The concentration game: differential effects of bioactive signaling in 2D and 3D culture
PERSPECTIVE
The concentration game: differential effects of bioactive signaling in 2D and 3D culture
Traumatic injuries to the central nervous system, such as traumatic brain injury, spinal cord injury and stroke, have a high prevalence, enormous financial costs and lack clinical treatments that restore neurological function (Ma et al., 2014). These injuries trigger a series of secondary biochemical and cellular responses that ultimately lead to cellular death and the maintenance of an unsupportive extracellular matrix (ECM) for tissue regeneration (Silva et al., 2014). Artificial ECM or scaffolds represent a way to alter this unsupportive environment to improve the efficacy of stem cell therapies and enhance neural tissue regeneration (Figure 1). Scaffold use could lead to greater improvements in neurological function (such as improved bladder control, increased dexterity and body control, etc.) than observed with the implantation of cells alone. To date, the inclusion of basic scaffolds with stem cell therapy treatments have shown increased efficacy in rodent models (Yasuda et al., 2010). More advanced scaffolds could better mimic the chemical, physical and mechanical properties of the ECM to promote cellular survival, adhesion, proliferation and differentiation. Altering the injured ECM to mitigate the barriers to axon invasion, myelination and cellular maturation further and lead to even greater gains in neurological function.
To expedite the development of such scaffolds, combinatorial methods, which have long been used in drug discover, are being applied to tissue engineering. Although many combinatorial methods have been developed (Nakajima et al., 2007; Smith Callahan et al., 2013), few are capable of both two- (2D) and three-dimensional (3D) culture necessary for scaffold development. There are many scaffold properties (stiffness, architecture, bioactive signaling concentrations, etc.) that affect cellular behavior (Callahan et al., 2013) and need to be studied systematically in order to develop scaffolds for the treatment of central nervous system injuries. A continuous gradient strategy within hydrogel scaffolds is one combinatorial method that can be used to maximize the number of material parameters tested in both 2D and 3D culture in order to quickly identify the optimal scaffold conditions.
Laminin, a major constituent of the basal lamina, has been associated with axon extension into the lesion in long term survivors of spinal cord injury (Buss et al., 2007), and cellular migration, differentiation, and axon extension during normal development. These characteristics make laminin and laminin derived bioactive peptides prime candidates for inclusion in scaffolds developed for central nervous system applications. Bioactive peptides are often chosen over whole proteins, such as laminin, for inclusion in scaffolds because they are easier to tether with regio- and chemo-selectivity and spatially disburse in 3D materials. Bioactive peptides often provide a similar biological effect as the whole protein.
A recent study (Yang et al., 2015) used a continuous concentration gradient approach to identify the optimal concentration of IKVAV, a commonly studied bioactive peptide from laminin. A wide therapeutic concentration range of 10 µM to 2.6 mM has been reported for IKVAV in the literature (Li et al., 2014; Sur et al., 2014). However, Yang et al. (2015) found increased apoptosis in cells exposed to concentrations at or above 740 µM and 155 µM in 2D and 3D, respectively. A significant reduction in the therapeutic concentration range from what was reported in the literature.
Previous studies that sought to identify the optimal IKVAV concentration for neural cell types utilized only a few discrete samples. The optimal IKVAV concentrations for neurite extension and neural differentiation identified in these studies were in or near the apoptotic range found in the study by Yang et al. (2015) for both 2D and 3D culture (Gunn et al., 2005; Lam et al., 2015). These studies did not examine apoptosis, but the findings highlight an advantage of the continuous gradient strategy over discrete samples and arrays for material development. In a continuous gradient, every potential concentration in the range is present within the gradient. If a subset of concentrations is found to be an area of interest, the gradient range can be changed to examine that subset of concentrations in greater detail as Yang et al. (2015) did in their transition from 2D to 3D. If a study using discrete samples or an array strategy has an insufficient number of sampling points near the area of interest, the optimal concentration can be misidentified and the concentration range of interest may not be studied at all. This is especially likely to happen if the likely concentration range has not been previously identified by other studies when discrete samples and arrays are used. The variation in the reported optimal IKVAV concentration is more complex than combinatorial method selection. A number of factors likely contribute to the variation, including: differences in the cellular differentiation state and species of the cells utilized in the experiments; the polymers and carbohydrates used for scaffold fabrication; and the chemistry used to the tether bioactive signaling and for scaffold gelation. Understanding how these fabrication changes cause shifts in cellular response to the scaffolds, and how changes in cellular maturation state, lineage and species affect cellular response to scaffolds will be invaluable for scaffold optimization. However, many more systematic studies of cell-biomaterial and cell-extracellular matrix interactions will be necessary to achieve the biological understanding necessary to enable optimal scaffold design for clinical use in the central nervous system.
Slight alterations in the concentration of bioactive tethered peptides, such as IKVAV, can have significant effect on cellular response in both 2D and 3D culture (Figure 2). Neurite extension and neural gene expression increased with increased in IKVAV concentation in 2D culture. Maximal neurite extension and neural gene expression occurred on hydrogels containing 570 µM of IKVAV (Yang et al., 2015). While in 3D culture, neurite extension was delayed and then only observed at 60 µM of IKVAV. This represents at least a 9.5 fold reduction in the optimal signaling concentration from 2D to 3D culture. Since 60 µM was the lowest concentration examined in the study, the reduction in the optimal IKVAV concentration for neural differentiation may actually be higher. Lower IKVAV concentrations may lead to further increases in the cellular neural differentiation. Looking at the IKVAV concentrations for initiating increased apoptosis detected in the same study by Yang et al., the reduction of concentration was only four fold between 2D and 3D culture. Within the same scaffold system, different reductions in concentration are being observed in response to the stimulation of different cellular pathways in the same cell type. This speaks to the complexity of neural differentiation, and complicates accurate predictions of the optimal 3D environment from 2D data until the biological causes of these cellular responses are better understood.

Figure 1 Schematic of brain extracellular matrix (ECM) for native, injured and artificial matrix supported injured.
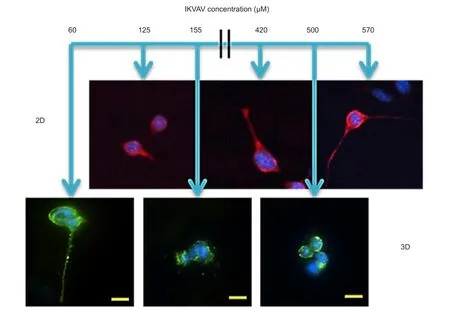
Figure 2 Mouse embryonic stem cell response to a continuous concentration gradient of IKVAV during neural differentiation in 2D and 3D culture.
One can think of the transition from a 2D to 3D culture as moving from laying on a cot, where only one’s back is directlytouching one’s sleeping surface to being in a mummy style sleeping bag where one is now completely surrounded. One’s head, back, front, sides and feet are interacting with the sleeping surface as would happen in a hydrogel environment. Unlike the human body, cells dynamically change their shape in response to their culture environment. The amorphous and changing nature of cell shape in response to biomaterials makes calculating the reduction in total bioactive signaling in the scaffold due to changes in cellular contact area difficult. As multiple layers or clumps of cells form, the calculations would be further complicated. Direct contact with other cells, or secreted cytokines and extracellular matrix proteins from those cells would alter the contact area of the original cell being studied with the biomaterial and perhaps its response to the biomaterial.
Changes in the scaffold can affect the concentration of bioactive signaling presented to the cell over time. Random movements of the polymer chains composing the scaffold can change the local concentration of bioactive signaling and alter the concentration of bioactive signaling available to individual cells. Depending on processing method used, the tethered bioactive signaling can either be sequestered in the bulk of the polymer, reducing the concentration available for the cell to interact with, or concentrated at the liquid interface, increasing the concentration available to the cell from what was originally calculated. These changes in bioactive signal presentation can then be further altered as the scaffold degrades. However, changes in bioactive signal presentation by the scaffold are more predictable than cellular response to biomaterials at this time.
As one transitions from 2D to 3D culture a host of additional factors begin to affect cellular behavior as the local microenvironment changes for the cell. The scaffold reduces intercellular diffusion and transport in 3D culture compared to 2D culture. This affects cellular viability and the concentration of cytokines in the local extracellular milieu. As the concentration and persistence of these cytokines change, so does the stimulation of signaling pathways within the cell and cellular behavior. The scaffold itself can act as a barrier to neurite and dendrite extension. However, conflicting reports of this in non-degradable scaffolds in the literature indicate that the issue is more complex than just a physical obstruction. Tethered bioactive signaling presented on multiple sides of the cells changes the clustering pattern of focal adhesions, causing changes to cytoskeletal shape and tension, which are thought to affect cellular differentiation. The concentration of the tethered bioactive signal can lead the cell to perceive a local bioactive signaling gradient, which facilitates neurite extension.
The common thread too many of the changes between 2D and 3D culture is a real or perceived change in the concentration of elements (cytokines, tethered bioactive signaling, etc.) by the cell in its extracellular milieu. The complex nature of how the concentration changes interact with cells to change cellular survival, attachment, and differentiation throughout central nervous system development is not yet well understood. Unraveling the effects of these concentration changes and breaking the cellular code so that the cellular effects can begin to be predicted will lead to a better understanding of tissue development, homeostasis, and repair. This knowledge will allow for truly rational treatment design and enable the effective manipulation of the extracellular environment with scaffolds, drugs, stem cells, etc. in order to restore neurological function after traumatic brain, spinal cord injuries and stroke.
Work was funded in part by Mission Connect, a program of TIRR foundation, the University of Texas Health Science Center at Houston Bentsen Stroke Center and Department of Neurosurgery William Stamps Farish Fund.
Laura A. Smith Callahan*
Te Vivian L. Smith Department of Neurosurgery & Center for Stem Cell and Regenerative Medicine, University of Texas Health Science Center at Houston, Houston, TX, USA
*Correspondence to: Laura A. Smith Callahan, Ph.D., Laura.A.SmithCallahan@uth.tmc.edu.
Accepted: 2015-08-26
orcid: 0000-0001-9234-1053 (Laura A. Smith Callahan)
Buss A, Pech K, Kakulas BA, Martin D, Schoenen J, Noth J, Brook GA (2007) Growth-modulating molecules are associated with invading Schwann cells and not astrocytes in human traumatic spinal cord injury. Brain 130:940-953.
Gunn JW, Turner SD, Mann BK (2005) Adhesive and mechanical properties of hydrogels influence neurite extension. J Biomed Mater Res A 72A:91-97.
Lam J, Carmichael ST, Lowry WE, Segura T (2015) Hydrogel design of experiments methodology to optimize hydrogel for iPSC-NPC culture. Adv Healthc Mater 4:534-539.
Li X, Liu X, Josey B, Chou CJ, Tan Y, Zhang N, Wen X (2014) Short laminin peptide for improved neural stem cell growth. Stem Cells Transl Med 3:662-670.
Ma VY, Chan L, Carruthers KJ (2014) Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil 95:986-995.e981.
Nakajima M, Ishimuro T, Kato K, Ko IK, Hirata I, Arima Y, Iwata H (2007) Combinatorial protein display for the cell-based screening of biomaterials that direct neural stem cell differentiation. Biomaterials 28:1048-1060.
Silva NA, Sousa N, Reis RL, Salgado AJ (2014) From basics to clinical: A comprehensive review on spinal cord injury. Prog Neurobiol 114:25-57.
Smith Callahan LA, Ma Y, Stafford CM, Becker ML (2013) Concentration dependent neural differentiation and neurite extension of mouse ESC on primary amine-derivatized surfaces. Biomater Sci 1:537-544.
Smith Callahan LA, Xie S, Barker IA, Zheng J, Reneker DH, Dove AP, Becker ML (2013) Directed differentiation and neurite extension of mouse embryonic stem cell on aligned poly(lactide) nanofibers functionalized with YIGSR peptide. Biomaterials 34:9089-9095.
Sur S, Guler MO, Webber MJ, Pashuck ET, Ito M, Stupp SI, Launey T (2014) Synergistic regulation of cerebellar Purkinje neuron development by laminin epitopes and collagen on an artificial hybrid matrix construct. Biomater Sci 2:903-914.
Yang YH, Khan Z, Ma C, Lim HJ, Smith Callahan LA (2015) Optimization of adhesive conditions for neural differentiation of murine embryonic stem cells using hydrogels functionalized with continuous Ile-Lys-Val-Ala-Val concentration gradients. Acta Biomater 21:55-62.
Yasuda H, Kuroda S, Shichinohe H, Kamei S, Kawamura R, Iwasaki Y (2010) Effect of biodegradable fibrin scaffold on survival, migration, and differentiation of transplanted bone marrow stromal cells after cortical injury in rats: Laboratory investigation. J Neurosurg 112:336-344.
10.4103/1673-5374.165303 http∶//www.nrronline.org/
How to cite this article: Smith Callahan LA (2016) The concentration game: differential effects of bioactive signaling in 2D and 3D culture. Neural Regen Res 11(1):66-68.
- 中国神经再生研究(英文版)的其它文章
- Direct reprogramming of somatic cells into neural stem cells or neurons for neurological disorders
- Vascular endothelial growth factor: an attractive target in the treatment of hypoxic/ischemic brain injury
- Glucocorticoids and nervous system plasticity
- RhoA/Rho kinase in spinal cord injury
- The potential of neural transplantation for brain repair and regeneration following traumatic brain injury
- Letter from the Editors-in-Chief

