微波消融同步联合TACE治疗大肝癌和巨块型肝癌的临床疗效分析
司增梅 钱 晟 刘 嵘 瞿旭东 龚高全 王小林 颜志平 王建华 △
(1上海市影像医学研究所 上海 200032; 2 复旦大学附属中山医院介入科 上海 200032)
微波消融同步联合TACE治疗大肝癌和巨块型肝癌的临床疗效分析
司增梅1,2钱晟1,2刘嵘1,2瞿旭东1,2龚高全1,2王小林1,2颜志平1,2王建华1,2 △
(1上海市影像医学研究所上海200032;2复旦大学附属中山医院介入科上海200032)
目的评价微波消融(microwave ablation,MWA)同步联合经动脉化疗栓塞(transarterial chemoembolization,TACE)治疗大肝癌(直径>5 cm)和巨块型肝癌(直径>10 cm)的安全性和有效性。方法回顾性分析2013年3月至2014年12月期间在复旦大学附属中山医院行经皮MWA同步联合TACE治疗的65例大肝癌或巨块型肝癌患者(肿瘤直径5~18 cm),治疗程序为先行动脉数字减影血管造影(digital subtraction angiography,DSA),接着超声引导下行经皮MWA,最后行再次血管造影并选择TACE治疗。结果同步治疗耐受性好。造影剂外渗3例,经栓塞后消失。术后仅见轻微肝功能受损和血液毒性及少量的胸腔积液,未见脓肿、胆道损伤、肝功能衰竭及其他手术相关的严重并发症。TACE碘油栓塞剂用量平均为8 mL。随访2~21个月,65例患者无疾病进展生存时间为(6.3±1.5)个月,中位生存时间为14个月,6、12及18个月的生存率分别为91.3%、81.5%及48%。2例患者获得II期手术切除。结论MWA联合同步TACE治疗大肝癌和巨块型肝癌安全、有效,既可扩大消融的适应证,又能减少TACE栓塞剂的用量,是值得推广的肝癌治疗新模式。
肝癌;经动脉化疗栓塞;微波消融;同步联合治疗;疗效评价
肝细胞肝癌(hepatocellular carcinoma,HCC)是常见的恶性肿瘤,起病隐匿,仅20%~25%的患者适合手术切除[1]。经动脉化疗栓塞(transarterial chemoembolization,TACE)是不能手术切除的中晚期HCC患者首选治疗方法[2-5]。大肝癌和巨块型肝癌由于动脉血供丰富,TACE治疗可取得一定的局部疗效,但由于患者肿瘤负荷大,单次TACE治疗疗效有限,需多次重复TACE治疗。即使经多次TACE治疗,大部分患者的肿瘤仍不能完全坏死,极易在治疗期间出现肝内和远处转移,从而严重影响TACE的疗效和患者生存期。因此,采用以TACE为基础的联合治疗能进一步提高大肝癌和巨块型肝癌介入治疗的疗效。
肿瘤热消融治疗主要指射频消融和微波消融(microwave ablation,MWA),具有操作简单、创伤小、对患者肝功能影响小及并发症少的特点,已成为直径<3 cm的HCC根治性治疗方法[6]。但对于大肝癌,消融治疗很难使肿瘤完全坏死,尤其是边缘部位容易残留肿瘤组织。先采用TACE再序贯消融治疗的联合治疗模式明显优于单一消融治疗或TACE治疗。Liu等[7]对比了TACE序贯MWA与单一TACE治疗直径>5 cm的HCC患者,发现联合治疗在病灶缩小率和生存期方面均明显优于单一 TACE 治疗。Xu等[8]报道在直径>5 cm的HCC治疗中,TACE序贯联合MWA治疗能明显延长患者总体生存期和肿瘤疾病进展时间。
目前国内外尚未见到MWA同步联合TACE治疗大肝癌和巨块型肝癌的报道。本文总结了65例接受MWA同步联合TACE治疗的大肝癌(直径>5 cm)和巨块型肝癌(直径>10 cm)患者,探讨该法治疗大肝癌和巨块型肝癌的安全性和有效性。
资 料 和 方 法
研究对象本研究经复旦大学附属中山医院伦理学委员会批准。收集自2013年3月至2014年12月在我院行 MWA 同步 TACE 治疗的大肝癌和巨块型肝癌患者。入选标准:病理证实或符合肝癌临床诊断标准的HCC患者且肿瘤直径>5 cm[9];肝功能 Child A或B级;ECOG(Eastern Cooperative Oncology Group)评分≤2分;无出血倾向、凝血功能正常或凝血功能障碍经治疗得以纠正者。排除标准:弥漫型肝癌;合并门脉主干至二级分支癌栓或肝静脉癌栓;合并活动性感染,尤其是胆管系统炎症等。
动脉造影采用改良Seldinger 法行股动脉穿刺,将5F或4F导管(RH,日本泰尔茂公司)插管至腹腔干或肠系膜上动脉造影(德国拜尔先灵制药有限公司),明确肿瘤大小、数目、分布以及供血情况。
超声引导下MWA根据CT、MRI或超声检查,再结合肝动脉造影确定拟消融病灶,消融主要针对肝内主瘤病灶。B超(型号IPC-1530,日本ALOKA公司)引导下把微波针直接插入肿瘤内并接近最远部,进针选择避开大血管、胆管、胆囊和肠管的最短路径,入路上至少应有1 cm 的正常肝实质,开启电源,MWA仪(型号ECO-100C,南京亿高医疗设备有限公司)采用水冷循环单极模式单极针,功率80~100 W,每个位点消融时间5~15 min,消融全程在B超监测下进行。前一位点消融结束后,根据消融情况退针2~4 cm至下一位点继续消融,两次消融区域要部分重合,上述过程重复进行,直至瘤体完全被强回声覆盖。患者一般局麻或全麻,根据肿瘤的大小及部位决定消融位点数和消融针用量,采用多灶重叠计算消融方案,一般采用双针多点消融,凝固时间足够,使坏死范围加大。消融结束后电凝状态下拔针,电凝穿刺道以防出血和穿刺道肿瘤种植。
再次肝动脉造影消融后再次行肝动脉造影,观察消融结果、残余病灶供血和肿瘤染色,有无造影剂外渗及肝动脉-静脉瘘等情况。针对残留病灶行TACE治疗,若出现造影剂外渗及肝动脉-静脉瘘等情况则进一步行相应的TACE治疗。
TACE治疗化疗药(奥沙利铂50~150 mg,表阿霉素20~50 mg)、超液化碘油(3 ~ 20 mL,法国Guerbet制药公司)及造影剂混合制成乳剂,通过微导管(商品名:Progreat,日本泰尔茂株式会社)注入残余肿瘤供血动脉,血流停滞时结束。对出现造影剂外渗、肝动脉-静脉瘘患者,给予合适粒径的明胶海绵颗粒/栓塞微球(商品名:Embosphere,美国Biosphere Medical公司)或微弹簧圈栓塞,如果胸廓内动脉、膈动脉等其他血管参与肿瘤供血,则同时进行栓塞。
术后处理和随访术后24 h监测生命体征,同时进行保肝、碱化尿液、预防感染、营养支持和止痛等治疗。术后3~7天复查肝肾功能、电解质、血常规以及平扫 CT,对消融程度和并发症进行评估。术后6~7周,患者复查肝功能、肿瘤标志物(AFP、CEA、CA199等)以及增强 CT 或MRI。按照改良的实体瘤疗效评价标准(modified response evaluation criteria in solid tumor,m-RECIST)[10]评估疗效,若术后复查未出现肿瘤复发或肝内转移,则患者每7~9周随访复查;若随访期间发现肿瘤坏死不完全或复发,则根据患者具体情况再次行 TACE、MWA或 MWA 联合 TACE。
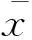
结 果
患者一般资料共有65例(男55例,女10例)患者纳入本次研究分析,其中AFP≥400 ng/mL42例,Child-Pugh A级52例,B级13例,肿瘤直径≥10 cm 23例,术前合并动静脉瘘4例。患者一般资料见表1。

表1 65例患者的临床基本资料
术中并发症所有患者均能耐受手术,TACE碘油栓塞剂用量平均为8 mL(3~20 mL),栓塞后肿瘤染色基本消失(图1)。消融后动脉造影见造影剂外渗3例,给予明胶海绵和微弹簧圈栓塞后均消失(图2),消融后新增动静脉瘘5例,给予明胶海绵栓塞后均消失。
术后并发症术后3天内21例患者出现轻至中度上腹部疼痛,经对症处理后缓解。部分患者出现不同程度的发热、恶心、呕吐和便秘,经药物对症治疗后缓解。2例患者消融穿刺点附近出现瘀斑,未经特殊处理后痊愈。
术后3~7天复查CT,9例患者出现少量胸腔积液,均无呼吸困难等临床症状,经短时间观察后积液均消失。65例患者术前丙氨酸氨基转移酶(alanine aminotransferase,ALT)和天门冬氨酸氨基转移酶(aspartate aminotransferase,AST)分别为(42.3±7.6)U/L和(67.3± 6.3)U/L,术后3天复查肝功能示ALT和AST为(150.3±82.0)U/L和135.3± 25.4)U/L,较术前均有明显升高(P值分别为0.009和0.075);同步治疗1个月后 ALT、AST 分别降至(32.3±4.2)U/L和(60.9 ±3.9)U/L(P值分别为0.074和0.092),差异无统计学意义。介入治疗前后白蛋白和胆红素差异无统计学意义(P值分别为0.42和0.36)。术后患者均未出现胆道损伤、周围脏器损伤和肝肾功能衰竭等严重并发症。
A:Contrast-enhanced CT showed a large lesion (15.2 cm×11.0 cm) in the right lobe of liver (arrow).B:Typical tumor staining was clearly demonstrated by angiography.C:Two 14-G antennas were showed under fluoroscopy in the MWA procedure.D:After MWA-TACE procedure,a selective angiography demonstrated complete devascularization of the targeted lesion.E:Axial contrast-enhanced CT scan obtained 3 month after combined treatment revealed tumor necrosis (arrow) and lipiodol deposition (star) in the location of original tumor.
图134岁女性因肝右叶巨块型肝癌行 DSA-B超引导下 MWA 同步联合 TACE 治疗
Fig 1MWA combined with TACE was performed on a 34-year-old female with large-size HCC in the right lobe of liver
临床疗效及生存期术前42例 AFP 升高患者中,手术1月后34例(81%)降至正常,7例有所下降(17%),1例增高。根据 m-RECIST 标准,完全缓解(complete response,CR)患者15例,部分缓解(partial response,PR) 46例,病情进展(progress disease,PD) 4例,有效率为93.8%。本研究随访截止日期为2015年7月23日,随访时间2~21个月(中位随访时间13个月),65例患者存活52例,死亡13例,其中4例死于肝内弥漫转移和复发,2例死于肝肿瘤破裂,3例死于肝肾功能衰竭,2例死于恶病质,1例死于全身转移,1例死于脑梗死。患者总体无疾病进展生存时间为(6.3±1.5)个月,中位生存时间为14个月,6、12及18个月的生存率分别为91.3%、81.5%及48%,2例患者经同步治疗后获得II期手术切除,至随访截止日期未见肿瘤复发。
A:Contrast-enhanced CT showed a large lesion in the right lobe of liver.B:Angiography after MWA showed contrast agent extravasation (arrow).C:No abnormal tumor staining was displayed after TACE with gelatin sponge particles.
图265岁女性因肝右叶巨块型肝癌(直径约10 cm)行DSA-B超引导下MWA同步联合TACE治疗
Fig 2MWA combined with TACE was performed on a 65-year-old female with large-size HCC in the right lobe of liver (diameter≈10 cm)
讨 论
对于大肝癌和巨块型肝癌,单纯 TACE 常难以使肿瘤完全坏死,疗效有限,患者易出现肝内和远处转移,长期疗效并不理想[11-12]。近年来热消融成为除 TACE 治疗外最常用的非手术治疗方法,在小肝癌治疗方面疗效确切,可作为与外科手术、肝移植等同的根治性治疗。与射频消融相比,MWA具有导入能量更大、升温效率更高、消融范围相对更大、相同消融范围所需时间更短、肿瘤局部灭活更彻底等优点,尤其对大肝癌和巨块型肝癌,MWA治疗具有更强的可操作性,省时、多针多点可达到更大消融范围,使肿瘤灭活更彻底[13]。对于直径≤3 cm 的肝癌,MWA与射频消融都可以取得与手术切除相同的效果。对于直径较大的肿瘤,难以保证肿瘤边缘均达到坏死[14-16],易发生肿瘤残存和复发转移。对于大肝癌,MWA 完全消融率仅达69%~81.8%[17-18]。Liu等[19]报道直径>5 cm的病灶经MWA后局部复发率(40.9%)明显高于直径<5 cm的病灶(14%),两者的局部复发率比较差异有统计学意义(P=0.026)。
MWA序贯联合TACE治疗HCC已被临床证实疗效显著优于单一MWA治疗或TACE治疗[7,20-22]。目前临床上序贯联合为先行 TACE 治疗,2~4周后行单一MWA治疗,TACE可使肿瘤组织血供减少,降低消融时热量流失,扩大肿瘤MWA的坏死范围,但由于TACE与MWA之间有较长的间隔时间,TACE治疗后可能出现侧支形成、血管再通等情况,因此序贯联合不是严格意义上的即时性联合治疗,没有充分发挥TACE与MWA之间的协同治疗作用,并且单一MWA后缺乏DSA造影,不能及时发现并处理消融后出血等可能危及患者生命的严重并发症。
本研究采用MWA同步联合TACE的方式治疗大肝癌(直径>5 cm)和巨块型肝癌(直径>10 cm),即先行MWA治疗再行TACE治疗,取得了良好的临床疗效。同步治疗先行MWA灭活大部分肿瘤,明显减少了TACE时碘油和颗粒型栓塞剂及化疗药物的用量,减轻不良反应和肝肾功能的损伤,MWA后行同步TACE所做得DSA造影,可进一步明确消融后残留肿瘤染色情况,靶向超选择性插管于残留肿瘤的供养动脉分支行进一步治疗。本组65例患者经MWA治疗后 TACE 碘油栓塞剂用量均在20 mL以内(平均 8 mL),碘油剂用量较单一 TACE 治疗明显减少。65例患者有效率(CR+PR)为93.8%。患者总体无疾病进展生存时间为(6.3±1.5)个月,中位生存期14个月,6、12及18个月的生存率分别为91.3%、81.5%及48%,其中2例患者经同步治疗后获得II期手术切除机会,至随访截止日期未见肿瘤复发。另外,MWA同步联合TACE治疗能及时发现并处理消融后可能出现的并发症(如出血、动静脉瘘等),本组65例患者消融后3例出现造影剂外渗,5例出现动静脉瘘,给予即时栓塞后均消失。因此,MWA同步联合TACE治疗能有效灭活肿瘤组织,减少TACE治疗时栓塞剂和药物的使用,减少因大剂量化疗带来的并发症,患者获益明显。与单一TACE或MWA治疗相比,MWA同步TACE治疗可以有效地控制疾病进展、延长患者生存时间[23-25]。
本组病例同步治疗后未见皮肤烧伤、出血、肝肾功能衰竭等严重并发症,术中消融后肝动脉造影见造影剂外渗3例,可能与术中穿刺有关,给予即时栓塞后消失。术后大多数患者只有轻度肝功能受损、栓塞后综合征(低热、疼痛、呃逆等)、便秘及少量的胸腔积液,经保肝、对症药物治疗后均缓解。相比单一TACE或MWA,联合治疗的凝固性坏死更加彻底,肝继发性脓肿的发生率更低。本组研究结果显示MWA同步联合TACE是安全的,与单一MWA或TACE比较并未明显增加不良反应及严重并发症的发生率,与Liang等[26]和Ding等[27]的报道相仿。
综上所述,MWA同步联合TACE治疗大肝癌和巨块型肝癌安全、有效,与单一治疗相比并未增加其他不良反应及严重并发症;同步治疗可扩大 MWA 的适应证,及时处理 MWA 所致的并发症(如出血、动静脉瘘),提高肿瘤坏死率、延长患者总体生存期。本研究的不足之处:(1) 本研究为回顾性研究,随访时间短,不足以准确地评价长期疗效;(2) 样本量较小,可能对研究结果有一定影响。将来拟纳入较多的样本行多中心随机对照研究。
[1]SIEGEL RL,MILLER KD,JEMAL A.Cancer statistics,2015[J].CA Cancer J Clin,2015,65(1):5-29.
[2]ZHANG L,YIN X,GAN YH,et al.Radiofrequency ablation following first-line transarterial chemoembolization for patients with unresectable hepatocellular carcinoma beyond the Milan criteria[J].BMC Gastroenterol,2014,14(1):11-17.
[3]MABED M,ESMAEEL M,EL-KHODARY T,et al.A randomized controlled trial of transcatheter arterial chemoembolization with lipiodol,doxorubicin and cisplatin versus intravenous doxorubicin for patients with unresectable hepatocellular carcinoma[J].Eur J Cancer Care (Engl),2009,18(5):492-499.
[4]LLOVET JM,REAL MI,MONTANA X,et al.Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma:a randomised controlled trial[J].Lancet,2002,359(9319):1734-1739.
[5]KOO JE,KIM JH,LIM YS,et al.Combination of transarterial chemoembolization and three-dimensional conformal radiotherapy for hepatocellular carcinoma with inferior vena cava tumor thrombus[J].Int J Radiat Oncol Biol Phys,2010,78(1):180-187.
[6]SHIOMI H,NAKA S,SATO K,et al.Thoracoscopy-assisted magnetic resonance guided microwave coagulation therapy for hepatic tumors[J].Am J Surg,2008,195(6):854-860.
[7]LIU C,LIANG P,LIU FY,et al.MWA Combined with TACE as a combined therapy for unresectable large-sized hepotocellular carcinoma[J].Int J Hyperthermia,2011,27(7):654-662.
[8]XU LF,SUN HL,CHEN YT,et al.Large primary hepatocellular carcinoma:transarterial chemoembolization monotherapy versus combined transarterial chemoembolization-percutaneous microwave coagulation therapy[J].J Gastroenterol Hepatol,2013,28(3):456-463.
[9]BRUIX J,SHERMAN M,AMERICAN ASSOCIATION FOR THE STUDY OF LIVER D.Management of hepatocellular carcinoma:an update[J].Hepatology,2011,53(3):1020-1022.
[10]LENCIONI R,LLOVET JM.Modified RECIST (mRECIST) assessment for hepatocellular carcinoma[J].Semin Liver Dis,2010,30(1):52-60.
[11]TAKAYASU K,ARII S,KUDO M,et al.Superselective transarterial chemoembolization for hepatocellular carcinoma.Validation of treatment algorithm proposed by Japanese guidelines[J].J Hepatol,2012,56(4):886-892.
[12]TERZI E,PISCAGLIA F,FORLANI L,et al.TACE performed in patients with a single nodule of hepatocellular carcinoma[J].BMC Cancer,2014,14(1):601-614.
[13]LENCIONI R,CROCETTI L.Local-regional treatment of hepatocellular carcinoma[J].Radiology,2012,262(1):43-58.
[14]GOLDBERG SN,GRASSI CJ,CARDELLA JF,et al.Image-guided tumor ablation:standardization of terminology and reporting criteria [J].J Vasc Int Radiol,2009,20(7 Suppl):S377-S390.
[15]QIAN GJ,WANG N,SHEN Q,et al.Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma:experimental and clinical studies[J].Eur Radiol,2012,22(9):1983-1990.
[16]ABDELAZIZ A,ELBAZ T,SHOUSHA HI,et al.Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma:an Egyptian multidisciplinary clinic experience[J].Surg Endosc,2014,28(12):3429-3434.
[17]JIAO DC,ZHOU Q,HAN XW,et al.Microwave ablation treatment of liver cancer with a 2,450-MHz cooled-shaft antenna:pilot study on safety and efficacy[J].Asian Pac J Cancer Prev,2012,13(2):737-742.
[18]POGGI G,MONTAGNA B,DI CESARE P,et al.Microwave ablation of hepatocellular carcinoma using a new percutaneous device:preliminary results[J].Anticancer Res,2013,33(3):1221-1227.
[19]LIU Y,ZHENG Y,LI S,et al.Percutaneous microwave ablation of larger hepatocellular carcinoma[J].Clin Radiol,2013,68(1):21-26.
[20]SEKI T,TAMAI T,NAKAGAWA T,et al.Combination therapy with transcatheter arterial chemoembolization and percutaneous microwave coagulation therapy for hepatocellular carcinoma[J].Cancer,2000,89(6):1245-1251.
[21]陈刚,唐晓军,李宏波,等.肝动脉化疗栓塞联合经皮微波消融治疗中晚期肝癌的疗效评价[J].临床放射学杂志,2012,31(5):710-713.
[22]GU L,LIU H,FAN L,et al.Treatment outcomes of transcatheter arterial chemoembolization combined with local ablative therapy versus monotherapy in hepatocellular carcinoma:a meta-analysis[J].J Cancer Res Clin Oncol,2014,140(2):199-210.
[23]NI JY,SUN HL,CHEN YT,et al.Prognostic factors for survival after transarterial chemoembolization combined with microwave ablation for hepatocellular carcinoma[J].World J Gastroenterol,2014,20(46):17483-17490.
[24]YIN XY,XIE XY,LU MD,et al.Percutaneous Thermal ablation of medium and large hepatocellular carcinoma long-term outcome and prognostic factors[J].Cancer,2009,115(9):1914-1923.
[25]FORNER A,REAL MI,VARELA M,et al.Transarterial chemoembolization for patients with hepatocellular carcinoma[J].Hepatol Res,2007,37(1):S230-S237.
[26]LIANG P,WANG Y,YU XL,et al.Malignant liver tumors:treatment with percutaneous microwave ablation-complications among cohort of 1136 Patients[J].Radiology,2009,251(3):933-940.
[27]DING J,JING X,LIU J,et al.Complications of thermal ablation of hepatic tumours:Comparison of radiofrequency and microwave ablative techniques[J].Clin Radiol,2013,68(6):608-615.
E-mail:wang.jianhua@zs-hospital.sh.cn
Microwave ablation combined with simultaneous transarterial chemoembolization for the treatment of large and massive hepatocellular carcinoma
SI Zeng-mei1,2, QIAN Sheng1,2, LIU Rong1,2, QU Xu-dong1,2, GONG Gao-quan1,2, WANG Xiao-lin1,2, YAN Zhi-ping1,2, WANG Jian-hua1,2△
(1Shanghai Institute of Medical Imaging,Shanghai 200032,China;2Department of Interventional Radiology, Zhongshan Hospital,Fudan University,Shanghai 200032,China)
ObjectiveTo assess the safety and efficacy of microwave ablation (MWA) combined with simultaneous transarterial chemoembolization (TACE) for large (diameter>5 cm) and massive (diameter>10 cm) hepatocellular carcinoma (HCC).MethodsWe reviewed the records of 65 patients with large or massive HCC treated with ultrasound-guided percutaneous MWA with simultaneous TACE between March 2013 and December 2014.The treatment procedure consisted of:initiative digital subtraction angiography (DSA),followed percutaneous MWA under ultrasound and subsequential angiography plus TACE.ResultsThe combination therapy was well tolerated in all patients with transitory hepatic and hematological toxicity and asymptomatic pleural effusion.Contrast agent extravasation was detected in 3 patients and dealt with embolization in time.There was no liver abscess,bile duct injury and other procedure related major complications.The average dosages of lipiodol were 8 mL.During the follow-up period (range 2-21 months),the mean progression-free survival time was (6.3±1.5) months.The median survival time was 14 months.The 6-,12- and 18-month overall survival rates were 91.3%,81.5%and 48%,respectively.Two patients underwent Ⅱ-stage surgical resection after immediate combination therapy.ConclusionsMWA combined simultaneous TACE therapy can be performed safely and effectively in patients without major complications for large and massive HCC.It enlarged the indications of MWA ,and was worth to be promoted.
hepatocellular carcinoma;transarterial chemoembolization;microwave ablation;simultaneous combination therapy;therapeutic evaluation
R735.7
Adoi: 10.3969/j.issn.1672-8467.2016.05.009
2016-01-05;编辑:段佳)

