Enterogenous infection of Candida albicans in immunocompromised rats under severe acute pancreatitis
Xiang-wang Zhao, Lei Yan, Dan Xu, Yu-hui Cui, Chun-hui Yang, Yan-jun Zhou, Jian-guo Tang
1Department of Intensive Care Unit, Shandong Cancer Hospital, Shandong University, Jinan 250117, China
2Department of Emergency Medicine, Zhongshan Hospital, Fudan University, Shanghai 200032, China
3Department of Trauma-Emergency & Critical Care Medicine, Shanghai Fifth People's Hospital, Fudan University, Shanghai 200240, China
4Division of Swine Infectious Diseases, Shanghai Veterinary Research Institute, CAAS, Shanghai 200240, China
Corresponding Author: Jian-guo Tang, Email: tangjianguo@5thhospital.com
Enterogenous infection of Candida albicans in immunocompromised rats under severe acute pancreatitis
Xiang-wang Zhao1, Lei Yan2, Dan Xu3, Yu-hui Cui3, Chun-hui Yang3, Yan-jun Zhou4, Jian-guo Tang3
1Department of Intensive Care Unit, Shandong Cancer Hospital, Shandong University, Jinan 250117, China
2Department of Emergency Medicine, Zhongshan Hospital, Fudan University, Shanghai 200032, China
3Department of Trauma-Emergency & Critical Care Medicine, Shanghai Fifth People's Hospital, Fudan University, Shanghai 200240, China
4Division of Swine Infectious Diseases, Shanghai Veterinary Research Institute, CAAS, Shanghai 200240, China
Corresponding Author: Jian-guo Tang, Email: tangjianguo@5thhospital.com
BACKGROUND: Opportunistic infection of Candida albicans (C. albicans) has become a serious problem in immunocompromised patients. The study aimed to explore the mechanism of enterogenous infection of C. albicans in immunocompromised rats under severe acute pancreatitis (SAP).
METHODS: Sprague Dawley (SD) rats (n=100) were randomly assigned into 5 groups as the following: blank group, cyclophosphamide+ceftriaxone+SAP group, cyclophosphamide+ceftriaxone group, cyclophosphamide+SAP group, and cyclophosphamide group. The rats were sacrifi ced at 5 and 10 days, and their jejunum, colon, mesenteric lymph nodes, pancreas, intestinal content, and blood were quickly collected to detect C. albicans. A region of the 25S rRNA gene was chosen and amplifi ed by polymerase chain reaction (PCR) to differentiate C. albicans genotypes. The amplified products were further sequenced and compared to judge their homology.
RESULTS: Compared with the Cyclophosphamide group, the combination of immunosuppressants and broad-spectrum antibiotics significantly increased the colonization of C. albicans in intestine in 5 and 10 days. Pure SAP stress did not increase the opportunistic infection of C. albicans. The PCR products of C. albicans isolates all belonged to the genotype A family, and sequence alignment showed that the amplified fragments were homologous.
CONCLUSION: The damage of immune system and broad-spectrum antimicrobial agents are important risk factors for opportunistic fungal infection. Intestinal tract is an important source for genotype-A C. albicans to translocate and invade into bloodstream.
Candida albicans; Immunosuppression; Severe acute pancreatitis; Genotype
World J Emerg Med 2016;7(4):294–299
INTRODUCTION
The increasing incidence of acquired immunodeficiency syndrome (AIDS) and the recent development of new and more aggressive treatment procedures for patients with malignancies and organ transplants have resulted in an increase in the number of immunocompromised patients with fungal infections.[1,2]Among the Candida species, Candida albicans (C. albicans) is still considered as the most important fungal pathogen.[1]Compared with patients discharged without a diagnosis of invasive fungal infection (IFI), those diagnosed with IFI are associated with a signifi cantly longer hospital stay and higher mean cost.[3]In the United States, approximately $1.89 billion in annual hospital costs may be attributable to IFIs.[3]
It is generally believed that the main reservoir for C. albicans in humans is the gastrointestinal tract (GI) and that systemic infections predominantly originate from this reservoir.[4,5]Under normal conditions when the intestinal mucosal barrier is intact, and the host's innate immune system is functioning, C. albicans behaves like commensal members of the gastrointestinal microflora. There is a homeostasis between C. albicans and the host.[6]However,when the balance is disrupted, the yeast can break through the intestinal mucosal barrier and cause invasive candidiasis and candidemia.[6]Under critical illness such as severe acute pancreatitis (SAP), the structure and function of intestinal mucosa would be damaged, leading to gut barrier dysfunction.[7,8]
Although recent microbiological studies have been focused on the pathogenic role of intestinal bacterial sepsis, the presence and activity of fungi have not been extensively explored, and what would happen in immunocompromised patients under SAP has puzzled researchers for years. In this paper, we tried to explain the phenomenon of enterogenous infection of C. albicans in immunocompromised rats under SAP.
METHODS
Animals
The experimental protocol and procedures used in this study were in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Experimental Animal Ethics Committee of Fudan University. A total of 100 SD rats (body weight, 170–220 g) were collectively housed in cages in the experimental room for at least 3 days before the start of experiments.
Experimental design
The rats were randomly assigned into 5 treatment groups with 20 in each group. The rats of each group were further divided into 2 subgroups with 10 in each subgroup according to different experiment time (5 or 10 days). The four groups were as follows:
1) Blank (B): Rats in this group didn't receive any treatment during the experiment.
2) Cyclophosphamide+ceftriaxone+SAP (CCS): Rats in this group all received intraperitoneal (IP) injection of cyclophosphamide (200 mg/kg) on the 1st day of experiment. Meanwhile, they were injected with ceftriaxone (100 mg/kg) through the tail vein once a day during the whole experiment. On the 4th day, rats in the 5-day subgroup received IP injection of L-arginine (2.0 g/kg) twice to establish SAP model, which was established on the 9th day in the 10-day subgroup.
3) Cyclophosphamide+ceftriaxone (CC): Rats in this group all received IP injection of cyclophosphamide (200 mg/kg) on the 1st day of the experiment. Meanwhile, they were injected with ceftriaxone (100 mg/kg) through the tail vein once a day during the whole experiment.
4) Cyclophosphamide+SAP (CS): Rats in this group all received IP injections of cyclophosphamide (200 mg/kg) on the 1st day of the experiment and intravenous saline once a day during the whole experiment. On the 4th day, rats in the 5-day subgroup received IP injection of L-arginine (2.0 g/kg) twice to establish SAP model, which was established on the 9th day in the 10-day subgroup.
5) Cyclophosphamide (C): Rats in this group all received IP injections of cyclophosphamide (200 mg/kg) on the 1st day of experiment and intravenous saline once a day during the whole experiment.
We observed the behavioral changes of rats in each treatment group after the experiment. Each change would be recorded, such as appetite, weight, excrement, hair coat and emotion.
Histologic changes of intestinal mucosa and pancreas
After the animals were sacrificed by cervical dislocation, the specimens of pancreas and gut were fi xed in formalin solution and embedded in paraffi n. Sections were routinely chipped and stained with hematoxylin and eosin (HE) for morphologic analysis by light microscopy.
Detecting the presence of C. albicans in multiple organs
As soon as the experimental rats were sacrificed, the blood was collected by cardiac puncture. Then, the jejunum, colon, mesenteric lymph nodes, pancreas and intestinal content were quickly collected. The intestinal tissues were cleaned with PBS to minimize surface contamination from organisms present in the lumen. The homogenized tissues and blood were plated into Sabouraud dextrose agar (SDA). The proliferation of C. albicans was observed after 48-hour incubation at 37 °C.
Molecular analysis of C. albicans isolates
The isolates were identified as C. albicans on the basis of their cultural and morphological characteristics on SDA and colony color on CHROM agar Candida.
DNAs were extracted using the Fungal DNA Kit (OMEGA) according to the manufacturer's instructions.
The primer pairs whose sequences span the site of the transposable intron in the 25S rDNA were as described by McCullough.[9]The primer sequences were as follows: CA-INT-L, 5'-ATAAGGGAAGTCGGCAAAA TAGATCCGTAA-3' and CA-INT-R, 5'-CCTTGGCTGTG GTTTCGCTAGATAGTAGAT-3'. The reactions contained 1 L of each primer (100 pM), 2 L DNA template, 5.0 L 10×Ex Taq buffer, 4 L dNTP mixture, 0.5 L ExTaq DNA polymerase (TaKaRa, Japan), and the total volume was adjusted to 50 L with H2O. The PCR conditions were as follows: denaturation at 94 °C for 5 minutes, 30 cycles of94 °C for 30 seconds, 60 °C for 30 seconds, and 72 °C for 1 minute, and a final extension at 72 °C for 10 minutes. All reaction products were characterized by electrophoresis on 1.5% agarose gels in 1×TAE (Tris-acetate-EDTA) at 140 V for 30 minutes and stained with ethidium bromide for visualization using an ultraviolet transilluminator.
The target fragments in the gels were extracted with a Gel Extraction Kit (OMEGA, USA), cloned into pMD18-T vector (TaKaRa, Japan), and then sequenced using a 3730 XL DNA analyzer (Applied Biosystems, USA) at Life Technologies (Shanghai, China). The sequence analysis and multiple alignments were performed using DNAStar Software.
Statistical methods
All data were compared with Fisher's exact test. Differences were considered to be statistically signifi cant if the P value was less than 0.01. All analysis was performed with Stata 10 software.
RESULTS
The behavioral changes of rats in each treatment group
The rats in group C showed no remarkable changes after the experiment. However, the animals in other groups changed sharply as follows: 1) they ate and drank less, resulting in weight loss; 2) their excrement increased and became loose; 3) their hair coat shed and tarnished; and 4) they became agitated and irritable.
Presence of C. albicans in multiple tissues
The culture results have been summarized in Table 1. Regardless the experiment time (5 or 10 days), the combination of immunosuppressants and broad-spectrum antibiotics significantly increased C. albicans colonization in intestine than single use of cyclophosphamide (P<0.01) (Table 1). However, no significant difference was observed between the groups C and CS (P>0.01). There were no statistical differences in positive rate of C. albicans from intestinal content between the 10-day subgroup and the 5-day one in each treatment group (P>0.01) (Table 1).
In the 5-day subgroup, rats from the group CC seemed to be more apt to suffer Candidemia than those in group C (P=0.011). With the extension of experiment days, this statistic difference disappeared, and there was no significant difference between the groups CC and C. It seemed that SAP stress or the combination of SAP and ceftriaxone did not increase the risk of Candidemia compared with group C. As for the culture result of jejunum and colon, the positive rates in groups CCS, CS, and CC seemingly increased in comparison with group C, though there was no statistic difference between these groups with Fisher's exact test (Table 1).
Histologic changes of intestinal mucosa and pancreas
There were no significant pathological changes in pancreatic tissues from groups C and CC. However, edema, acinar and fat necrosis, hemorrhage, as well as monocyte and neutrophil infiltration were observed in the pancreas of all rats from groups CS and CCS (Figure 1). The intestinal mucosa of rats without SAP showed normal villous architecture and glands, with no hemorrhage (Figure 2). Significant pathological changes in the intestinal tissues were observed in SAP models, presenting with mucosal and submucosal swelling, congestion, and inflammatory cell infiltration, shedding of some villi, and mucosal erosion (Figure 2).
Genotyping of C. albicans isolates
Among the 600 samples, C. albicans could be isolated from 104 samples (Table 1). We made the gene analysis of all C. albicans strains isolated from multiple tissues. The PCR products of representative genotypes of C. albicans were exemplifi ed in Figure 3. The PCR products appeared as a single band at 450 bp, defi ning the genotype A.
Analysis and comparison of genomic sequences

Figure 1. Pancreatic histology in the blank group and SAP groups (HE staining; original magnification×100). A: normal pancreatic histology of rats in the blank group; B: destruction of acinar structures, with visible scattered bleeding spots, saponifi cation spots, and inflammatory infiltrates after SAP induction.
The sequences spanning the site of the transposable group I intron of the 25S rRNA gene were chosen for alignment. By amplifying and comparing the sequence of 25S rRNA gene of C. albicans isolated from each cultured tissue (blood, mesenteric lymph nodes, pancreas, intestinal content, jejunum, and colon), we found that these sequences were almost identical (Table 2), demonstrating that the intron was partially present throughout their genomes.
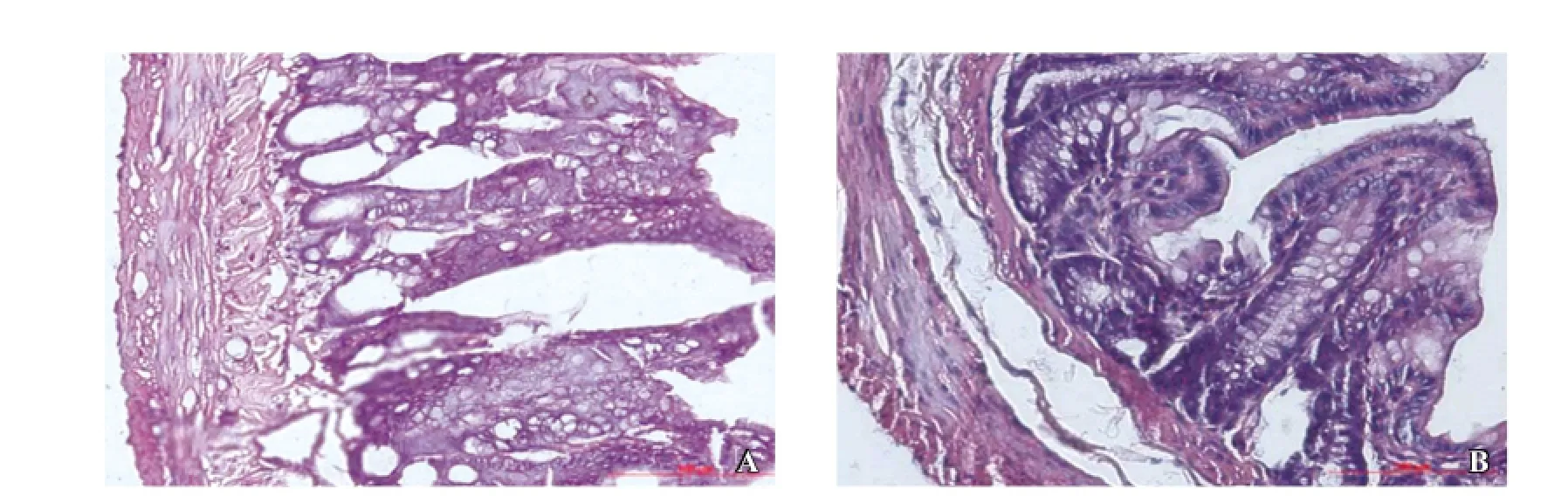
Figure 2. Intestinal histology in the blank group and SAP groups (HE staining; original magnification×100). A: normal intestinal histology of rats in the blank group; B: mucosal and submucosal swelling, congestion, inflammatory cells infiltration, shedding of some villi, mucosal erosion in SAP group.
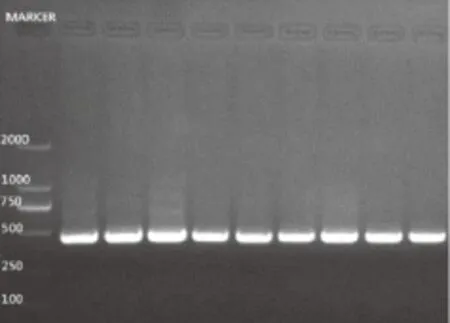
Figure 3. Genotype of C. albicans isolates from multiple tissues. All lanes were genotype A (450 bp).
DISCUSSION
C. albicans is a ubiquitous commensal organism and has been considered as a major pathogen for immunocompetent as well as immunocompromised patients. Excessive use of antifungal agents has been implicated in the emergence of antifungal resistance in C. albicans and constitutes a serious clinical problem in hospitals by affecting the natural balance of the intestinal microfl ora in patients.[10]
In this study, obvious C. albicans colonization and proliferation were observed in intestine and circulation of rats whose immune system was damaged by cyclophosphamide and intravenously continuously injected with ceftriaxone for 5 days. Previous studies have discovered that broadspectrum antimicrobial agents customarily used for the treatment of bacterial infection in patients and animals substantially alter the bacterial flora of the gut, and thus facilitate the uninhibited growth of yeasts.[11]Among these agents, ceftriaxone is excreted in the bile, resulting in high intestinal concentrations, and can significantly increase the alimentary tract colonization by yeasts in experimental animals and human.[12]Antineoplastic agents causing neutropenia and mucositis, such as cyclophosphamide, damage the natural host defenses and predispose the patients to disseminated fungal infection.[12]Our study found that the rate of optimistic infection of C. albicans did not decrease with the extension of ceftriaxone exposure time, implying that over use of broad-spectrum antimicrobial agents was an important risk factor of optimistic infection of C. albicans, and this harm could not be reduced with the prolonging of time.
Studies in both animal models and humans have documented that gut permeability is increased shortly after the onset of pancreatitis, and clinically the magnitude ofgut barrier failure has been shown to correlate with the development of sepsis, multiple organ failure syndrome, and an increased risk of death.[13]In SAP, waterfall-style release of inflammatory factors such as TNF-α led to ischemiareperfusion injury of gut mucosa which resulted in serious oxidative stress and activation of caspase-3 pathway and severe apoptosis of gut mucosa.[8]However, in our study, although SAP could have a destructive effect on the intestinal mucosa barrier as shown in the pathological images (Figure 2), the rate of opportunistic colonization and blood stream dissemination of fungi in groups CCS and CS did not increase compared with group CC (P>0.01). Unlike bacteria, which were thought to be the main cause of superinfection leading to pancreatic necrosis that peaks during the first 4 days following the onset of symptoms, C. albicans could not break through intestinal mucosa barrier and invade into distant organs as free as bacteria after SAP, suggesting that inherent intestinal mucosa still keeps its barrier function against the yeast in some ways.[14,15]Hence, when clinicians are faced with infection after SAP, pathogenic bacteria, not fungi, should be taken into fi rst account.
Molecular typing of an infectious agent is important for epidemiological studies and for the development of appropriate infection control strategies. Different methods have been developed to differentiate isolates of C. albicans and to determine the relationships between the genotypes and the diseases.[16,17]McCullough developed a PCR-based method using a primer pair designed to span the region covering the site of the transposable group I intron of the 25S rRNA gene (rDNA).[9]This method has been shown to be able to classify C. albicans strains into 5 genotypes based on the length of the amplified PCR product as genotype A (450 bp), B (840 bp), C (450 and 840 bp), D (1 080 bp), and E (1 400 bp).[9]The classifi cation of genotypes using this form of PCR relies on the presence of group I introns of varying sizes in the 25S rRNA. The method detecting the presence and the size of the intron in the 25S rDNA is particularly easily adapted for use in reference laboratories for the rapid identification of large numbers of isolates.[16]In this study, a PCR primer pair designed to span the 25S rRNA gene was employed to genotype C. albicans isolated from various anatomical sites of infected rats. The results showed that the PCR products appeared as a single band at 450 bp, which meets the criteria of genotype A and in accordance with the researches of Adachi.[18–20]Previous studies have shown that C. albicans of genotype A are more prone to invade the blood stream and more virulent than the other genotypes.[19]Meanwhile, C. albicans of genotypic subgroup A were the dominant strains in the biofilm and more resistant to the antifungal agent flucytosine.[9,21]The prevalence of genotypes B and C was higher in non-invasive C. albicans strains. In our research, the PCR products of C. albicans isolated from multiple tissues were a single band of 450 bp, suggesting that C. albicans of genotype A were more likely to proliferate and invade into important organs in the immunocompromised rat model. Meanwhile, it also indicated that genotype A may have a greater tendency to be transmitted between rats in the same environment.[22]The present study confirmed the invasiveness of C. albicans of genotype A and indicated that C. albicans originated from intestine tract would be more resistant to the antifungal agent flucytosine. When antifungal agents are used for the treatment of C. albicans infection in such patients, the antifungal effect should be evaluated, and the dose should be optimized.
Researchers commented that the presence or absence of transpsable group I intron in 25S rDNA gene would be important in the detection of invasiveness of C. albicans.[19]The aim of this study was to determine the correlation between C. albicans isolated from multipletissues of infected rats. By amplifying and comparing the sequence of PCR products of C. albicans isolated from each cultured tissue (blood, mesenteric lymph nodes, pancreas, intestinal content, jejunum, and colon), we found that the amplified gene segments were almost same regardless the anatomical sites of isolation, suggesting that these C. albicans might be originated from the same strain or translocated from the same infected source. Therefore, GI remains to be an important source for disseminated infections of C. albicans in immunocompromised rats.

Table 2. Sequence alignment of the PCR products
In conclusion, GI is an important source for C. albicans of genotype A to break through the intestinal mucosa barrier and translocate to other organs. The damage of immune system and broad-spectrum antimicrobial agents are important risk factors for opportunistic fungal infection. Clinically significant C. albicans infection could not be induced by pure stress factors like SAP. To understand the mechanisms of C. albicans-bacterial interactions in the circulation and exploit it for therapeutic strategies, further studies using in vitro or animal model systems as well as large-scale, multicentre clinical data are needed.
Funding: This work was supported by grants from the Research Foundation of Shanghai Minhang District Municipal Commission of Health and Family Planning (2013MW12) and from the Research Foundation of Shanghai Municipal Commission of Health and Family Planning (201540136). We express sincere thanks to Professor Guangzhi Tong for technical assistance.
Ethical approval: All applicable international, national, and/ or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Conflicts of interest: All authors have seen and approved the content of “Instructions to Authors“ and the submission version of the paper. All authors declare that they have no confl ict of interest.
Contributors: Zhao XW conceived the study. Lei Y analyzed all data and wrote the fi nal version of the manuscript. Dan X, Cui YH and Yang CH assisted in animal experiment. Zhou YJ participated in study design and coordination. Tang JG helped to draft the manuscript. Zhao XW and Yan L contributed equally to this paper.
近日,针对俞敏洪有关女性的错误言论,全国妇联、北京市妇联等均发声予以批驳。11月20日,俞敏洪专程来到全国妇联机关,向广大女同胞诚恳道歉。
REFERENCES
1 Yang ZT, Wu L, Liu XY, Zhou M, Li J, Wu JY, et al. Epidemiology, species distribution and outcome of nosocomial Candida spp. bloodstream infection in Shanghai. BMC Infect Dis 2014; 14: 241.
2 Yapar N. Epidemiology and risk factors for invasive candidiasis. Ther Clin Risk Manag 2014; 10: 95–105.
3 Menzin J, Meyers JL, Friedman M, Perfect JR, Langston AA, Danna RP, et al. Mortality, length of hospitalization, and costs associated with invasive fungal infections in high-risk patients. Am J Health Syst Pharm 2009; 66:1711–1717.
4 Nucci M, Anaissie E. Revisiting the source of candidemia: skin or gut? Clin Infect Dis 2001; 33: 1959–1967.
5 Miranda LN, van der Heijden IM, Costa SF, Sousa AP, Sienra RA, Gobara S, et al. Candida colonisation as a source for candidaemia. J Hosp Infect 2009; 72: 9–16.
6 Schulze J, Sonnenborn U. Yeasts in the gut: from commensals to infectious agents. Dtsch Arztebl Int 2009; 106: 837–842.
7 Zhang J, Yuan C, Hua G, Tong R, Luo X, Ying Z. Early gut barrier dysfunction in patients with severe acute pancreatitis: attenuated by continuous blood purifi cation treatment. Int J Artif Organs 2010; 33: 706–715.
8 Tian R, Tan JT, Wang RL, Xie H, Qian YB, Yu KL. The role of intestinal mucosa oxidative stress in gut barrier dysfunction of severe acute pancreatitis. Eur Rev Med Pharmacol Sci 2013; 17: 349–355.
9 McCullough MJ, Clemons KV, Stevens DA. Molecular and phenotypic characterization of genotypic Candida albicans subgroups and comparison with Candida dubliniensis and Candida stellatoidea. J ClinMicrobiol 1999; 37: 417–421.
10 Yan L, Yang C, Tang J. Disruption of the intestinal mucosal barrier in Candida albicans infections. Microbiol Res 2013; 168: 389–395.
11 Chi HW, Yang YS, Shang ST, Chen KH, Yeh KM, Chang FY, et al. Candida albicans versus non-albicans bloodstream infections: the comparison of risk factors and outcome. J Microbiol Immunol Infect 2011; 44: 369–375.
12 Samonis G, Karyotakis NC, Anaissie EJ, Barbounakis E, Maraki S, Tselentis Y, et al. Effects of cyclophosphamide and ceftriaxone on gastrointestinal colonization of mice by Candida albicans. Antimicrob Agents Chemother 1996; 40: 2221–2223.
13 Fishman JE, Levy G, Alli V, Zheng X, Mole DJ, Deitch EA, et al. The intestinal mucus layer is a critical component of the gut barrier that is damaged during acute pancreatitis. Shock 2014; 42: 264–270.
14 Tarpila E, Nystrom PO, Franzen L, Ihse I. Bacterial translocation during acute pancreatitis in rats. Eur J Surg 1993; 159: 109–113.
15 Sun X, Shao Y, Jin Y, Huai J, Zhou Q, Huang Z, et al. Melatonin reduces bacterial translocation by preventing damage to the intestinal mucosa in an experimental severe acute pancreatitis rat model. Exp Ther Med 2013; 6: 1343–1349.
16 Gurbuz M, Kaleli I. Molecular analysis of Candida albicans isolates from clinical specimens. Mycopathologia 2010; 169: 261–267.
17 Tamura M, Watanabe K, Mikami Y, Yazawa K, Nishimura K. Molecular characterization of new clinical isolates of Candida albicans and C. dubliniensis in Japan: analysis reveals a new genotype of C. albicans with group I intron. J Clin Microbiol 2001; 39: 4309–4315.
18 Millar BC, Xu J, McMullan R, Walker MJ, Hedderwick S, Moore JE. Frequency and distribution of group I intron genotypes of Candida albicanscolonising critically ill patients. Br J Biomed Sci 2005; 62: 24–27.
19 Karahan ZC, Güriz H, Ağirbaşli H, Balaban N, Göçmen JS, Aysev D, et al. Genotype distribution of Candida albicans isolates by 25S intron analysis with regard to invasiveness. Mycoses 2004; 47: 465–469.
20 Adachi H, Shimizu K, Hattori H, Tanaka R, Chibana H, Takagi Y, et al. Genotyping of Candida albicans by fragment analysis of microsatellites combined with 25S rDNA and RPS-based strategies. Nihon Ishinkin Gakkai Zasshi 2009; 50: 167–174.
21 Yang XQ, Zhang Q, Lu LY, Yang R, Liu Y, Zou J. Genotypic distribution of Candida albicans in dental biofilm of Chinese children associated with severe early childhood caries. Arch Oral Biol 2012; 57: 1048–1053.
22 Hammarskjöld F, Mernelius S, Andersson RE, Berg S, Hanberger H, Löfgren S, et al. Possible transmission of Candida albicans on an intensive care unit: genotype and temporal cluster analyses. J Hosp Infect 2013; 85: 60–65.
Accepted after revision August 28, 2016
10.5847/wjem.j.1920–8642.2016.04.010
Original Article
April 6, 2016
 World journal of emergency medicine2016年4期
World journal of emergency medicine2016年4期
- World journal of emergency medicine的其它文章
- The state and future of emergency medicine in Macedonia
- Amiodaron in atrial fi brillation: post coronary artery bypass graft
- Short lessons in basic life support improve self-assurance in performing cardiopulmonary resuscitation
- Is current training in basic and advanced cardiac life support (BLS & ACLS) effective? A study of BLS & ACLS knowledge amongst healthcare professionals of North-Kerala
- Effects of a general practitioner cooperative co-located with an emergency department on patient throughput
- Acute Care/Trauma Surgeon's role in obstetrical/ gynecologic emergencies (The OBCAT Alert)
