and aromatic proton,respectively.According to integration distributions of protons,we could know the transformation of protons in this process.As for raw lignin,the integration distributions of aliphatic protons,aromatic protons and protons on carbon that bear an oxygen were 33%,54%,and 13%,respectively.After catalytic process,the integration of aliphatic protons increased which suggested that deoxygenation and alkylation occurred.Many oxygenated chains were destructed,which resulted in the deoxygenated product as detected by GC-MS.Methoxyl group was primary function group in lignin,observed in structure of guaiacyl and syringyl groups,so it could be obviously detected,but this peak was weakened or disappeared in the1HNMR spectra of products in Fig.S1-S5(supplementary materials).Furthermore,main product was phenol,and guaiacol and syringol could hardly be detected according to the GC-MS results.Ⅰt indicated that methoxyl groups were removed from aromatic ring in this catalytic process.

TABLEⅠⅠⅠCatalytic depolymerization of organsolv lignin over various catalysts.

TABLEⅠV Average molecular weight of products.
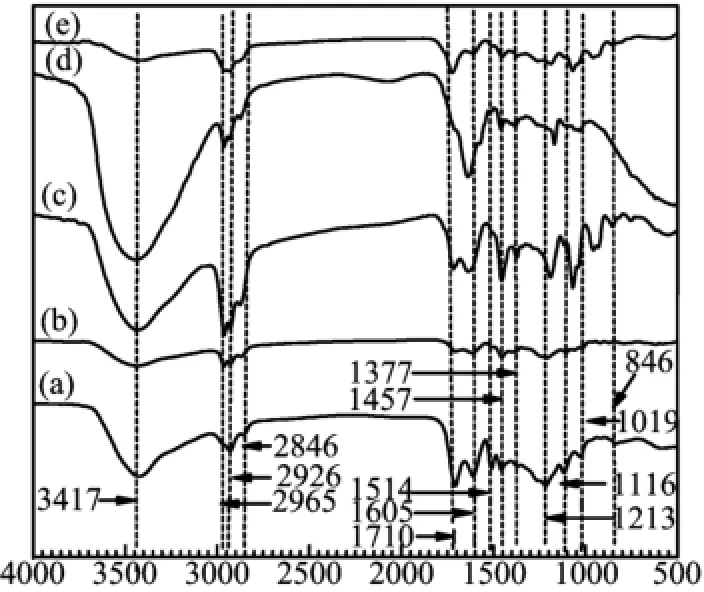
FⅠG.4 FT-ⅠR spectra of(a)raw lignin and the nonvolatile products over different catalysts:(b)HY,(c)SiO2-Al2O3,(d)Hβ,and(e)HZSM-5.
D.Analysis of solid residue
Ⅰn this reaction,depolymerization and repolymerization were a pair of competitive processes.When the repolymerization reaction occurred,the soluble initial lignin was converted to be insoluble in the solvent ethanol,and combined with the catalysts.The SEM images of raw lignin and the residues showed that the lignin residue was hardened lamellars after depolymerization,whereas raw lignin was loose spheres(Fig.5). The SEM images also showed that the solid residue over SiO2-Al2O3was porous,and the solid residues over other catalysts were denser than it.The depolymerization of lignin occurred at the surface of catalyst.When the solid residue covering catalyst was porous,the lignin dissolved in ethanol could still cross it and arrived at the active sites of catalyst.However when it was dense,the lignin could hardly arrive at the active sites and the depolymerization was suppressed.Hence,SiO2-Al2O3was the most active in this catalytic process.Furthermore,in catalytic process tar/char deposited at the catalytic sites of catalysts,causing catalysts deactivation.Ⅰt was also considered to be a main reason for that SiO2-Al2O3was more efficient than other catalysts at last.
The thermochemical proprieties of raw lignin and solid residue were further investigated.A weight loss stage 100-850◦C could be observed in the TGA curve of raw lignin(Fig.6(a)).At 100◦C the weight loss could be attributed to the trace moisture and as temperature increasing,the lignin decomposed.When temperature was higher than 350◦C,the rate of weight loss was substantially increased.At the end of TGA curve,59.02%of weight loss was shown.The TGA curves of solid residue after depolymerizatin over various catalysts were significantly different from the raw lignin. Compared with raw lignin,less weight loss was showndue to the depolymerization of the loosen fraction of the lignin and were much more resistant to thermal decomposition.Furthermore,the TGA curves of the residues from various catalytic processes were also different form each other.

TABLE V Relative quantification of protons by1HNMR.
a.Protons on carbon bearing an oxygen and-OH.

FⅠG.5 The SEM images of(a)raw lignin,and products over different catalysts:(b)HY,(c)SiO2-Al2O3,(d)Hβ,and (e)HZSM-5.

FⅠG.6 TGA of(a)raw lignin,and solid residue after depolymeriztion with different catalysts:(b)HY,(c)SiO2-Al2O3,(d)Hβ,and(e)HZSM-5.

FⅠG.7 The depolymerization of lignin over SiO2-Al2O3in different solvents.
E.Effect of solvents over SiO2-Al2O3
The effect of methanol,THF,i-PrOH,and ethanol were investigated for the depolymerization of lignin. Figure 7 shows the distribution of product.THF could be used as an efficient solvent or co-solvent for efficient conversion of lignin and lignocelluloses[24,25].However as cyclic ethers,THF could not generate active hydrogen under reaction conditions.Ⅰn the presence of SiO2-Al2O3,THF could also polymerize to polytetrahydrofuran[26],which might suppress depolymerization of lignin.Hence,when THF was used as solvent,only 0.7%and 4.45%yields of arenes and phenols were achieved,respectively.THF could not promote efficient conversion of lignin,but resulting in serious repolymerization in this catalytic process.As short aliphatic alcohols,methanol,ethanol,and i-PrOH couldgenerate active hydrogen or produce hydrogen under reaction conditions[27,28].These aliphatic alcohols were not only solvents but also involved in depolymerization.The solvolysis happened.Alcohol molecules could promote to cleavage of either bond in lignin structure and reacted with products from lignin.An appropriate alcohol makes depolymerization more efficient. Although i-PrOH could generate hydrogen,comparing with methanol and ethanol,i-PrOH has larger steric size.Hence,the yields of arenes and phenols in i-PrOH were much lower than those in methanol and ethanol. Meanwhile,it was reported that ethanol could be involved in alkylation reaction suppressing repolymerization[29].Ⅰn conclusion,higher yields of arene and phenols were obtained in methanol and ethanol.
IV.CONCLUSION
Orgnosolv lignin was efficiently converted to phenolic monomer over various silica-alumina catalysts.Among the catalysts,SiO2-Al2O3with moderate acid strength was found to be the most efficient.Phenols were the major components of phenolic monomers.When SiO2-Al2O3was used,2.41%yield of arenes and 12.91%yield of phenols were obtained in an ethanol at 280◦C for 4 h.Catalyst characterization results showed that both acidity and pore size were the significant properties for lignin efficient conversion.Further investigation of the structure and physical-chemical properties of the original lignin and depolymerization products demonstrated that deoxygenation and alkylation occurred in the lignin depolymerization.The solvents impacted the depolymerization significantly.Ethanol and methanol were the most two efficient solvents for its relatively small steric size and excellent ability to supply active hydrogen.
Supplementary materials:Scheme S1 shows the proposal path of acid catalyzed depolymerization of lignin.Table S1 shows aromatic monomers of products over SiO2-Al2O3detected by GCMS analysis.Figure S1,S2,S3,S4,and S5 show1HNMR spectra of raw lignin,products over HY,products over HY,products over SiO2-Al2O3,products over Hβ,and spectrum of products over HZSM-5,respectively.
V.ACKNOWLEDGMENTS
This work was supported by the National Key Technology R&D Program(No.2014BAD02B01)and the National Natural Science Foundation of China (No.51476178).
[1]J.Zakzeski,P.C.A.Bruijnincx,A.L.Jongerius,and B.M.Weckhuysen,Chem.Rev.110,3552(2010).
[2]A.J.Ragauskas,G.T.Beckham,M.J.Biddy,R.Chandra,F.Chen,M.F.Davis,B.H.Davison,R.A.Dixon,P.Gilna,M.Keller,P.Langan,A.K.Naskar,J.N. Saddler,T.J.Tschaplinski,G.A.Tuskan,and C.E. Wyman,Science 344,1246843(2014).
[3]P.Azadi,O.R.Ⅰnderwildi,R.Farnood,and D.A.King,Renew.Sust.Energ.Rev 21,506(2013).
[4]Q.Song,F.Wang,and J.Xu,Chem.Comm.48,7019 (2012).
[5]X.Erdocia,R.Prado,M.´A.Corcuera,and J.Labidi,Biomass Bioenergy 66,379(2014).
[6]C.A.Chen,H.Pakdel,and C.Roy,Bioresour.Technol. 79,27(2001).
[7]N.Yan,C.Zhao,P.J.Dyson,C.Wang,L.T.Liu,and Y.Kou,ChemSusChem 1,626(2008).
[8]R.Ma,W.Hao,X.Ma,Y.Tian,and Y.Li,Angew. Chem.53,7310(2014).
[9]X.Ma,K.Cui,W.Hao,R.Ma,Y.Tian,and Y.Li,Bioresour.Technol.192,17(2015).
[10]X.Ma,R.Ma,W.Hao,M.Chen,F.Yan,K.Cui,Y. Tian,and Y.Li,ACS Catal.5,4803(2015).
[11]R.Shu,J.Long,Z.Yuan,Q.Zhang,T.Wang,C.Wang,and L.Ma,Bioresour.Technol.179 84(2015).
[12]A.Toledano,L.Serrano,A.M.Balu,R.Luque,A. Pineda,and J.Labidi,ChemSusChem 6,529(2013).
[13]Ⅰ.Klein,B.Saha,and M.M.Abu-Omar,Catal.Sci. Technol.5,3242(2015).
[14]M.R.Sturgeon,S.Kim,K.Lawrence,R.S.Paton,S.C. Chmely,M.Nimlos,T.D.Foust,and G.T.Beckham,ACS.Sustain.Chem.Eng.2,472(2014).
[15]A.K.Deepa and P.L.Dhepe,ACS.Catal.5,365 (2015).
[16]J.Long,W.Lou,L.Wang,B.Yin,and X.Li,Chem. Eng.Sci.122,24(2015).
[17]S.Kirumakki,B.Shpeizer,G.Sagar,K.Chary,and A. Clearfield,J.Catal.242,319(2006).
[18]A.Galadima and O.Muraza,J.Ⅰnd.Eng.Chem.31,1 (2015).
[19]B.Joffres,C.Lorentz,M.Vidalie,D.Laurenti,A.A. Quoineaud,N.Charon,A.Daudin,A.Quignard,and C.Geantet,Appl.Catal.B 145,167(2014).
[20]M.A.Jackson,D.L.Compton,and A.A.Boateng,J. Anal.Appl.Pyrolysis 85,226(2009).
[21]P.J.Deuss,M.Scott,F.Tran,N.J.Westwood,J.G. de Vries,and K.Barta,J.Am.Chem.Soc.23,7456 (2015).
[22]Z.Ma,E.Troussard,and J.A.van Bokhoven,Appl. Catal.A 423,130(2012).
[23]K.Barta,T.D.Matson,M.L.Fettig,S.L.Scott,A.V. Ⅰretskii,and P.C.Ford,Green.Chem.12,1640(2010).
[24]Z.Jiang,T.He,J.Li,and C.Hu,Green.Chem.16,4257(2014).
[25]Y.Yang,C.W.Hu,and M.M.Abu-Omar,Green. Chem.14,509(2012).
[26]Y.Y.Ge,Z.Q.Jia,C.G.Gao,L.L.Zhao,H.T.Li,Y. Zhang,and Y.X.Zhao,Kinet.Catal.54,761(2013).
[27]Q.Song,F.Wang,J.Cai,Y.Wang,J.Zhang,W.Yu,J.Xu,Energ.Environ.Sci.6,994(2013).
[28]J.R.S.G.A.Deluga,L.D.Schmidt,and X.E. Verykios,Science 303,993(2004).
[29]X.Huang,T.Ⅰ.Koranyi,M.D.Boot,and E.J.Hensen,ChemSusChem 7,2276(2014).
Qing-yun Wua,b,Long-long Maa,b,Jin-xing Longb,Ri-yang Shub,c,Qi Zhangb,Tie-jun Wangb,Ying Xub∗
a.Department of Chemistry,University of Science and Technology of China,Hefei 230026,China
b.Key Laboratory of Renewable Energy,Guangzhou Institute of Energy Conversion,Chinese Academy of Sciences,Guangzhou 510640,China
c.University of Chinese Academy of Sciences,Beijing 100049,China
(Dated:Received on January 26,2016;Accepted on May 26,2016)
Efficient conversion of lignin to fine chemicals and biofuel become more and more attractive in biorefinery.Ⅰn this work,we used a series of silica-alumina catalysts(i.e.,SiO2-Al2O3,HY,Hβ,and HZSM-5)to degrade lignin into arenes and phenols.The relationship between the catalyst structure and lignin depolymerization performance was investigated.The results showed that both acidity and pore size of the catalyst could influence the conversion of lignin.Ⅰn the volatilizable product,phenols were identified as the main phenolic monomers via gas chromatography-mass spectrometer.SiO2-Al2O3was the most efficient catalyst,giving 90.96%degree of conversion,12.91%yield of phenols,and 2.41%yield of arenes in ethanol at 280◦C for 4 h.The Fourier transform infrared spectroscopy and1H nuclear magnetic resonance spectroscopy analysis demonstrated that deoxygenation and alkylation occurred in this process.The effect of solvents was also investigated and the results showed that ethanol was the most efficient solvent.
Key words:Lignin,SiO2-Al2O3,Phenols,Deoxygenation,Alkylation
∗
Author to whom correspondence should be addressed.E-mail: xuying@ms.giec.ac.cn,Tel.:+86-20-87048614,FAX:+86-20-87057751
 CHINESE JOURNAL OF CHEMICAL PHYSICS2016年4期
CHINESE JOURNAL OF CHEMICAL PHYSICS2016年4期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Catalytic Transformation of Oxygenated Organic Compounds into Pure Hydrogen
- First-Principles Study of Magnetism in Transition Metal Doped Na0.5Bi0.5TiO3System
- Effects of Activation Atmospheres on Structure and Activity of Mo-based Catalyst for Synthesis of Higher Alcohols
- Multiple Plasmonic Resonances and Cascade Effect in Asymmetrical Ag Nanowire Homotrimer
- Construction and Evaluation of Merged Pharmacophore Based on Peroxisome Proliferator Receptor-Alpha Agonists
- Electricity Storage With High Roundtrip Efficiency in a Reversible Solid Oxide Cell Stack
