A novel bioactive nerve conduit for the repair of peripheral nerve injury
Bin-bin Li, Yi-xia Yin,, Qiong-jiao Yan, Xin-yu Wang, Shi-pu Li
1 State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan, Hubei Province, China
2 Biomedical Materials and Engineering Research Center of Hubei Province, Wuhan University of Technology, Wuhan, Hubei Province, China
RESEARCH ARTICLE
A novel bioactive nerve conduit for the repair of peripheral nerve injury
Bin-bin Li1,2, Yi-xia Yin1,2,*, Qiong-jiao Yan1,2, Xin-yu Wang1,2, Shi-pu Li1,2
1 State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan, Hubei Province, China
2 Biomedical Materials and Engineering Research Center of Hubei Province, Wuhan University of Technology, Wuhan, Hubei Province, China
Graphical Abstract
orcid: 0000-0002-0326-1658 (Yi-xia Yin)
The use of a nerve conduit provides an opportunity to regulate cytokines, growth factors and neurotrophins in peripheral nerve regeneration and avoid autograft defects. We constructed a poly-D-L-lactide (PDLLA)-based nerve conduit that was modified using poly{(lactic acid)-co-[(glycolic acid)-alt-(L-lysine)]} and β-tricalcium phosphate. The effectiveness of this bioactive PDLLA-based nerve conduit was compared to that of PDLLA-only conduit in the nerve regeneration following a 10-mm sciatic nerve injury in rats. We observed the nerve morphology in the early period of regeneration, 35 days post injury, using hematoxylin-eosin and methylene blue staining. Compared with the PDLLA conduit, the nerve fibers in the PDLLA-based bioactive nerve conduit were thicker and more regular in size. Muscle fibers in the soleus muscle had greater diameters in the PDLLA bioactive group than in the PDLLA only group. The PDLLA-based bioactive nerve conduit is a promising strategy for repair after sciatic nerve injury.
nerve regeneration; polylactic acid; poly{(lactic acid)-co-[(glycolic acid)-alt-(L-lysine)]}; β-tricalcium phosphate; nerve conduit; nerve fiber; neural regeneration
Introduction
The two detached ends of an injured peripheral nerve cannot rejoin successfully in the absence of an external aid. A nerve-like device is necessary for the repair of the injured nerve to reduce the loss of muscle function and sensory disorder (Evans et al., 1999; Li et al., 2014). The application of a nerve conduit is considered as an effective method that avoids the shortfalls associated with an autograft. It can also provide an opportunity to regulate the responses to the cytokines and neurotrophins during the peripheral nerve regeneration (Mohammad et al., 2000; Kehoe et al., 2012; Azizi et al., 2015). To resolve these issues, composite materials with excellent biodegradability and biocompatibility are employed to imitate the structure and function of naturalnerves (Mligiliche et al., 1999; Chen et al., 2005; Luis et al., 2007; Subramanian et al., 2009; Das et al., 2013). Poly-DL-lactide (PDLLA) was employed in this study as the nerve conduit due to its excellent biodegradability and biocompatibility. However, the degradation products of PDLLA can lower the local pH which is harmful to the surrounding cells and tissues. The addition of β-tricalcium phosphate (β-TCP) is beneficial not only because of its good biocompatibility, biodegradability and non-toxicity, but also because its basic degradation products restore the local pH to its normal value. The peptide Gly-Arg-Gly-Asp-Gly (RGD) has been shown to enhance Schwann cell attachment and elongation in vitro (Yan et al., 2012; de Luca et al., 2013), and thereby facilitate axon growth in the early stage in vivo (Liu et al., 2009).
Most previous studies focused on the long-term (over 3 months) nerve regeneration after nerve conduit implantation (Den Dunnen et al., 1993; Toba et al., 2002; Bian et al., 2009; Xu et al., 2011, 2014; Yan et al., 2012), but there have been fewer reports on the morphology of regenerated nerves in the early stages of nerve regeneration (Yang et al., 2001; Jaminet et al., 2013; Kawasaki et al., 2013; Schrems-Hoesl et al., 2013; Seo et al., 2013; Qiu et al., 2014; Li et al., 2015).
In this study, both RGD and β-TCP were first used to modify a PDLLA conduit to offer a bioactive microenvironment for nerve regeneration using a biomimetic method. We planned to analyze the biological performance of the nerve conduit in the repair of a 10-mm deletion of the sciatic nerve in rats by observing the changes at an early stage (35 days) during nerve regeneration.
Materials and Methods
Preparation of PDLLA-based bioactive nerve conduit
A polymer RGD peptide (GL Biochem, Shanghai, China) modification of poly{(lactic acid)-co-[(glycolic acid)-alt-(L-lysine)]} (PRGD) was fabricated by the following steps. Firstly, (3S)-3-[4-(benzyloxycarbonylamino) butyl] morpholine-2,5-dione (BMD) was synthesized by bromoacetyl bromide and Nε-(benzyloxycarbonyl)-L-lysine. Secondly, poly(lactic acid)-co-[(glycolic acid)-alt-(Nε-benzyloxycarbonyl-L-lysine)] was obtained by copolymerization of D, L-lactide and BMD. Then, poly{(lactic acid)-co-[(glycolic acid)-alt-(L-lysine)]} (PLGL) was synthesized by catalytic hydrogenation. Finally, PLGL was modified with RGD peptide. PRGD (0.05 g) and PDLLA (0.9 g) (molecular weight 250,000, synthesized in the State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology in China) were dissolved in ethyl acetate at a concentration of 0.05 g/L β-TCP (0.05 g) (synthesized in the State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology) was then added to the ethyl acetate solution and mixed thoroughly. The PRGD, PDLLA and β-TCP were used to prepare PDLLA-based bioactive composite using a solvent volatilization method (Zhang et al., 2015). The PDLLA-based composite and PDLLA membranes were fabricated and then rolled to form the PDLLA-based composite or PDLLA bioactive nerve conduits. The product was 14 mm long, 2 mm in diameter and 0.2 mm thick. The nerve conduits scheduled for bridging sciatic nerve defects were sterilized with ultraviolet light for 60 minutes. The material characterizations and in vitro evaluations of these conduits have been reported in our previous study (Zhang et al., 2015).
Ethics statement and animals
The study protocol was approved by the Animal Care and Use Committees of Wuhan University of Technology, China and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (No.85-23, revised 1996). Precautions were taken to minimize the number of animals used and their suffering.
Adult male Wistar rats, weighing 200-250 g, 8 weeks old, specific pathogen-free level, were purchased from the Centers for Disease Control and Prevention of Hubei Province (China) (license No. SCXK(E)2015-0018). The rats were randomly divided into three groups each with 10 rats: PDLLA conduit group; PDLLA-based bioactive conduit group and normal nerve group.
Sciatic nerve injury model establishment and nerve conduit repair
The rats were anesthetized with 50 mg/kg pentobarbital sodium by intraperitoneal injection. The right sciatic nerve was exposed after a skin incision was made and the muscles around the nerve tissues were separated using blunt dissection. Subsequently, the right sciatic nerve was severed into proximal and distal segments at the center of the right thigh. Both the proximal and distal stumps were sutured with 9-0 nylon sutures to a depth of 1 mm into the conduits, leaving a 10-mm gap between the stumps, bridged by the nerve conduits (Figure 1). The muscle and skin layers were re-approximated using 6-0 nylon sutures. In the normal nerve group, no surgery was carried out on the sciatic nerve.
Hematoxylin-eosin staining
At 35 days after conduit implantation, the rats were anesthetized again with 50 mg/kg pentobarbital sodium to expose the right sciatic nerve. The conduits were opened and samples of the regenerated nerves, or normal nerves, and small pieces of the soleus muscle in all groups were collected. All the rats were sacrificed by cervical dislocation after all the samples had been collected. The soleus muscle and nerve specimens were fixed in a solution containing 1% paraformaldehyde, 1.25% glutaraldehyde and 0.1 M cacodylate buffered to pH 6.5-7.0, then dehydrated and embedded in paraffin. Sections (5 µm thick) were stained with hematoxylin and eosin and observed using an inverted microscope (IX71, Olympus, Tokyo, Japan). The muscle fiber size in each group was analyzed by selecting 200 muscle fibers in 10 random areas. The average diameter of the muscle fibers was analyzed using an image analysis system (Image-Pro Plus, Media Cybernetics, San Francisco, CA, USA).
Toluidine blue staining
To evaluate the physiological status of axons and myelin sheath regeneration at 35 days after conduit implantation, the regenerated nerve specimens were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffered saline for 2 hours and postfixed in 1% osmium tetroxide for 1.5 hours. Then they were dehydrated in a graded ethanol series, and embedded in paraffin. The specimens were cut into 1-µm-thick cross-sections with an ultramicrotome (MT-XL, RMC Inc., New York, NY, USA), stained with toluidine blue, and observed by inverted microscopy. The fiber sizes in all groups were analyzed by selecting 200 nerve fibers in 10 random areas. The average diameter of the regenerated nerve fibers was analyzed using the image analysis system (Image-Pro Plus, Media Cybernetics).
Statistical analysis
Data are expressed as the mean ± SD. Experimental data were processed using the statistical software SPSS 10.0 (SPSS Inc., Chicago, IL, USA), and analyzed with one-way analysis of variance followed by a Bonferroni post-hoc test. P values less than 0.05 were considered statistically significant.
Results
The biocompatibility of the PDLLA-based bioactive conduits
All the rats used in this study appeared to be well by their daily behavior. Macroscopically, axonal sprouts were found at both the distal and proximal ends, and these were successfully connected by the 35thday. The conduit was well integrated with the sciatic nerve and had not yet degraded.
Regenerated nerve morphology after rat sciatic nerve repair with PDLLA-based bioactive conduits
To observe the morphology of the nerve in the early stage of regeneration, hematoxylin-eosin staining was done 35 days post surgery. These hematoxylin-eosin images illustrate that Figure 2C appears to have denser packing of nerve fibers than either Figure 2A or B. Also, the fiber bundles in Figure 2C are more regular than those in Figure 2B, which in turn are more regular than those in Figure 2A. Figure 2B shows less in-growth of irregular connective tissues from the sciatic nerve sheath than that in Figure 2A. The regenerated nerve fibers of sciatic nerves in the rats (Figure 2D, E) were smaller in diameter and less uniform in morphology than those in the normal nerve group (Figure 2F). Those in the PDLLA conduit group were thinnest and most irregular with the most in-growth of connective tissues (Figure 2D). The sections in the two conduit groups showed more activated Schwann cells and blood vessels than those in the normal nerve group. These findings showed that the axons were actively supported and that the neurotrophic substances were delivered to the lesion for the nerve regeneration.
Results of toluidine blue staining showed that nerve fibers were densely packed in all groups (Figure 3A-C), the myelinated fibers in the PDLLA-based bioactive conduit group had more compact and uniform structures than those in the PDLLA conduit group, but less than those in the normal nerve group. The average fiber diameter analysis showed that the mean fiber diameter in the PDLLA-based bioactive conduit group was significantly smaller than that in the normal nerve group, but 1.42 times larger than that in the PDLLA conduit group (Figure 3D).
Morphology of the soleus muscles after rat sciatic nerve repair with PDLLA-based bioactive conduits
To evaluate the nerve function recovery, the soleus muscles in all groups were subjected to hematoxylin-eosin staining. Compared with the normal nerve group (Figure 4C), the soleus muscles following surgery had degenerated in the PDLLA conduit and PDLLA-based bioactive conduit groups, and presented smaller fiber diameters in the same area. The muscle atrophy and connective tissues in the PDLLA-based bioactive conduit group (Figure 4B) had re-grown, showing a better morphology, while the muscles in the PDLLA conduit group (Figure 4A) were still in a poor condition. The mean diameter of muscle fibers in the PDLLA-based bioactive conduit group was 1.34-times larger than that in the PDLLA conduit group, yet still smaller than that in the normal nerve group (Figure 4D).
Discussion
The RGD peptide has been proven to enhance Schwann cell attachment and elongation in vitro (Yan et al., 2012; de Luca et al., 2013), and RGD in vivo facilitates the axonal regeneration in the early period after sciatic nerve injury in rats (Liu et al., 2009). The presence of RGD-coated conduits in the early phase of peripheral nerve regeneration provides a permissive surface for activated Schwann cells around the lesion to secrete vital trophic factors to support axon regeneration (Rafiuddin Ahmed and Jayakumar, 2003; Liu et al., 2009). The other supplement, β-TCP, upregulates the mRNA expression of cytoskeletal protein and has been proved to be nontoxic (Qiu et al., 2014).
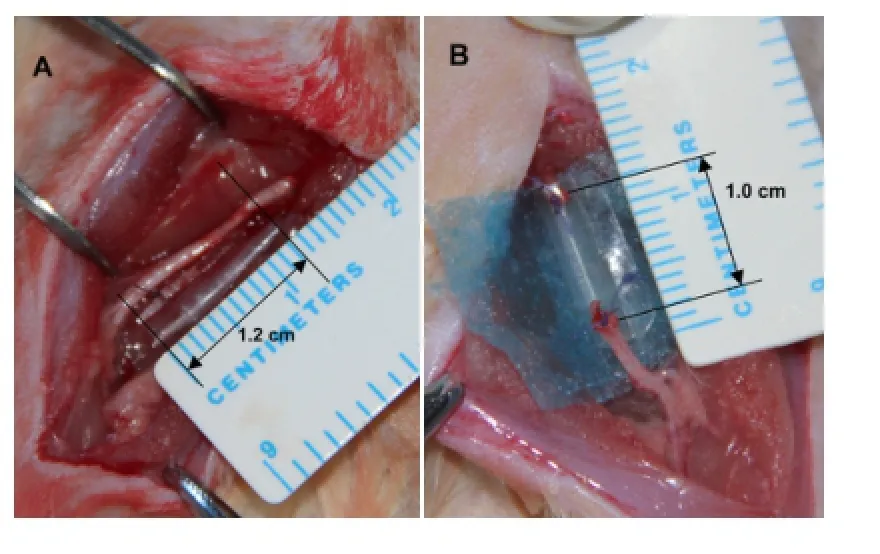
Figure 1 Sciatic nerve defects bridged by poly-D-L-lactide (PDLLA)-based bioactive conduits.
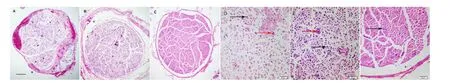
Figure 2 Morphology of regenerated sciatic nerves in rats at 35 days after sciatic nerve injury (hematoxylin-eosin staining).
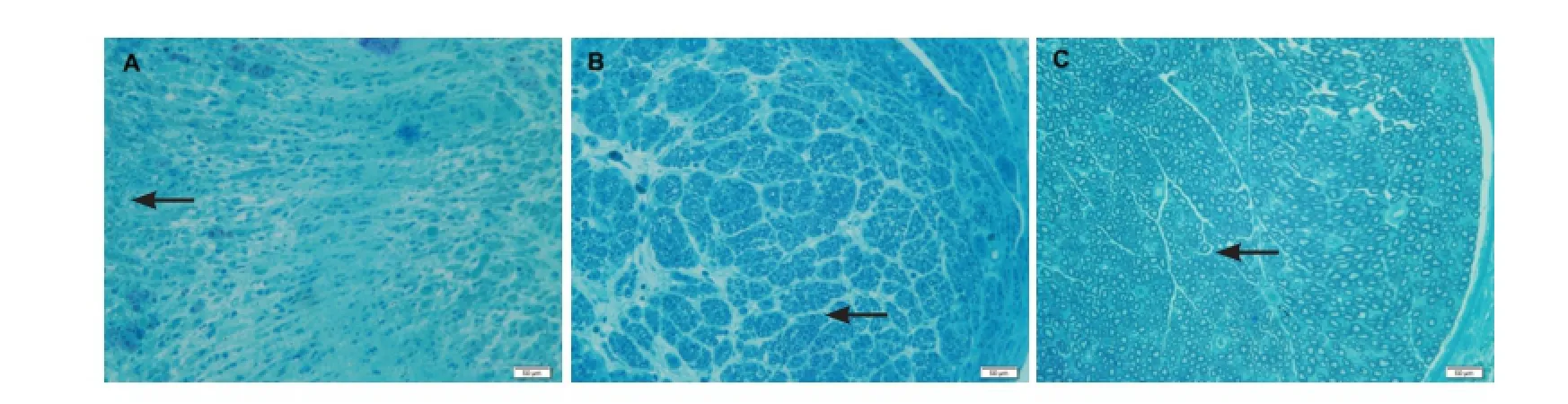
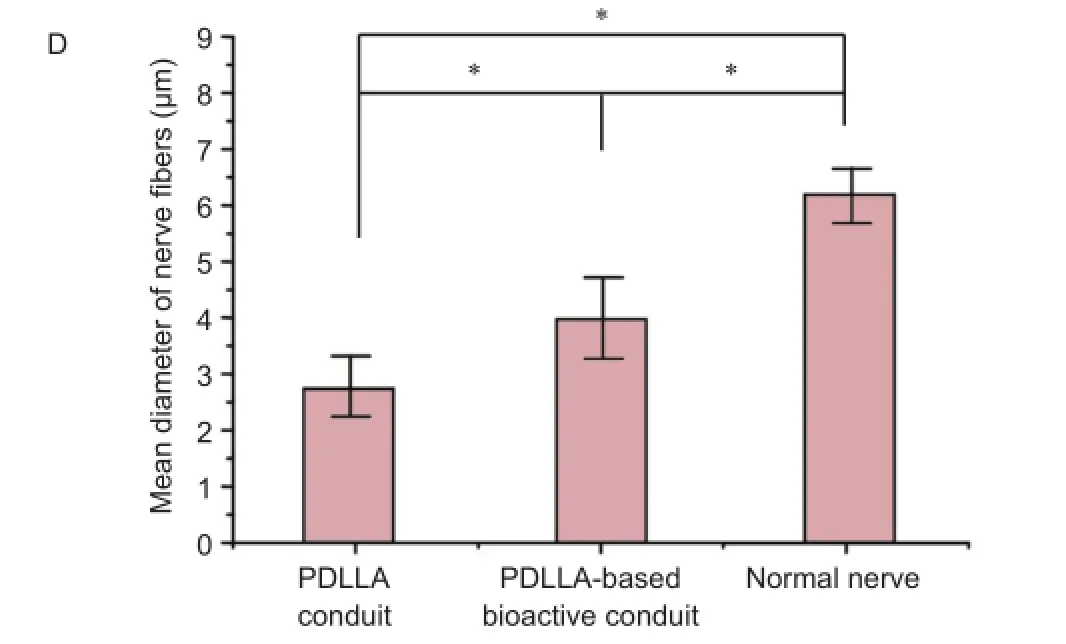
Figure 3 Morphology of regenerated sciatic nerves in rats at 35 days after sciatic nerve injury (toluidine blue staining).


Figure 4 Morphology of the soleus muscle in rats at 35 days after sciatic nerve injury.
In this study, we aimed to observe the morphology of regenerated nerves bridged by the PDLLA-based bioactive nerve conduit in the early period of nerve regeneration. Thenerve morphology of nerve fibers at different stages would directly demonstrate the maturation of axons, which is also a marker for assessing the efficiency of indirect conduit bridging. Many of the fibers with small diameters could be nonconducting and degenerating rather than regenerating. As the nerve fibers regenerate distally and reach the appropriate target organs, the fiber diameter increases and the myelin sheath grows (Weiss, 1945; Schröder, 1972). If sprouting axons are incapable of establishing a suitable connection with the target organ, they are deprived of vital growth factors and degenerate.
The morphology and size analysis of nerve fibers have demonstrated that the PDLLA-based bioactive nerve conduits promote early-stage peripheral nerve regeneration by enhancing the nerve regeneration rate and significantly increase the myelinated fiber and soleus muscle fiber density compared with PDLLA conduit controls. At 35 days after sciatic nerve surgery, the fibroblasts and macrophages concentrated around the periphery of the newly formed nerve tissues (Brown et al., 1991; Zhou and Snider, 2006). Schwann cells and endothelial cells moved into the lesion and secreted the necessary cytokines and neurotrophins to enhance synthesis of new nerve tissue and axon elongation (Markus et al., 2002; Leibinger et al., 2009; Liu et al., 2011). The injury detected by the neuronal body switched the axons from the normal state to growth mode with an associated gene expression and protein synthesis (Kretz et al., 2005; Agthong et al., 2006; Miao et al., 2006; Luo et al., 2007; Trenchi et al., 2009; Yamazaki et al., 2009; Liu et al., 2011). Concurrently, cell-adhesion molecules, myelin proteins, and extracellular matrix proteins around the lesion supported the growth cone sprouting and axons remodeling (Skene et al., 1986; Fernandes et al., 1999; Janke and Bulinski, 2011). The nerve fiber morphology would be different in this early stage during nerve regeneration because of the different cytokines and gene regulation. Therefore, we concluded that the PDLLA-based bioactive nerve conduit might promote axon growth and soleus muscle recovery in the early stage of nerve regeneration.
Our evidence indicated that a PDLLA-based conduit modified with bioactive compounds enhanced regeneration of the injured nerve during the first 35 days. However, the regenerating nerve morphology should be explored at other time points. Similarly, research at the molecular level is necessary to explore how the bioactive conduit affects the changes in the cytokines and neurotrophins.
In this study, we developed a novel PDLLA-based bioactive nerve repair conduit, which we used in in vivo trials for the repair of rat sciatic nerve injury with a 10 mm gap. We evaluated the outcomes of nerve regeneration by observing the nerve morphology with histological staining in the early period. Our results demonstrated that compared with the PDLLA conduit group, the nerve recovery in the PDLLA-based bioactive conduit group showed larger diameter nerve fibers in more ordered array. Soleus muscle fibers were also larger. This enhanced nerve conduit offers new opportunities for research in the field of nerve regeneration.
Author contributions: BBL wrote the paper. All authors designed the study, provided critical revision of the paper and approved the final version of this paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Agthong S, Kaewsema A, Tanomsridejchai N, Chentanez V (2006) Activation of MAPK ERK in peripheral nerve after injury. BMC Neurosci 7:45.
Azizi S, Heshmatian B, Amini K, Raisi A, Azimzadeh M (2015) Alpha-lipoic acid loaded in chitosan conduit enhances sciatic nerve regeneration in rat. Iran J Basic Med Sci 18:228-233.
Bian YZ, Wang Y, Aibaidoula G, Chen GQ, Wu Q (2009) Evaluation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) conduits for peripheral nerve regeneration. Biomaterials 30:217-225.
Brown MC, Hugh Perry V, Ruth Lunn E, Gordon S, Heumann R (1991) Macrophage dependence of peripheral sensory nerve regeneration: Possible involvement of nerve growth factor. Neuron 6:359-370.
Chen YS, Chang JY, Cheng CY, Tsai FJ, Yao CH, Liu BS (2005) An in vivo evaluation of a biodegradable genipin-cross-linked gelatin peripheral nerve guide conduit material. Biomaterials 26:3911-3918.
Das B, Chattopadhyay P, Mandal M, Voit B, Karak N (2013) Bio-based biodegradable and biocompatible hyperbranched polyurethane: a scaffold for tissue engineering. Macromol Biosci 13:126-139.
de Luca AC, Stevens JS, Schroeder SLM, Guilbaud JB, Saiani A, Downes S, Terenghi G (2013) Immobilization of cell-binding peptides on poly-ε-caprolactone film surface to biomimic the peripheral nervous system. J Biomed Mater Res A 101:491-501.
Den Dunnen WF, Van der Lei B, Schakenraad JM, Blaauw EH, Stokroos I, Pennings AJ, Robinson PH (1993) Long-term evaluation of nerve regeneration in a biodegradable nerve guide. Microsurgery 14:508-515.
Evans GR, Brandt K, Widmer MS, Lu L, Meszlenyi RK, Gupta PK, Mikos AG, Hodges J, Williams J, Gürlek A, Nabawi A, Lohman R, Patrick C W Jr (1999) In vivo evaluation of poly(l-lactic acid) porous conduits for peripheral nerve regeneration. Biomaterials 20:1109-1115.
Fernandes KJ, Fan DP, Tsui BJ, Cassar SL, Tetzlaff W (1999) Influence of the axotomy to cell body distance in rat rubrospinal and spinal motoneurons: Differential regulation of GAP-43, tubulins, and neurofilament-M. J Comp Neurol 414:495-510.
Jaminet P, Köhler D, Rahmanian-Schwarz A, Lotter O, Mager A, Fornaro M, Ronchi G, Geuna S, Rosenberger P, Schaller HE (2013) Expression patterns and functional evaluation of the UNC5B receptor during the early phase of peripheral nerve regeneration using the mouse median nerve model. Microsurgery 33:216-222.
Janke C, Bulinski JC (2011) Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol 12:773-786.
Kawasaki T, Oka N, Yagi H, Akiguchi I (2013) Cyclin/INS;D1 expression in Schwann cell nucleus associated with the stage of nerve regeneration. J Neurol Sci 333:e705.
Kehoe S, Zhang XF, Boyd D (2012) FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury 43:553-572.
Kretz A, Happold CJ, Marticke JK, Isenmann S (2005) Erythropoietin promotes regeneration of adult CNS neurons via Jak2/Stat3 and PI3K/AKT pathway activation. Mol Cell Neurosci 29:569-579.
Leibinger M, Müller A, Andreadaki A, Hauk TG, Kirsch M, Fischer D (2009) Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J Neurosci 29:14334-14341.
Li B, Qiu T, Iyer KS, Yan Q, Yin Y, Xie L, Wang X, Li S (2015) PRGD/ PDLLA conduit potentiates rat sciatic nerve regeneration and the underlying molecular mechanism. Biomaterials 55:44-53.
Li R, Liu Z, Pan Y, Chen L, Zhang Z, Lu L (2014) Peripheral nerve injuries treatment: a systematic review. Cell Biochem Biophys 68:449-454.
Liu K, Tedeschi A, Park KK, He Z (2011) Neuronal intrinsic mechanisms of axon regeneration. Annu Rev Neurosci 34:131-152.
Liu WQ, Martinez JA, Durand J, Wildering W, Zochodne DW (2009) RGD-mediated adhesive interactions are important for peripheral axon outgrowth in vivo. Neurobiol Dis 34:11-22.
Luis AL, Rodrigues JM, Amado S, Veloso AP, Armada-Da-silva PA, Raimondo S, Fregnan F, Ferreira AJ, Lopes MA, Santos JD, Geuna S, Varejão AS, Maurício AC (2007) PLGA 90/10 and caprolactone biodegradable nerve guides for the reconstruction of the rat sciatic nerve. Microsurgery 27:125-137.
Luo JM, Cen LP, Zhang XM, Chiang SW, Huang Y, Lin D, Fan YM, Van Rooijen N, Lam DS, Pang CP, Cui Q (2007) PI3K/akt, JAK/STAT and MEK/ERK pathway inhibition protects retinal ganglion cells via different mechanisms after optic nerve injury. Eur J Neurosci 26:828-842.
Markus A, Patel TD, Snider WD (2002) Neurotrophic factors and axonal growth. Curr Opin Neurobiol 12:523-531.
Miao T, Wu D, Zhang Y, Bo X, Subang MC, Wang P, Richardson PM (2006) Suppressor of cytokine signaling-3 suppresses the ability of activated signal transducer and activator of transcription-3 to stimulate neurite growth in rat primary sensory neurons. J Neurosci 26:9512-9519.
Mligiliche NL, Tabata Y, Ide C (1999) Nerve regeneration through biodegradable gelatin conduits in mice. East Afr Med J 76:400-406.
Mohammad J, Shenaq J, Rabinovsky E, Shenaq S (2000) Modulation of peripheral nerve regeneration: a tissue-engineering approach. The role of amnion tube nerve conduit across a 1-centimeter nerve gap. Plast Reconstr Surg 105:660-666.
Qiu T, Yin Y, Li B, Xie L, Yan Q, Dai H, Wang X, Li S (2014) PDLLA/ PRGD/β-TCP conduits build the neurotrophin-rich microenvironment suppressing the oxidative stress and promoting the sciatic nerve regeneration. J Biomed Mater Res A 102:3734-3743.
Rafiuddin Ahmed M, Jayakumar R (2003) Peripheral nerve regeneration in RGD peptide incorporated collagen tubes. Brain Res 993:208-216.
Schröder JM (1972) Altered ratio between axon diameter and myelin sheath thickness in regenerated nerve fibers. Brain Res 45:49-65.
Schrems-Hoesl LM, Schrems WA, Cruzat A, Shahatit BM, Bayhan HA, Jurkunas UV, Hamrah P (2013) Cellular and subbasal nerve alterations in early stage Fuchs’ endothelial corneal dystrophy: an in vivo confocal microscopy study. Eye (Lond) 27:42-49.
Seo SY, Min SK, Bae HK, Roh D, Kang HK, Roh S, Lee S, Chun GS, Chung DJ, Min BM (2013) A laminin-2-derived peptide promotes early-stage peripheral nerve regeneration in a dual-component artificial nerve graft. J Tissue Eng Regen Med 7:788-800.
Skene JH, Jacobson RD, Snipes GJ, McGuire CB, Norden JJ, Freeman JA (1986) A protein induced during nerve growth (GAP-43) is a major component of growth-cone membranes. Science 233:783-786.
Subramanian A, Krishnan UM, Sethuraman S (2009) Development of biomaterial scaffold for nerve tissue engineering: Biomaterial mediated neural regeneration. J Biomed Sci 16:108.
Toba T, Nakamura T, Lynn AK, Matsumoto K, Fukuda S, Yoshitani M, Hori Y, Shimizu Y (2002) Evaluation of peripheral nerve regeneration across an 80-mm gap using a polyglycolic acid (PGA)--collagen nerve conduit filled with laminin-soaked collagen sponge in dogs. Int J Artif Organs 25:230-237.
Trenchi A, Gomez Guillermo A, Daniotti Jose L (2009) Dual acylation is required for trafficking of growth-associated protein-43 (GAP-43) to endosomal recycling compartment via an Arf6-associated endocytic vesicular pathway. Biochem J 421:357-369.
Weiss P (1945) Experiments on cell and axon orientation in vitro; the role of colloidal exudates in tissue organization. J Exp Zool 100:353-386.
Xu H, Yan Y, Li S (2011) PDLLA/chondroitin sulfate/chitosan/NGF conduits for peripheral nerve regeneration. Biomaterials 32:4506-4516.
Xu H, Holzwarth JM, Yan Y, Xu P, Zheng H, Yin Y, Li S, Ma PX (2014) Conductive PPY/PDLLA conduit for peripheral nerve regeneration. Biomaterials 35:225-235.
Yamazaki T, Sabit H, Oya T, Ishii Y, Hamashima T, Tokunaga A, Ishizawa S, Jie S, Kurashige Y, Matsushima T, Furuta I, Noguchi M, Sasahara M (2009) Activation of MAP kinases, Akt and PDGF receptors in injured peripheral nerves. J Peripher Nerv Syst 14:165-176.
Yan Q, Yin Y, Li B (2012) Use new PLGL-RGD-NGF nerve conduits for promoting peripheral nerve regeneration. Biomed Eng Online 11:36.
Yang YG, Li CG, Guo P, Ren XS (2001) The early stage morphology study of the repairment of cauda equina injury with peripheral nerve transplantation in rats. Zhongguo Jizhu Jisui Zazhi 11:168-170.
Zhang Z, Wu XP, Yin YX, Zhao Z, Li SP (2015) Fabrication, characterization and biological evaluation of PRGD/PDLLA/β-TCP scaffold for nerve regeneration. J Fiber Bioeng Inform 8:133-142.
Zhou FQ, Snider WD (2006) Intracellular control of developmental and regenerative axon growth. Philos Trans R Soc Lond B Biol Sci 361:1575-1592.
Copyedited by Dawes EA, Edanz QC, Yu J, Wang L, Li CH, Song LP, Zhao M
10.4103/1673-5374.175062
How to cite this article: Li BB, Yin YX, Yan QJ, Wang XY, Li SP (2016) A novel bioactive nerve conduit for the repair of peripheral nerve injury. Neural Regen Res 11(1)∶150-155.
Funding: This study was supported by a grant from the National Key Basic Research Program of China, No. 2011CB606205; a grant from the National Natural Science Foundation of China, No. 51403168; a grant from the Major Scientific and Technological Research Projects of the Ministry of Education of China, No. 313041; and a grant from the Scientific and Technological Cooperation Projects of Hong Kong, Macao and Taiwan, China, No. 2015DFH30180.
http://www.nrronline.org/
Accepted: 2015-12-16
*Correspondence to: Yi-xia Yin, yinyixia@whut.edu.cn.
- 中国神经再生研究(英文版)的其它文章
- Direct reprogramming of somatic cells into neural stem cells or neurons for neurological disorders
- Vascular endothelial growth factor: an attractive target in the treatment of hypoxic/ischemic brain injury
- Glucocorticoids and nervous system plasticity
- RhoA/Rho kinase in spinal cord injury
- The potential of neural transplantation for brain repair and regeneration following traumatic brain injury
- Letter from the Editors-in-Chief

