Lipid mediators of inflammation in neurological injury: shifting the balance toward resolution
PERSPECTIVE
Lipid mediators of inflammation in neurological injury: shifting the balance toward resolution
Acquired neurological injuries initiate a pathological cascade of secondary injury processes, including inflammation, which continue for days to weeks following injury. Injury-induced neuroinflammation acts as a host defense mechanism contributing to the neutralization of the insult (removing offending factors) and restoring structure and function of the brain (establish homeostasis). The timing of these protective functions of the immune response is vital, since chronic inflammation has been associated with progressive cell loss and neurotoxicity (for review, see Faden and Loane, 2015). The pathophysiology of traumatic brain injury (TBI) includes arachidonic acid derived lipid mediators driving inflammatory conditions that promote the activation of resident microglia and infiltration of neutrophils through the disrupted blood-brain barrier (Figure 1A). A separate sub-class of lipid mediators, termed specialized pro-resolving mediators (SPMs), functions to resolve inflammation (Figure 1B). Endogenous SPMs, notably those derived from omega-3 fatty acids, may represent a valuable target in shifting the balance of neuroinflammatory processes from inflammation-driving to inflammation-resolving conditions in the injured central nervous system (CNS). Enthusiasm for a therapeutic approach involving SPMs comes from the natural routes of administration, such as dietary supplementation of their metabolic precursors, exogenous SPMs, and adjunctive interventions focused on increasing the availability of SPMs after injury.
Biochemically, lipid mediators represent a diverse family of endogenous bioactive molecules enzymatically derived from fatty acid substrates. Prostaglandins, a family of extensively studied lipid mediators, are synthesized from arachidonic acid and are elevated after acquired neurological injury, such as TBI (Yang and Gao, 1999). For years, the predominantly pro-inflammatory nature of prostaglandins contributed to the perspective that lipid mediators singly promote inflammation. Affirming this, administration of other arachidonic-acid derived families, such as leukotrienes, have shown pro-inflammatory effects after TBI, while their inhibition has shown improvement in outcome (Hartig et al., 2013). In contrast, SPMs derived from docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), including families of resolvins and protectins, have demonstrated a role in the resolution of inflammation (for review, Recchiuti and Serhan, 2012). Comprehensive mechanisms of action for SPMs have not been identified, but data indicate g-protein-coupled receptors on leukocytes bind SPMs to reduce infiltration and promote tissue regeneration (Recchiuti and Serhan, 2012). While both prostaglandins and SPMs are lipid mediators, they are distinguished by their roles in promoting versus resolving inflammation, respectively (Hartig et al., 2013). This broader perspective on lipid mediators has established a platform to further investigate the balance between pro-inflammatory and pro-resolving lipids in mediating the course of inflammation in the wake of acquired neurological injury, potentially through dietary supplementation or exogenous administration.
Dietary supplementation with metabolic precursors of SPMs has a potential for increasing the availability of SPMs and resolving inflammation after neurological injury. In the brain, fatty acid metabolic precursors of lipid mediators are incorporated into cell membranes (Figure 1). A western diet is especially high in omega-6 fatty acids, such as arachidonic acid, which displace omega-3 fatty acids, such as DHA and EPA, in cell membranes (Bradbury, 2011). In this situation, omega-6 derived lipid mediators, including prostaglandin E2 and leukotriene B4, may prolong inflammation and injury. With omega-3 supplementation, the balance of lipid mediator metabolic precursors can be restored or reversed by providing substrates for SPM production. Metabolic precursors of the inflammation-resolving SPMs, including DHA, have demonstrated potent anti-inflammatory effects in animal models of ischemic stroke (Belayev et al., 2009) and TBI (Wu et al., 2011). In one study, rats were subjected to middle cerebral artery occlusion (MCAO) and subsequently treated intravenously with high (70 mg/kg), medium (16 or 35 mg/kg), or low (3.5 mg/kg) doses of DHA (Belayev et al., 2009). The low and medium doses, but not high dose, of DHA resulted in significant tissue sparing in the peri-infarct region. Treated rats showed significantly better performance in neurological function up to 7 days following MCAO. Following experimental TBI in rats, DHA-enriched diet for 12 days preserved otherwise depleted brain derived neurotrophic factor (BDNF) and improved learning performance (Wu et al., 2011). While the mechanisms of action of DHA in the wake of neurological injury remain widely unknown, its therapeutic potential is compelling.
Further support for the efficacy of dietary supplementation is demonstrated by the increased production of SPMs following supplementation after injury. After cellular insult, membrane fatty acids, including DHA, are released and become more readily available for metabolism into SPMs (Martin et al., 2000). To test the endogenous production of SPMs, DHA was administered intravenously after the onset of ischemic stroke in the rat and brain levels of lipid metabolites were measured (Belayev et al., 2011). Mass spectrometric analysis revealed that neuroprotectin D1 (NPD1) was biosynthesized in the peri-infarct region of DHA-treated rats. This study demonstrates that SPM synthesis in the brain can be enhanced through precursor supplementation, which lead to therapeutic efficacy on behavioral performance and histological outcome following neurological injury. While DHA is not a lipid mediator, investigations into its therapeutic effects in neurological injury suggest its effects may be attributable in part to the biosynthesis of SPMs.
In addition to dietary supplementation, administration of exogenous SPMs may improve outcome from neurological injury. Using different SPMs and models of neurological injury, two studies demonstrated therapeutic efficacy of exogenous SPMs. Following MCAO, rats were administered DHA, NPD1, or saline. Both DHA and NPD1 treatments independently reduced infarct volume and attenuated behavioral deficits measured by neurological assessment (Eady et al., 2012). Next, our group tested the efficacy of aspirin-triggered resolvin D1 (AT-RvD1) and resolvin E1 (RvE1) on amelioration of functional deficits after diffuse TBI in mice (Harrison et al., 2015). AT-RvD1, but not RvE1, treatment mitigated motor deficits in rotarod performance and cognitive deficits in the novel object recognition task. Together, these experimental data in stroke and TBI indicate a standalone role for SPMs as therapeutics for acquired neurological injury.
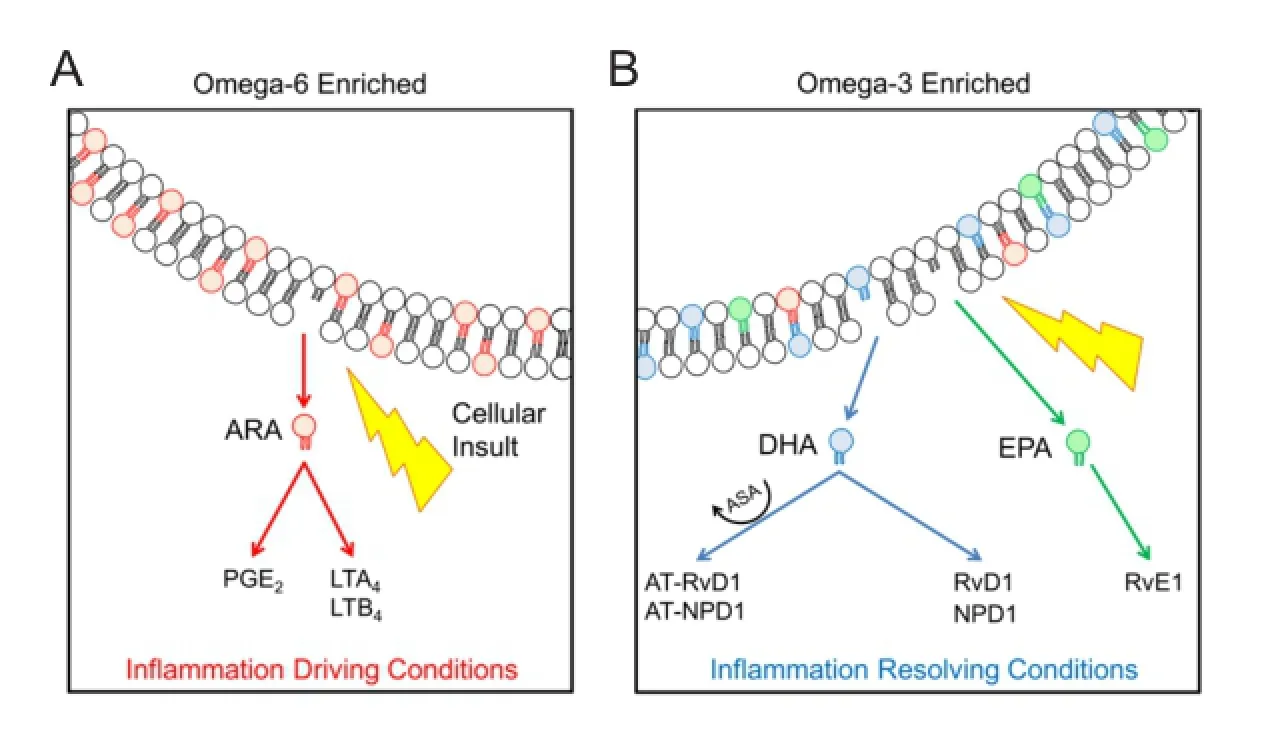
Figure 1 Membrane fatty acid profiles contribute to the balance of inflammation driving and resolving lipid mediators.
In light of the outlined therapeutic potential of SPMs through diet and direct administration, practical considerations in the therapeutic use of SPMs are warranted. Effective therapeutic strategies may include continuous prophylactic dietary supplementation of fatty acid precursors, direct administration of exogenous SPMs, or even physical medicine approaches to potentiate SPM production. These strategies are presented in order of patient accessibility, where direct SPM administration may require specialized knowledge, inventory, and administration by a healthcare professional. On the other hand, dietary omega-3 fatty acid supplementation is a tangible home healthcare strategy, but requires time for fatty acids to accumulate into cell membranes, particularly in the brain. Since acquired neurological injuries occur without warning, supplementation after injury may not be effective or practical. Populations at higher risk for stroke and TBI, such as athletes and soldiers, may benefit from continuous prophylactic supplementation with omega-3 fatty acids. Physical medicine approaches to potentiate endogenous production of SPMs have not been investigated, but could include approaches shown to be therapeutic after acquired neurological injury and secondary inflammation, such as remote ischemic conditioning (Joseph et al., 2015). Complementary approaches directed to antagonism or depletion of inflammation-driving prostaglandins or leukotrienes may contribute to shifting the inflammatory balance toward resolution.
In conclusion, SPMs provide a cellular target for therapeutic approaches to limit secondary injury processes after acquired neurological injury. The potent anti-inflammatory properties of SPMs in peripheral diseases (see review: Recchiuti and Serhan, 2012) and the therapeutic efficacy of their fatty acid precursors in neurological disease provide a sound basis for further exploration of their neuroprotective efficacy in acquired neurological injury. Of particular value to the clinical problems of stroke and TBI are therapies which are rapid and accessible. The endogenous nature of SPMs makes them promising candidates for readily accessible therapies, which could shift the inflammatory balance toward resolution of cellular pathophysiology and limit the extent of injury.
The authors have no conflicts of interest to declare. JLH, RKR and JL contributed to manuscript content and style.
Jordan L. Harrison, Rachel K. Rowe, Jonathan Lifshitz*
BARROW Neurological Institute at Phoenix Children’s Hospital, Phoenix, AZ, USA (Harrison JL, Rowe RK, Lifshitz J)
Interdisciplinary Graduate Program in Neuroscience, Arizona State University, Tempe, AZ, USA (Harrison JL, Lifshitz J)
Department of Child Health, University of Arizona College of Medicine - Phoenix, Phoenix, AZ, USA (Harrison JL, Rowe RK, Lifshitz J)
Phoenix Veteran Affairs Healthcare System, Phoenix, AZ, USA (Rowe RK, Lifshitz J)
*Correspondence to: Jonathan Lifshitz, Ph.D., jlifshitz@email.arizona.edu.
Accepted: 2015-10-28
Belayev L, Khoutorova L, Atkins KD, Bazan NG (2009) Robust docosahexaenoic acid-mediated neuroprotection in a rat model of transient, focal cerebral ischemia. Stroke 40:3121-3126.
Belayev L, Khoutorova L, Atkins KD, Eady TN, Hong S, Lu Y, Obenaus A, Bazan NG (2011) Docosahexaenoic acid therapy of experimental ischemic stroke. Transl Stroke Res 2:33-41.
Bradbury J (2011) Docosahexaenoic acid (DHA): an ancient nutrient for the modern human brain. Nutrients 3:529-554.
Eady TN, Belayev L, Khoutorova L, Atkins KD, Zhang C, Bazan NG (2012) Docosahexaenoic acid signaling modulates cell survival in experimental ischemic stroke penumbra and initiates long-term repair in young and aged rats. PLoS One 7:e46151.
Faden AI, Loane DJ (2015) Chronic neurodegeneration after traumatic brain injury: Alzheimer disease, chronic traumatic encephalopathy, or persistent neuroinflammation? Neurotherapeutics 12:143-150.
Harrison JL, Rowe RK, Ellis TW, Yee NS, O’Hara BF, David Adelson P, Lifshitz J (2015) Resolvins AT-D1 and E1 differentially impact functional outcome, post-traumatic sleep, and microglial activation following diffuse brain injury in the mouse. Brain Behav Immun 47:131-140.
Hartig W, Michalski D, Seeger G, Voigt C, Donat CK, Dulin J, Kacza J, Meixensberger J, Arendt T, Schuhmann MU (2013) Impact of 5-lipoxygenase inhibitors on the spatiotemporal distribution of inflammatory cells and neuronal COX-2 expression following experimental traumatic brain injury in rats. Brain Res 1498:69-84.
Joseph B, Pandit V, Zangbar B, Kulvatunyou N, Khalil M, Tang A, O’Keeffe T, Gries L, Vercruysse G, Friese RS, Rhee P (2015) Secondary brain injury in trauma patients: the effects of remote ischemic conditioning. J Trauma Acute Care Surg 78:698-705.
Martin RE, Wickham JQ, Om AS, Sanders J, Ceballos N (2000) Uptake and incorporation of docosahexaenoic acid (DHA) into neuronal cell body and neurite/nerve growth cone lipids: evidence of compartmental DHA metabolism in nerve growth factor-differentiated PC12 cells. Neurochem Res 25:715-723.
Recchiuti A, Serhan CN (2012) Pro-resolving lipid mediators (SPMs) and their actions in regulating mirna in novel resolution circuits in inflammation. Front Immunol 3:298.
Wu A, Ying Z, Gomez-Pinilla F (2011) The salutary effects of DHA dietary supplementation on cognition, neuroplasticity, and membrane homeostasis after brain trauma. J Neurotrauma 28:2113-2122.
Yang SY, Gao ZX (1999) Determination and clinical significance of plasma levels of prostaglandins in patients with acute brain injury. Surg Neurol 52:238-245.
10.4103/1673-5374.175046 http∶//www.nrronline.org/
How to cite this article: Harrison JL, Rowe RK, Lifshitz J (2016) Lipid mediators of inflammation in neurological injury: shifting the balance toward resolution. Neural Regen Res 11(1):77-78.
- 中国神经再生研究(英文版)的其它文章
- Direct reprogramming of somatic cells into neural stem cells or neurons for neurological disorders
- Vascular endothelial growth factor: an attractive target in the treatment of hypoxic/ischemic brain injury
- Glucocorticoids and nervous system plasticity
- RhoA/Rho kinase in spinal cord injury
- The potential of neural transplantation for brain repair and regeneration following traumatic brain injury
- Letter from the Editors-in-Chief

