Primary Question and Hypothesis Testing in Randomized Controlled Clinical Trials
Jiangtao LUO
·Biostatistics in psychiatry (33)·
Primary Question and Hypothesis Testing in Randomized Controlled Clinical Trials
Jiangtao LUO
hypothesis, randomize, clinical trial, primary question
1. Primary and secondary questions
Medical research is always about answering scientific questions related to treatments or preventions of diseases in special population of patients, who are defined by inclusion and exclusion criteria and treatments or preventions are performed among them.These questions are often divided into primary and secondary questions. Usually we have only one primary question in a study and it is the key and central question that we want to answer. We should state our primary question in advance rather than define it during our trial process later. Our study should be designed to answer the primary question and our sample size calculation should be based on it. It is unacceptable if a proposal lacks clear statement about primary question. Some research questions are important in public health and clinical practice, but we cannot answer them all due to limited technology and resources. So answerable is another criterion in choosing primary question.Therefore, at the design stage we must choose a primary question that we are able to answer via clinical trials.For example, in a study on ‘Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia’[1], the primary question is to find the treatment difference between ibrutinib and chlorambucil for ‘previously untreated older patients with CLL or small lymphocytic lymphoma’. All the other research questions are secondary.
Secondary questions are closely related to the primary question. A study may have several secondary questions, which must be stated in advance or at design stage. We do not recommend mining data after the trial although data mining has become increasingly important in other settings. The number of secondary questions should be limited as well. Otherwise we may not have sufficient power to answer truly important questions and effectively control false positive rate. In Physicians’ Health Study[2], the primary question is total mortality rate between aspirin and placebo groups. The secondary question is fatal and nonfatal myocardial infarction.
Once the primary question has been determined,the next step is to define the primary outcome that is used to describe the primary question. We must pay special attention to primary outcome and it must be evaluated in each of all study participants in the same way. Also the evaluation must be unbiased. Our recommendation is to use double blind, hard endpoint,and independent assessment. All study participants should have primary outcomes when the study ends[3,4].
2. Hypothesis testing
The methods for answering scientific questions from data collected in clinical trials belong to statistical inference. An important part of statistical inference is hypothesis testing, the foundation of which was laid by Fisher, Neyman, and Pearson among others[5]. Hypotheses consist of null hypothesis (H0)and alternative hypothesis (H1). The H1is our scientific hypothesis, which is what we want to collect data for.To test if H1is true, we start from H0, our straw man,since we usually already know from our pilot data,animal model or other approaches that H1is true. So we assume that H0is true and show that the probability that the observed data satisfies the null H0is very small (<0.05, usually). Thus, the null H0is opposite to the scientific hypothesis, the alternative H1, such as H0: cure rate for the standard therapy is equivalent to that for the experimental intervention vs. H1: cure rate for the standard therapy is different from that of the experimental intervention.
If we use µ1and µ2to represent the cure rates of the standard therapy and the experimental intervention,the H0and H1can be quantitatively expressed as: Null hypothesis (H0): µ1- µ2= 0 (no difference) vs. Alternative hypothesis (H1): µ1- µ2≠ 0 (difference)
Of course, our alternative hypothesis should be stated as µ1<µ2, if we are sure that µ1>µ2will not happen. Such one-sided alternative increases power for the same sample size. If we already have data, we can easily test the above hypotheses[6]. Hypothesis testing in clinical trials is analogous to trials by juries in court cases in which the null hypothesis is that a defendant is presumed innocent, while the alternative hypothesis is that the defendant is proven guilty. Our inference of rejecting the null and accepting the alternative hypothesis at type I error α = 0.05 corresponds to a judicial decision that finds the defendant guilty beyond reasonable doubt. Likewise, a non-conviction decision frees the defendant, it does not imply that the defendant truly does not commit the prosecuted crime, but rather only indicates that there is not enough evidence for the conviction. Therefore, we have two types of errors in making the decision. The type I error or false positive alpha (α) refers to the error in convicting the innocent or, in a clinical trial, conclude the treatments differ, when, in fact, they are the same.The type II error or false negative beta (β) is the error in freeing the guilty or failing to conclude the treatments differ, when, in fact, they are different. In statistics,we often use 1-β, or statistical power, which is the probability for statistically detecting difference when true difference exists. We must control or minimize both types of errors or have small false positive rate and high power for a credible trial. High proportion of early published negative trials did not have adequate power due to small sample sizes and Freiman et al.[7]showed that 50 of 71 negative trials that they had surveyed could miss a 50% benefit. We need a sample size large enough to have small false positive rate and high power in detecting the difference for the primary outcome.
3. Sample size
Sample size for hypothesis testing of the primary question is a function of the type I error rate or significance level (usually 0.05 or smaller), power (1-β,80% or larger), minimal clinically significant difference in primary outcome by treatments (set by investigators),and measure of variability (usually from pilot or related studies) in primary outcome. Suppose we have a continuous response variable from 2 independent samples for testing H0: µ1- µ2= 0 vs. H1: µ1- µ2≠ 0, 2-sided alternative. Our total sample size[8]is

where N=2N1=2N2(N1and N2are corresponding group sample sizes), Zais critical value corresponding to 2-sided, Type I error rate, Zbis critical value corresponding to Type II error rate, d is effect size or the minimal detectable difference, and s is the standard deviation for the primary outcome. We can see the relationship among sample size, power (Type II error),Type I error, effect size, and standard deviation. Often we may not have any estimate for effect size d and standard deviation s. Cohen defines
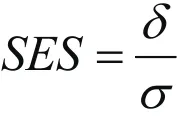
as standardized effect size (SES) when outcome standard deviation estimates are not available[9]. Then the formula for total sample size becomes

The SES is widely used to guide power analysis, with the general guideline: small effect size, SES=0.2, medium effect size, SES=0.5, and large effect size, SES=0.8. We may use this guideline for estimating our sample size accordingly if we do not have any preliminary data.
4. Equivalent test and other extensions
Another popular type of hypothesis testing is equivalence. The new treatment may not be better than the standard, but offers other benefits, such as cost saving, fewer side effects, or easier to administer, etc. In such a study, the hypotheses are set as following:
Null hypothesis: Cure rate for new treatment is worse than that for standard therapy.
Alternative hypothesis: Cure rate for new treatment is“clinically equivalent” to that for standard therapy:
Null hypothesis: |µ1 - µ2| > Δ vs Alternative hypothesis:|µ1 - µ2| ≤ Δ
where Δ is a minimum clinically-meaningful difference.Note here that Δ is defined by clinicians according to their experience and not by any statistical methods.Please see[10]for a recent example on equivalent test of thrombolytic therapy for acute ischemic stroke.
Readers are referred to[8]for methods of sample size calculations for testing different kinds of hypotheses.Also see[11]for advanced topics on testing statistical hypotheses.
In many studies, we often continuously monitor the trials for important differences in toxicity among treatments, for “definitive” differences in outcome among treatments, and/or for unlikely important differences. The goal of interim analyses is to assess the need to stop study early for severe side effects,efficacy, or futility, or to modify protocol (which belongs to adaptive design). Theoretically, we may have as many interim analyses as we want. But the more interim analyses we perform at say α =0.05, the more likely we declare “statistical significance”, increasing the type I error. So we need to adjust our significance level according to the number of interim analyses performed. Please see[12]for statistical techniques on such adjustments. In practice, interim analyses are quite costly, with 2 or 3 interim analyses more popular than 5 or 6. Interim analyses are common for studies with high risk treatments such as cancer, especially in Children’s Oncology Group (COG) studies[13,14].
In this report, we have discussed components of traditional clinical trials, in which primary question and hypotheses do not change during the process of the trial. But the scenario is different in modern adaptive designs. ‘An adaptive design is referred to as a clinical trial design that uses accumulating data to decide on how to modify aspects of the study as it continues,without undermining the validity and integrity of the trial’[15]. The FDA’s definition is ‘An adaptive design for a medical device clinical study is defined as a clinical trial design that allows for prospectively planned modifications based on accumulating study data without undermining the trial’s integrity and validity’[16]. An adaptation is defined as a change or modification made to a clinical trial before and during the conduct of the study. Examples include relax inclusion/exclusion criteria, change study endpoints, modify dose and treatment duration, change primary question, change hypotheses etc.[17]. For Bayesian approach of adaptive design, please see[18].
Funding
Not available.
Conflict of interest statement
The authors report no conflict of interest
Acknowledgement
Thanks are given to the two anonymous editors for their valuable comments.
Reference
1. Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P,et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med. 2015; 373(25): 2425-2437. doi: http://dx.doi.org/10.1056/NEJMoa1509388
2. Steering Committee of the Physicains’ Health Study Research Group. Final report on the aspirin component of the ongoing physicians’ health study. N Engl J Med. 1989; 321(3): 129-135. doi: http://dx.doi.org/10.1056/NEJM198907203210301
3. Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical trials, 4thEd. New York; 2010
4. Meinert CL. Clinical Trials: Design, Conduct, and Analysis.New York: Oxford University Press; 2012
5. Lehmann EL. The fisher, neyman-pearson theories of testing hypotheses: One theory or two? J Am Stat Assoc. 1993;88(424): 1242-1249. doi: http://dx.doi.org/10.1080/016214 59.1993.10476404
6. van Belle G, Fisher LD, Heagerty PJ, Lumley T. Biostatistics: a Methodology for Health Sciences, 2ndEd. New Jersey: Wiley& Sons, Hoboken; 2004
7. Freiman JA, Chalmers TC, Smith Jr.H, Kuebler RR. The Importance of Beta, the Type II Error and sample size in the design and interpretation of the randomized control trial — survey of 71 negative trials. N Engl J Med.1978; 299(3): 690-694. doi: http://dx.doi.org/10.1056/NEJM197809282991304
8. Chow S, Shao J, Wang H. Sample Size Calculations in Clinical Researches. New York: Marcel Dekker; 2003
9. Cohen J. A power primer. Psychol Bull. 1992; 112(1): 155-159. doi: http://dx.doi.org/10.1037/0033-2909.112.1.155
10. Anderson CS, Robinson T, Lindley RI, Arima H, Lavados PM,Lee TH, et al. Low-dose versus standard-dose intravenous Alteplase in acute ischemic stroke. N Engl J Med. 2016;374(24): 2313-2323. doi: http://dx.doi.org/10.1056/NEJMoa1515510
11. Lehmann EL. Testing Statistical Hypotheses, 2ndEd. New York: Springer; 1986
12. Jennison C, Turnbull BW. Group sequential methods with applications to clinical trials. Boca Raton, FL: Chapman &Hall/CRC; 1999
13. Malempati S, Hawkins DS. Rhabdomyosarcoma: Review of the Children’s Oncology Group (COG) Soft-Tissue Sarcoma Committee Experience and Rationale for Current COG Studies. Pediatri Blood Cancer. 2012; 59(1): 5-10. doi:http://dx.doi.org/10.1002/pbc.24118
14. Rudzinski ER, Anderson JR, Hawkins DS, Skapek SX, Parham DM, Teot LA. The World Health Organization Classification of Skeletal Muscle Tumors in Pediatric Rhabdomyosarcoma:A Report from the Children’s Oncology Group. Arch Pathol Lab Med. 2015; 139(10): 1281-1287. doi: http://dx.doi.org/10.5858/arpa.2014-0475-OA
15. Gallo P, Chow CS, Dragalin V, Gaydos B, Krams M, Pinheiro J.Adaptive designs in clinical drug development—an executive summary of the PhRMA working group. J Biopharm Stat.2006; 16(3): 275-283
16. FDA. Adaptive Designs for Medical Device Clinical Studies:Draft Guidance. FDA; 2015 [cited 2016]. Available from: http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm446729.pdf
17. Chow SC, Chang M. Adaptive Design Methods in Clinical Trials. Boca Raton, FL: Chapman & Hall/CRC; 2007
18. Berry SM, Carlin BP, Lee JJ, Muller P. Bayesian Adaptive Design. Boca Raton, FL: Chapman & Hall/CRC; 2010

Dr. Jiangtao Luo obtained his PhD degree from the University of Florida in 2009. He started to work at the University of Nebraska Medical Center College of Public Health, USA in 2010, and is currently an assistant professor in the Department of Biostatistics. His research interests include design of medical studies, statistical genetics, Bayesian method, numerical optimization, and big data.
随机对照临床试验中的主要问题和假设检验
Jiangtao LUO
假设、随机、临床试验、主要问题
We briefly reviewed and provided cautions about some of the fundamental concepts used in the design of medical and public studies, especially primary question, hypothesis testing and sample size in this short note. We also talked about some of the extensions and development in the recent years.
[Shanghai Arch Psychiatry. 2016; 28(3): 177-180.
http://dx.doi.org/10.11919/j.issn.1002-0829.216057]
Department of Biostatistics, College of Public Health, University of Nebraska Medical Center, Omaha, NE 68198-4375 USA
correspondence: Luo Jiangtao. Mailing address: Department of Biostatistics, College of Public Health, University of Nebraska Medical Center, 984375 Nebraska Medical Center, Omaha, NE 68198-4375 USA. Postcode: NE 68198-4375 USA. E-mail: Jiangtao.luo@unmc.edu
概述:本文中我们简要地回顾并提供了医疗和公共研究设计中使用的一些基本概念的有关注意事项,特别是主要问题、假设检验、和样本量。此外,我们还讨论了最近几年的一些扩展和发展。
- 上海精神医学的其它文章
- The clinical value,principle,and basic practical technique of mindfulness intervention
- Sleep quality in university students with Premenstrual Dysphoric Disorder
- Psychometric properties of the Chinese version of the Arabic Scale of Death Anxiety
- An analysis of factors influencing drinking relapse among patients with alcohol-induced psychiatric and behavioral disorders
- Literature searches in the conduct of systematic reviews and evaluations
- Current problems in the research and development of more effective antidepressants

