Resultsof cryoenergy andradiofrequency-based catheterablation for treating ventricular arrhythmias arising from the papillary muscles of the left ventricle, guided by intracardiac echocardiography and image integration
Resultsof cryoenergy andradiofrequency-based catheterablation for treating ventricular arrhythmias arising from the papillary muscles of the left ventricle, guided by intracardiac echocardiography and image integration
The papillary muscles(PMs)from the left ventricle (LV)have been shown to be a potential site of origin of ventricular arrhythmias(VAs)in patients with and without structural heart disease.Catheter ablation has been described as an effective treatment for these arrhythmias,although radiofrequency delivery at these regions has been associated with poor manipulation and catheter stability compared with other VAs.This study compares procedural outcomes and recurrence rate after catheter cryoablation or radiofrequency ablation for the treatment of ventricular tachycardia(VT)and premature ventricular complexes(PVCs)localized at the PMs of the LV,with the aid of intracardiac echocardiography(ICE) and image integration.
A total of 21 patients with recurrent VAs originating at the PMs of the LV were identified from retrospective review of 189 consecutive patients with symptomatic sustained VT,nonsustained VT,or PVC referred for catheter ablation.The sites of origin of idiopathic VAs included the posteromedial PM(PMPM) in 19(10%),and anterolateral PM(ALPM)in 3(1.5%).
One operator treated the first 12 patients with cryoenergy,and a different operator treated the following 9 patients,using radiofrequency energy.Procedural outcomes suchas catheterstability,incidence of multiple VAs morphologies during energy delivery, acute success rate,and long-term recurrence rate were comparedbetweenthosepatientstreatedwith cryoenergy and those with radiofrequency.For mapping and pacing,standard multielectrode catheters were placed in the coronary sinus(CS),His bundle region, and RV apex through the right femoral vein.Arrhythmia inductionwasattemptedbyprogrammedelectric stimulation from the RV apex,RV outflow tract,and CS, with 1,2,and 3 extrastimuli introduced after an 8-beat drive train,if necessary,with the addition of an isoproterenol infusion.
Imaging
A 2-dimensional ICE probe was positioned toward the RV outflow tract and RV inflow tract to visualize the different LV structures,as shown in Figure 1.To specify VAs origin and catheter position,3 segments were attributed to each PM:the apex,at the point of insertion of the chords(distal third of the PM);the body(middle portion of the PM);and the base,at the LV wall insertion. Catheter position,contact,and stability were assessed through this method.Catheter stability was defined asthe absence of back and forth movement of the catheter during energy delivery at the effective lesion site.
Multidetector computed tomography(MDCT)was performed with a 64-detector<15 days before catheter ablation.No ionic contrast material was used and scanningwasperformedwithacollimatedslice thickness of 0.9 mm.

Figure 1A,Intracardiac echocardiography(ICE)image of the posteromedial papillary muscle(PMPM).A multipolar catheter is placed over the PMPM,for mapping the clinical arrhythmia.B,ICE image showing the Freezor Max 8 mm cryoablation catheter delivering cryoenergy at the base of the PMPM.C,Clinical ventricular tachycardia.D,Pace-mapping score of 24 at the site of effective lesion.E,Left anterior oblique and(F)right anterior oblique fluoroscopic projection of the cryocatheter at the effective lesion site.
Cryoablation
Cryoenergy was delivered at myocardial sites exhibiting the earliest bipolar activity or local unipolar QS pattern or at a Purkinje network with an early activity preceding the QRS onset for≥25 ms during the VA(Figure 2)at pace-mapping areas exhibiting QRS match of≥11 of 12.Focal ablation was performed with a 9-Fr/8-mm cryoablation catheter through a transeptal and transmitral approach.Transeptal access was obtained by performing a transeptal puncture with a Brockenbrough needle.Special attention was given to catheter manipulation inside the LV to avoid damage to the chords because the cryocatheter is stiffer than the radiofrequency catheter.This was continuously assessed by ICE.When a reduction in the incidence of VT or PVCs was observed,cryoenergy was delivered for≤240s with 2 freeze-thaw-freeze1cycles; otherwise,cryoenergy delivery was terminated,and the catheter was repositioned.
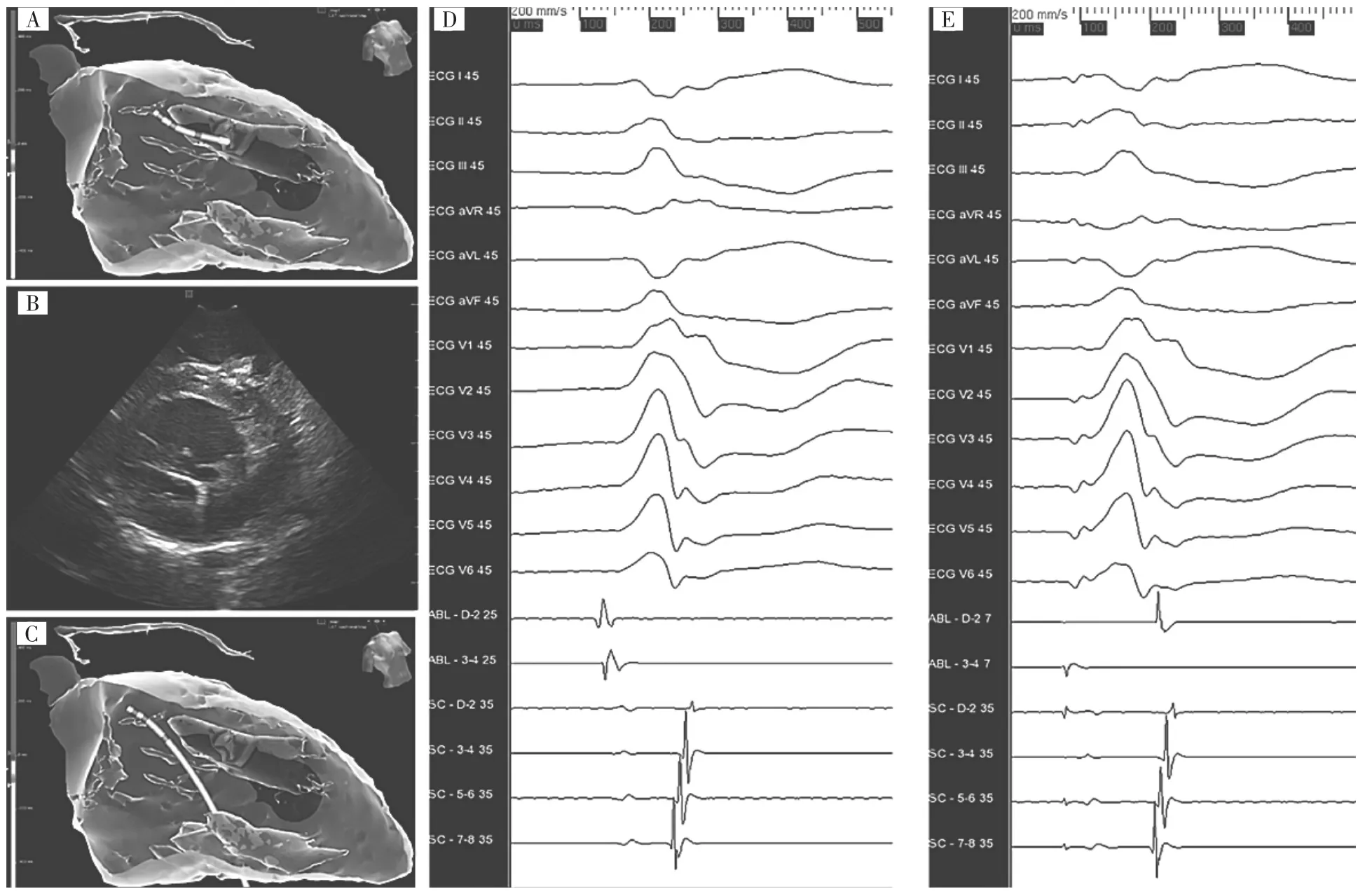
Figure 2Cryoablation.A,Activation map of the clinical arrhythmia at the body of the anterolateral papillary muscle(ALPM),in the 3-dimensional integrated left ventricle(LV)multidetector computed tomography model.The cryocatheter is positioned at the earliest activation site.B,Intracardiac echocardiography(ICE)visualization of the cryocatheter at the body of the ALPM.C,Melt-down effect during cryoenergy delivery at the ALPM. This corresponds to an artifact generated by the cryoenergy over the mapping system.The movement of the catheter is only apparent and continuous assessment of catheter position and stability during energy delivery is achieved by ICE.D,Clinical premature ventricular complex mapped at the body of the ALPM.The VEGM-QRS interval was measured to 35 ms.E,Pace mapping at the site of earliest activation,accounting for a score of 24.
Radiofrequency Ablation
Radiofrequency energy was delivered using the same criteria previously described.Focal ablation was performed with a 4-mm open irrigated radiofrequency ablation catheter through a transmitral or transaortic approach.When a reduction in the incidence of VT or PVCs was observed radiofrequency was delivered for≤90 s,with 2 posterior 45-s consolidation lesions at the same area;otherwise,radiofrequency delivery was terminated,and the catheter was repositioned.
The end point of catheter ablation was the eliminationandnoninducibilityofVAsduring isoproterenol infusion(2-10 μg/min)and burst pacing from the RV to a cycle length as short as 300 ms. Procedural acute success was defined as abolition of inducible or spontaneous VA.
Procedural Outcomes
Termination of the arrhythmia was observed in 19 patients without further inducibility.There were no intraoperativecomplications.Patientstreatedwith cryoenergy(n=12)showed a 100%acute success rate, whereasthosetreatedwithradiofrequency(n=9) presented a 78%success rate.Catheter stability was achieved in all patients treated with cryoenergy and only in 2 patients treated with radiofrequency.Incidence of multiple VA morphologies was observed in 7 patients treated with radiofrequency(77.7%),whereas none was observed in those treated with cryoenergy.All patients included in this study had single VA morphology before catheter ablation.
In the cryoablation group,VAs originating from the base of the PM were observed in 6 patients,the apex in3 patients,and the body in 3 patients.In the radiofrequency group,VAs originating from the base of the PM were observed in 6 patients,and 3 patients showed VAs from the body.Pace mapping showed≥11 of 12 match in all treated PMs at the site of effective lesion.Purkinje potentials(PP)were observed in 13 (62%)patients,mainly in VAs with origin at the base of the PM(base 100%versus body 16 versus apex 0%).
Follow-Up Outcomes
Median follow-up was 360 days(interquartile range,116-365)forcryoablationand87days (interquartile range,65-148)for radiofrequency.There was no increase in the incidence of mitral valve regurgitation,or an increase of MR severity after ablation,using either method.None of the patient treated with cryoablation showed VA recurrence during follow-up(recurrence rate of 0%).One patient from the cryoablation group showed a 50%VA Holter burden reduction,from 40%to 19%,during the first month. After 6 months,Holter burden was<5%and the patient remained asymptomatic,without the use of antiarrhythmic drugs.Four patients(44%)presented VA recurrence during follow-up in the radiofrequency group(P=0.03).VA Holter burden was decreased from 20±15%to 2.8±5%in the cryoablation group and from 21±12%to 11±8%in the radiofrequency group.
Discussion
Catheter irrigation is often used to cool the ablation electrode such that more power can be delivered without being limited by the formation of thrombus at the catheter-tissue interface.Amajorconcernduring radiofrequency ablation is mitral valve dysfunction by injury or rupture of the PMs,specially when using irrigated tip ablation catheters,although this has not yet been reported.Excessive intramyocardial heating can produce steam formation and abrupt volume expansion, which may be audible as steam pops.Pops are capable ofcausingdeeptissuetears,andpatientswith ventricular perforations are more likely to require surgical repair.
Catheter cryoablation was introduced into clinical electrophysiologyin1998.Cryoablationhasbeen reported as a safe alternative for catheter ablation in idiopathic VT arising from the RV outflow tract,aortic cusps,and epicardium.Cryothermal safety profile is attributed to the mechanism of tissue destruction. Histology of chronic lesions shows well-demarcated2lesions with minimal tissue disruption and preserved underlying architecture.Catheter stabilization during ablation at the PMs is a major consideration.Stabilization is achieved because of catheter-tissue adherence after reaching temperatures of-80℃.
词汇
integration n.整合,融合,集成
cryoablation n.冷冻消融
cryoenergy n.冷冻能量
probe n.&v.探测器,探针,探头;探索
specify v.具体指定,详细说明,列举
collimate v.使...平行,校准
thaw v,&n,融化,使...融化,变暖和;融化,缓和
consolidation n.巩固,合并,联合
pop adj.&v.&n.&adv.通俗的,流行的;敲击,取出;流行音乐,汽水,砰然声,枪击;突然地,砰地
cryothermal adj.冰冻的,冷冻的,冰温的
disruption n.破裂,混乱
architecture n.建筑学,建筑风格,架构
注释
1.freeze-thaw-freeze指“冷冻-融化-冷冻”过程,冷冻导管消融过程中常采用此方法,可扩大消融的创面,如Data in hepatocellular ablation show that repetitive freezethaw cycles create larger lesions than those obtainedby longer freezing at a certain temperature.肝细胞消融资料显示,在特定的温度下,重复冷冻-融化周期与较长时间的冷冻比较,能产生较大的病损。
2.well-demarcated指“界限明晰的”,如Histological analysisagainrevealedhomogeneouslesionswithwelldemarcated borders.组织学分析再次揭示有着界限明晰边缘的均质病损。
参考译文
第71课腔内心脏超声和图形整合指导下的左心室乳头肌起源室性心律失常的冷冻和射频消融
业已显示左心室乳头肌是伴或不伴心脏结构异常患者心律失常的潜在起源部位。与其他部位室性心律失常比较,这些区域的心律失常消融操作困难、导管稳定性差,但已有导管消融有效的报道。本研究借助心腔内心脏超声和图像整合,比较左心室乳头肌起源室性心动过速或期前搏动的冷冻和射频消融手术结果及复发率。
回顾性分析连续189例因症状性持续性室性心动过速、阵发性室性心动过速或室性期前搏动接受射频导管消融的患者,确定21例为左心室乳头肌起源的反复发作室性心律失常。这一特发性室性心律失常的起源部位包括后内侧乳头肌(19例)和前外侧乳头肌(3例)。
一位术者对最初12例患者行冷冻消融术,另一位术者对随后的9例患者行射频消融术。将冷冻消融术和射频消融术结果进行对比,内容涉及导管稳定性、消融过程中多种形态室性心律失常的发生率、即刻成功率和长期复发率。经右股静脉途径将标准多极导管放置到冠状窦、希氏束和右心室心尖,用于标测和起搏。经右心室心尖部、右心室流出道和冠状窦发放8次短阵刺激加1、2、3个额外刺激,诱发心律失常,必要时加用异丙肾上腺素滴注。
显像
二维心腔内心脏超声探头抵近右心室流出道和流入道,以便显示左心室不同结构,如图1所示。为阐述室性心律失常起源部位和导管位置,将每一乳头肌分成3个节段:尖部,腱索插入点(乳头肌远端1/3);体部(乳头肌中部);基部,左心室壁插入部。通过这种方法对导管的位置、接触和稳定性进行评估。导管的稳定性是指在有效靶点能量释放过程中导管无前后向移动。
于导管消融前15d内做64排CT检查。使用非离子对比剂,扫描层厚0.9mm。
冷冻消融
在起搏标测到QRS波群图形匹配≥11/12的区域,于呈现最早双极激动或局部单极QS图形的心肌部位、或在较室性心律失常QRS群起点提前≥25ms的浦肯氏网部位释放冷冻能量(图2)。采用9-Fr/8-mm冷冻消融导管跨间隔和跨二尖瓣方法进行冷冻消融。用Brockenbrough穿间隔针建立跨间隔通路。导管进入左心室的操作应特别小心,以免损伤腱索,因为冷冻导管比射频消融导管硬。心腔内心脏超声连续评估此过程。当观察到室性心动过速或室性期前搏动减少时,继续释放冷冻能量,不超过240s,重复冷冻-融化-冷冻两轮,否则,停止冷冻,调整导管位置。
射频消融
按照之前描述的同一标准释放射频能量。采用4mm开放式灌注射频消融导管经跨二尖瓣或主动脉瓣途径作病灶消融。当观察到室性心动过速或室性期前搏动减少时,继续释放射频能量,不超过90s,对同一区域追加2次45s的巩固消融;否则,停止消融,调整导管位置。
导管消融的终点是消除室性心律失常,且静脉滴注异丙肾上腺素(2~10μg/min)及右心室猝发起搏周长短至300ms下,不能诱发室性心律失常。手术即刻成功率定义为消除诱发的或自发的室性心律失常。
手术结果
19例患者心律失常得到中止且不能被进一步诱发。术中无并发症。冷冻治疗组(12例)手术即刻成功率100%,而射频治疗组(9例)成功率78%。冷冻治疗组所有患者导管稳定性好,而射频治疗组导管稳定性好的只有2例。射频治疗组7例(77.7%)发生多形室性心律失常,而冷冻治疗组无多形室性心律失常发生。本研究中所有患者消融前均呈单一形态的室性心律失常。
在冷冻消融组,室性心律失常起源于乳头肌基部6例,体部和尖部各3例。射频消融组,室性心律失常起源于乳头肌基部6例,体部3例。所有乳头肌有效部位起搏标测显示匹配达≥11/12。浦肯野电位见于13例(62%),主要见于乳头肌基部起源的室性心律失常(基部100%、体部16%、尖部0%)。
随访结果
冷冻消融组随访时间中位数360d,射频消融组87d。两组消融后均无增加二尖瓣脱垂的发生率或增加二尖瓣反流严重性。随访期间冷冻消融组无室性心律失常复发。冷冻消融组1例随访第1个月24h动态心电图即显示室性心律失常负荷下降50%,从40%下降到19%。6个月后,动态心电图负荷<5%,无症状,未服用抗心律失常药物。随访中射频消融组4例(44%)出现室性心律失常复发。动态心电图负荷冷冻消融组从(20±15)%降至(2.8±5)%,射频消融组从(21± 12)%降至(11±8)%。
讨论
导管灌注常用于冷却消融电极,有利于释放更大的能量,而不受导管-组织接触界面血栓形成的限制。射频消融中主要关注的是乳头肌损伤或断裂而导致二尖瓣功能不全,特别在使用灌注消融导管时,尽管尚未见报道。心肌内加热可产生蒸汽和突发容量膨胀,这可听到蒸汽爆破声。爆破可引起深部组织撕裂,心室穿孔的患者更多需要外科修补。
导管冷冻消融于1998年用于临床电生理学。有报告认为冷冻消融是右心室流出道、主动脉窦和心外膜起源特发性室性心动过速导管消融的安全替代方法。冷冻安全性取决于组织破坏的机制。慢性病损的组织学检查显示病损界限明晰,组织断裂小,保留基本架构。乳头肌部位消融主要关注的是导管稳定性。当温度达到-80℃时,导管-组织粘着而达到稳定。
图1A.后内测乳头肌(PMPM)心腔内心脏超声显像(ICE)。多极导管置于PMPM表面,用于标测临床心律失常。B.ICE显示Freezor Max 8 mm冷冻导管于PMPM基部释放冷冻能量。C.临床室性心动过速。D.有效部位起搏标测评分24分。E.有效部位冷冻导管左前斜位。F.右前斜位透视投照位。
图2冷冻消融。A.三维整合左心室多排CT模型中,于前外侧乳头肌(ALPM)体部激动标测临床心律失常。冷冻导管位于最早激动部位。B.ICE显示冷冻导管位于ALPM体部。C.ALPM部位冷冻消融过程中融化效应。这与标测系统上冷冻能量产生的伪影相一致。唯一明显的是导管移动,通过ICE连续评估能量释放过程中导管的位置和稳定性。D.ALPM体部标测到临床期前室性搏动。测得VEGM-QRS间期达35ms.E.最早激动部位起搏标测,评分24分。
[1]Rivera S,Ricapito Mde L,Tomas L,et al.Results of Cryoenergy and Radiofrequency-Based Catheter Ablation for Treating Ventricular Arrhythmias Arising From the Papillary Muscles of the Left Ventricle, Guided by Intracardiac Echocardiography and Image Integration[J]. Circ Arrhythm Electrophysiol,2016,9(4)∶e003874.
(童鸿)
《食管心脏电生理技术快速参考》由浙江省人民医院蔡卫勋、李忠杰主编,已于近期发行。本书重点介绍了食管心脏电生理技术的方法、技巧,并对可能遇到的诊断、操作等问题作整理解答。全书共5章,100个问答,便于电生理初学者快速参考。定价15元。购书请联系浙江省人民医院心电功能科蔡老师(0571-85893072)。
Lesson Seventy-one

