Ultra-structure of testes of rats born to dams treated withhydroxy-progesterone hexanoate
Samy Ismail Ahmed, Tahir Osman Ali, Adil Salim Elsheikh
1Department of Anatomy, Faculty of Medicine, Najran University, Saudi Arabia
2Faculty of Graduate Studies & Scientific Research, National Ribat University, Sudan
3Department of Applied Medical Sciences, Community College Najran, Saudi Arabia
Ultra-structure of testes of rats born to dams treated withhydroxy-progesterone hexanoate
Samy Ismail Ahmed1*, Tahir Osman Ali2, Adil Salim Elsheikh3
1Department of Anatomy, Faculty of Medicine, Najran University, Saudi Arabia
2Faculty of Graduate Studies & Scientific Research, National Ribat University, Sudan
3Department of Applied Medical Sciences, Community College Najran, Saudi Arabia
ARTICLE INFO
Article history:
Received
Received in revised form
Accepted
Available online
Prenatal exposure
Hydroxy-progesterone hexanoate
Male rats
Testis
Ultra-stature
Objective:To investigate the effects of prenatal exposure of male rats to hydroxy-progesterone hexanoate (HPH) on ultra-structures of testis.Methods:Ten male rat puppies born to dams treated with 10 mg/ kg body weight of HPH on the 1st, 7th and 14th day after conception were allowed to grow for 90 d; sacrificed, their testes dissected and prepared for electron microscope examination.Results:The testes of all rats born to dams treated with HPH had degenerated germ cells that lost their attachment to surrounding sertoli cells (SCs) and the basement membrane. The germinal cells were abnormal with cytoplasmic cavitations. SCs had small invaginated nucleus with peripheral nucleolus, folded cell membrane and large lipid droplets. The cytoplasm of SCs had small cavities and the mitochondriae appeared distended and had lost their cristae. The interstitial spaces were wider with few leydig cells (LCs) that had elongated nucleus with indented nuclear membrane and three prominent nucleoli with dense scattered chromatin. LCs cytoplasm contained abundant lipid droplets, numerous mitochondriae and cytoplasmic vacuoles. The seminiferous tubules germinal epithelium suffered severe degree of degeneration and there were no stages of spermatogenesis.Conclusion:Prenatal exposure of male rats to HPH induces ultra-structural changes in the seminiferous tubules, SCs and LCs as well as it hampers spermatogenesis. Thus the future fecundity of males born to females treated with HPH during pregnancy will inevitably be affected.
1. Introduction
The male reproductive health disorders are steadily increasing among humans[1]. Many manmade chemicals and medicines are accused of these disorders[2]. Synthetic progesterone is one of the medicines that have been accused of this problem,which is widely used in fertility clinics[3-5]. Homeostasis of progesteronewithin the body of the pregnant woman is very critical for maintenance of normal pregnancy. Accordingly worldwide, many females are being extensively exposed to this synthetic progesterone which is thought to elicit adverse effects on development and fertility of the next generation[6]. Many studies have confirmed that males born to females exposed to steroid hormones during pregnancy suffered varying degrees of testicular developmental disorders[7-10].
Males born to dams medicated with synthetic progesterone during pregnancywere reported to have small testicles and damagedseminiferous tubules (ST) with large lumen and devoid of sperm[11]. Histopathological studies of the testes of rats exposed tosynthetic progesteroneduring prenatal life revealed decrement in STs diameters, reduction in the number of spermatogenic cells, widening of tubular lumen with necrozoospermia and widening of the interstitial space[11-13].
Exposure of males to hydroxyl-progesterone caproate during embryonic development was associated with increment in the levels of FSH & LH hormones and reduction in the level of circulatingtestosterone suggesting a probable interference in the synthesis of this hormone[14,15]. Also prenatal exposure tohydroxy-progesterone resulted in permanent alteration in the spermatogenic cells function and suppressed the testicular steroidogenesis by decreasing the steroidogenic enzyme activity which in turns may suppress the reproductive activities in male rat[4]. Although many studies were conducted to study the influences of prenatal exposure to synthetic progesteroneon the function and histology of the testes of rats; ultramicroscopic studies are absent. Therefore the objective of the current study is to elucidate the effects of prenatal exposure of male rats to hydroxy-progesterone hexanoate (HPH) on the ultrastructures of STs, sertoli cells (SCs) and leydig cells (LCs).
2. Materials and methods
2.1. Animals and treatments
Eight to 10 wk old female albino Wistar rats (n=14) were grouped into two groups of 7 females. Every 7 females were kept with a male of proven fertility at 12:12 hlight/dark cycle at 28 ℃±7 ℃temperature, fed on commercial pellets and offered water ad libitum. The female rats were daily examined for the presence of cervical plugs and those with cervical plugs were grouped into 2 groups and kept in sterilized polypropylene cages (90×45×15) cm bedded with woody husk. Group I females were injected with HPH, 10 mg/ kg body weight; subcutaneously on the 1st, 7th and 14th day after the day of cervical plug formation (the probable day of pregnancy). The dose of the hormone was adjusted daily to the body weight of the dams. Group II females were injected on the same days with a placebo to serve as control. Ten male puppies born to HPH treated females and 10 male puppies born to the controls were allowed to grow for 90 d; thereafter they were sacrificed to collect the testicles.
2.2. Tissue collection and preparation
After 90 d of rearing the puppies were sacrificed with cervical dislocation and the testes were dissected[16]. The testes were decapsulated and cut into small pieces and rapidly fixed in 0.1 M cacodylate buffered in 3% gluteraldehyde at 4 ℃ then washed in the 0.1M cacodylate buffer overnight before they were post-fixed in 1% osmium tetroxide in cacodylate buffer for 2 h. Thereafter they were dehydrated in an ascending series of ethyl alcohol and cleared in propylene oxide and embedded in epoxy resin in an oven at 60 ℃ for 20 h to produce affirm block. Ultrathin sections were cut from the desired parts, mounted on perforated copper grids (Plano, Wetzlar, Germany) and stained with uranyl acetate and lead citrate[17]. The sections were examined under a transmission electron microscope with 80 KV acceleration voltages (JEOL, 1200 EXII, Tokyo, Japan).
3. Results
Electron microscopic examination of the STs and the interstitial spaces of the control rats revealed normal spermatogenic cells, SCs and LCs cells. As in Figure 1(A) the spermatogenic cells were in various normal stages of spermatogenesis. The testes of all rats born to dams treated with P4had degenerated germ cells that lost their attachment to surrounding SCs and the basement membrane. The spermatogonia cells of rats born to dams treated with HPHwere degenerated with vacuolated cytoplasm and small pyknotic nuclei. As in Figure 1(B) there were empty spaces left by the atrophied degenerated germ cells and occasionally the nuclear envelops of the primary spermatocytes had invaginations. The pachytene stage primary spermatocytesappeared smaller in size and the basal lamina appeared thick, folded and contained hypertrophied myoid cells. Thegerminal cells were abnormal with cytoplasmic cavitations (Figure 2). SCs had small invaginated nucleus with peripheral nucleolus, folded cell membrane and large lipid droplets. The cytoplasm of SCs had small cavities as a result of the degenerative processes that affected its micro-organelles. Figure 3(A) shows that the mitochondriae appeared distended and they had lost their cristae and the LCs had elongated nucleus, indented nuclear membrane, three prominent nucleoli and dense scattered chromatin. Additionally Figure 3(B) revealed the cytoplasm of LCs had abundant lipid droplets, numerous mitochondria and cytoplasmic vacuoles.
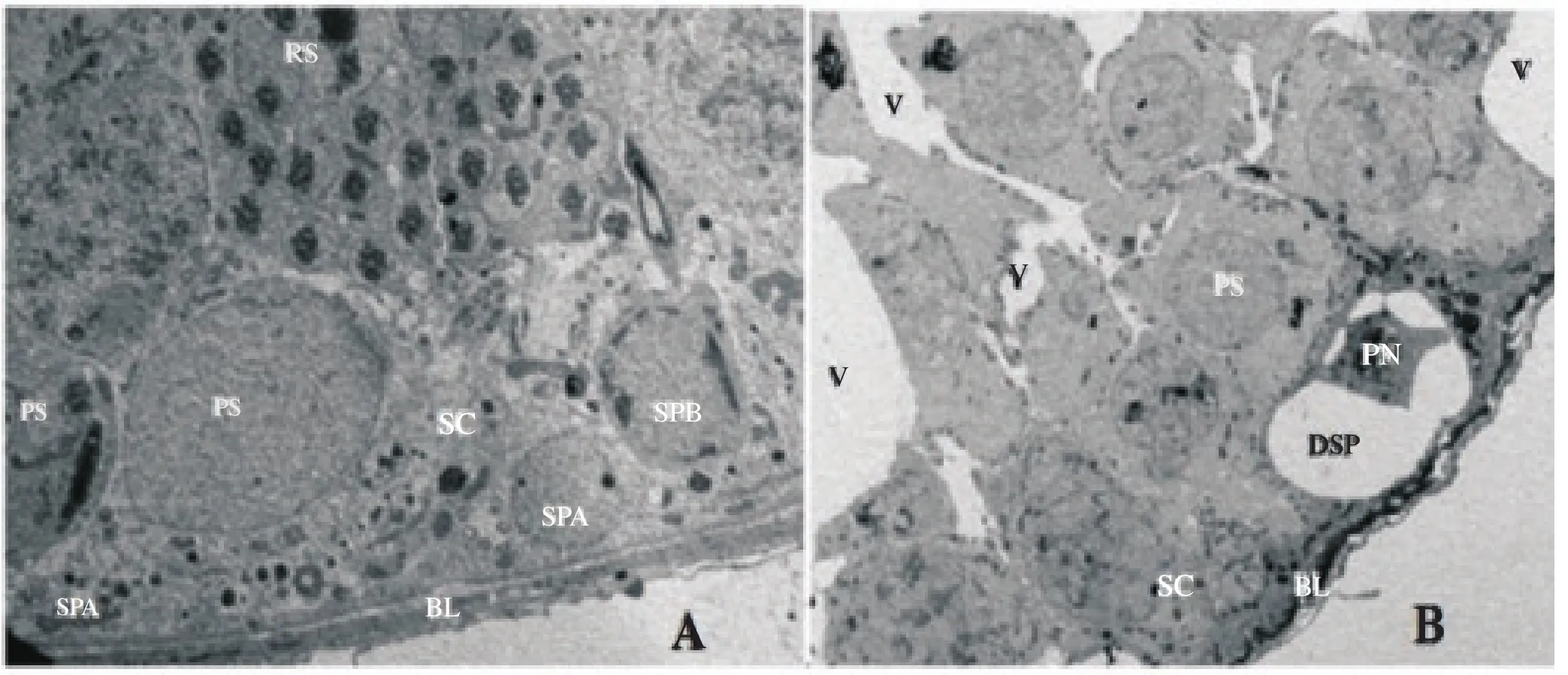
Figure 1. (A),(B): An electron photomicrograph of normal ST of a control rat with normal ultrastructure. SPA: spermatogonia type A; SPB: spermatogonia type B; PS: primary spermatocyte; SC: Sertoli cell; RS: round spermatids (RS), and BL: normal basal lamina. B: An electron photomicrograph of ST of a treatment rat with abnormal ultrastructure. V: vacuoles; PS: small size primary spermatocyte; DSP: degenerated spermatogonia with pyknotic nucleus (PN); SC: Sertoli cell and BL: folded basal lamina (×3000).
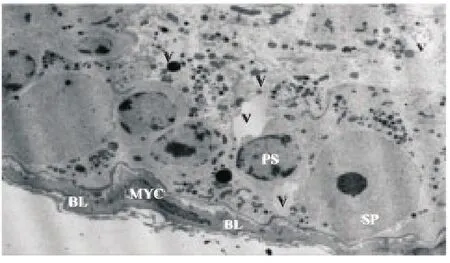
Figure 2. An electron photomicrograph of ST of a rat from the experimental group showing small size pachytene primary spermatocyte (PS) separated from adjoining cell by empty spaces (V), spermatogonia (SP), thick folded basal lamina (BL) and hypertrophied myoid cell (MYC) (×3000).
4. Discussion
The electron microscope examination of the testes of rats born to dams treated with HPH revealed that the testes of the rats had undergone different ultra-structural defects in the STs, SCs and LCs. The STs had undergone serious apoptotic changes especially the germinal epithelium, SCs and LCs.
Some authors reported that the STs of rats born to dams treated with HPH had undergone different degrees of degenerative histopathological changes[12,13,18-20]. The current study agrees to and confirms these findings. The most serious findings of this study are the apoptotic changes of SCs and LCs. It is well known that the development of the male reproductive traits and reproductive tract depends on normal differentiation and development of LCs and SCs during the prenatal life[21-23]. The function of the fetal LCs during prenatal life is to synthesize the androgens that are necessary for the development of the masculine characteristics and play an important role in development of the STs and LCs[6,24,25]. Also the androgens that are produced by the fetal LCs are essential for the healthiness of SCs that nurses the spermatogenic cells. Since in this study SCs and LCs were seriously affected by prenatal exposure to HPH and developed serious degenerative changes; the germination of the spermatogonia cells and spermatogenesis process were also affected. A similar phenomenon was reported in testes of rats born to dams treated with HPH during pregnancy[13,26]. The SCs is responsible of orchestrating spermatocytogenesis and spermatogenesis through sequential phases of mitosis, meiosis, and differentiation through hormonal, nutritional, and physical support.
The SCs of the experimental rats had undergone various apoptotic changes such as an ill-defined nucleolus, folded cell membrane, cytoplasmic vacuoles, distended mitochondria and increment of lipid droplet. Furthermore empty vacuolar spaces were observed in the places between SCs where spermatogonia and spermatocytes should differentiate and germinate. These vacuoles indicate the failure of spermatocytogenesis. The ultra-structural apoptotic changes of the germinal epithelium found here support the findings of Blanco et al.[20] who described apoptosis in hamster testis following treatment with anabolic androgenic steroids. It is well known that the elimination apoptotic process develop when drugsand toxic agents injure the SCs and reduce their supportive role in spermatogenesis[15]. These apoptotic and degenerative changes that occur in cells and tissues are due to oxidative stress caused by toxic agents[27]. From the results of the current study probably HPH and/ or its metabolites reached the fetal testicular circuit and induced an oxidative stress on the fetal testes. Consequently the antioxidant and peroxidation mechanisms within the testes were affected leading to the observed cellular changes[28].

Figure 3. (A),(B): An electron photomicrograph of SC of a rat born to dams treated with P4. The nucleus (N) is small in size, invaginated with ill-defined nucleolus (NU) and the cell membrane (CM) is folded. The cytoplasm contains large & small vacuoles (V), distended mitochondria (M) and lipid droplet (LD). B: LC of a rat born to dams treated with P4with elongated nucleus (N), indented nuclear membrane (NM), numerous mitochondria (M) and large amounts of lipid droplets (L) within the cytoplasm (×4000).
Conflict of interest statement
The authors declare that they have no conflict of interest.
[1] Pushpalatha T, Reddy PR, Reddy PS. Gestational exposure to hydroxyprogesteronecaproate suppresses reproductive potential in male rats. Naturwissenschaften 2005; 92(8): 385-388.
[2] Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. Br Med J 1992; 304: 609-613.
[3] Marianowski P, Radwańska E. Intramuscular vs vaginal progesterone for luteal support in cycles of in vitro fertilization. Ginekol Pol 2000; 71(9): 1064-1070.
[4] Pushpalatha T, Reddy PR, Reddy PS. Effect of prenatal exposure to hydroxyprogesterone on steroidogenic enzymes in male rats. Naturwissenschaften 2002; 90: 40-43.
[5] Vidaeff AC, Belfort MA. Critical appraisal of the efficacy, safety, and patient acceptability of hydroxyprogesteronecaproate injection to reduce the risk of preterm birth. Patient Prefer Adherence 2013; 7: 683-691.
[6] Palmlund I, Apfel R, Buitendijk S, Cabau A, Forsberg JG. Effect of diethylstilbestrol (DES) medication during pregnancy. J Psychosom Obstet Gynaecol 1993; 14:71-89.
[7] Fielden MR, Halgren RG, Fong CJ, Staub C, Johnson L, Chou K, et al. Gestational and lactational exposure of male mice to diethylstilbestrol causes long-term effects on the testis sperm fertilizing ability in vitro and testicular gene expression. Endocrinology 2002; 143: 3044-3059.
[8] Goyal HO, Robateau A, Braden TD, Williams CS, Srivastava KK, Ali K. Neonatal estrogen exposure of male rats alters reproductive functions at adulthood. Biol Reprod 2003; 68: 2081-2091.
[9] Storgaard L, Bonde JP, Olsen J. Male reproductive disorders in humans and prenatal indicators of estrogen exposure. A review of published epidemiological studies. Reprod Toxicol 2006; 21(1): 4-15.
[10] Delbes G, Levacher C, Habert R. Estrogen effects on fetal and neonatal testicular development. Reproduction 2006; 132: 527-538.
[11] Harini C, Sainath SB, Reddy PS. Recovery of suppressed male reproduction in mice exposed to progesterone during embryonic development by testosterone. Reproduction 2009; 137(3): 439-448.
[12] Pushpalatha T, Reddy RP, Trivikram G, Reddy SP. Reduced spermatogenesis in rats exposed trans-placently tohydroxyprogesterone. Cytologia 2003; 68: 369-373.
[13] Ahmed S I, Elsheikh AS, Attia GA, Ali TO. Prenatal progesterone exposure of male rats induces morphometric and histological changes in testes. Asian Pacific J Reprod 2016; 5(3): 204-209.
[14] Pushpalatha T, Reddy PR, Reddy PS. Impairment of male reproduction in adult rats exposed to hydroxyl-progesterone caproatein utero. Naturwissenschaften 2004; 91: 242-244.
[15] Northen AT, Norman GS, Anderson K, Anderson GD. Follow-up of children exposed in utero to 17 α-hydroxyprogesterone caproate compared with placebo. Obstet Gynecol 2007; 110: 865-872.
[16] Elsheikh AS, Takahashi Y, Hishinuma M, Kanagawa H. Effect of encapsulation on development of mouse pronuclear stage embryos in vitro. Anim Reprod Sci 1997; 48: 317-324.
[17] Bancroft JD. Fixation and fixatives. In: Theory and practice of histological techniques. New York: Churchill Livingstone; 2003. p.76.
[18] Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-Disrupting Chemicals. Endocr Rev 2009; 30: 293-342.
[19] Gopalkrishnan K, Gill-Sharma MK, Balasinor N, Padwal V, D'Souza S, Parte P, et al. Tamoxifen-induced light and electron microscopic changes in the rat testicular morphology and serum hormonal profile of reproductive hormones. Contraception 1998; 57: 261-270.
[20] Blanco A, Flores-Acuna F, Roldan-villalobos R, monterde JG. Testicular damage from anabolic treatments with the beta (2)-adrenergic agonist clenbuterol in pigs: a light and electron microscope study. Vet J 2002; 163: 292-298.
[21] Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun 1982; 107: 1198-1205.
[22] Husain K, Somani SM. Interaction of exercise training and chronic ethanol ingestion on testicular antioxidant system in rat. J Appl Toxicol 1998; 18: 421-429.
[23] Richburg JH. The relevance of spontaneous-and chemically-induced alterations in testicular germ cell apoptosis to toxicology. Toxicol Lett 2000; 15: 79-86.
[24] Abney TO. The potential roles of estrogens in regulating Leydig cell development and function: a review. Steroids 1999; 64: 610-617.
[25] O'Donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocr Rev 2001; 22(3): 289-318.
[26] Sharpe RM, Rivas A, Walker M, McKinnell C, Fisher JS. Effect of neonatal treatment of rats with potent or weak (environmental) oestrogens, or with a GnRH antagonist, on Leydig cell development and function through puberty into adulthood. Int J Androl 2003; 26: 26-36.
[27] Kavlock RJ, Daston GP, DeRosa C, Fenner-Crisp P, Gray LE, Kaattari S, et al. Research needs for the risk assessment of health and environmental effectsof endocrine disruptors: A report of the U.S. EPA-sponsored workshop. Environ Health Perspect 1996; 104(Suppl 4): 715-740.
[28] Schlappack OK, Delic JI, Harwood JR, Stanley JA. Protection from radiation-induced damage to spermatogenesis in the androgen pre-treated rats. Radiother Oncol 1988; 12: 219-224.
ment heading
10.1016/j.apjr.2016.10.010
*Corresponding author: Dr. Samy Ismail Ahmed Mustafa, Department of Anatomy, Faculty of Medicine, Najran University, Saudi Arabia.
E-mail: samyanatomist@yahoo.com
 Asian Pacific Journal of Reproduction2016年6期
Asian Pacific Journal of Reproduction2016年6期
- Asian Pacific Journal of Reproduction的其它文章
- Hormonal changes and spermatogenesis of male rat puppies born by mothers consuming soybean extract
- Factors affecting length of gestation in artificially inseminated Marwari Mares of India
- Nicotine effect toward the oocyte level of rats(Rattus novergicus)
- Effect of Thaumatococcus daniellii leaf rat-feed on potassium bromate induced testicular toxicity
- Prolificity of Portuguese Serrana Goats between 1987 and 2015
- The effect of freeze-drying media and storage temperature on ultrastructure and DNA of freeze-dried buffalo bull spermatozoa
