镁粉促进“一锅法”合成α,β-炔基酮类化合物
杨天宇, 黄丹凤, 王克虎, 苏瀛鹏, 胡雨来
(西北师范大学 化学化工学院,甘肃 兰州 730070)
·研究论文·
镁粉促进“一锅法”合成α,β-炔基酮类化合物
杨天宇, 黄丹凤, 王克虎, 苏瀛鹏, 胡雨来*
(西北师范大学 化学化工学院,甘肃 兰州 730070)
报道了镁粉(4)促进下,以Weinreb 酰胺(1a~1j, 1l~1n, 1p~1r)、苯乙炔(2)和正丁基溴(3)为原料,“一锅法”合成α,β-炔酮类化合物(5a~5j, 5l~5n, 5p~5r)的反应。结果表明:在最优反应条件(THF为溶剂,3 1.1 mmol, 4 1.25 mmol,混拌2 h;加入2 0.75 mmol, 搅拌1 h;加入1 0.5 mmol,于室温反应)下,5a~5j, 5l~5n, 5p~5r产率45%~86%,其结构经1H NMR和13C NMR确证。
一锅法合成; 镁粉促进; Weinreb酰胺; 苯乙炔;α,β-炔基酮
α,β-炔酮类化合物同时含有碳碳叁键和羰基两种官能团,反应性质活泼,是有机合成中重要的合成砌块,广泛用于杂环化合物的合成[1-5]。此外,许多天然产物、生物活性物种及有机中间体也含有炔酮结构单元[6-14]。寻找简单,便捷,高效的合成炔酮类化合物的方法成为化学家们关注的重点。
目前,合成该类化合物的方法主要有:(1)炔基化合物与醛发生加成氧化反应[15-18];(2)末端炔与卤化物通过Sonogashira羰基化反应[7,19-25];(3)末端炔或炔基化合物与羧酸衍生物发生Sonogashira酰基化反应[2,7,26-32];(4)高价碘类炔基试剂与醛类化合物发生自由基形式的炔基化反应[33-34];(5)α-酮酸的脱羧炔酮化反应[35];(6)末端炔与卤化物发生异腈插入反应[36]。
Weinreb酰胺(1)作为酰基化合成砌块广泛应用于有机合成中[37-44]。1与金属氢化物反应制得醛,与有机锂或镁试剂反应制得酮,金属试剂过量也不会导致产物进一步反应。这一特点使1在含羰基化合物的合成中占有重要地位。本课题组报道了镁粉(4)促进Weinreb酰胺和卤代烃经“一锅法”合成酮类化合物的反应[45],发现镁粉促进的“一锅法”反应可避免预先制备活泼卤代烃的Grignard试剂。转而通过镁粉与卤代烃的原位反应生成Grignard试剂,然后直接与其它反应物发生反应。
本文以Weinreb 酰胺(1a~1j, 1l~1n, 1p~1r)、苯乙炔(2)和正丁基溴(3)为原料,“一锅法”合成α,β-炔酮类化合物(5a~5j, 5l~5n, 5p~5r, Scheme 1),产率45%~86%,其结构经1H NMR和13C NMR确证。
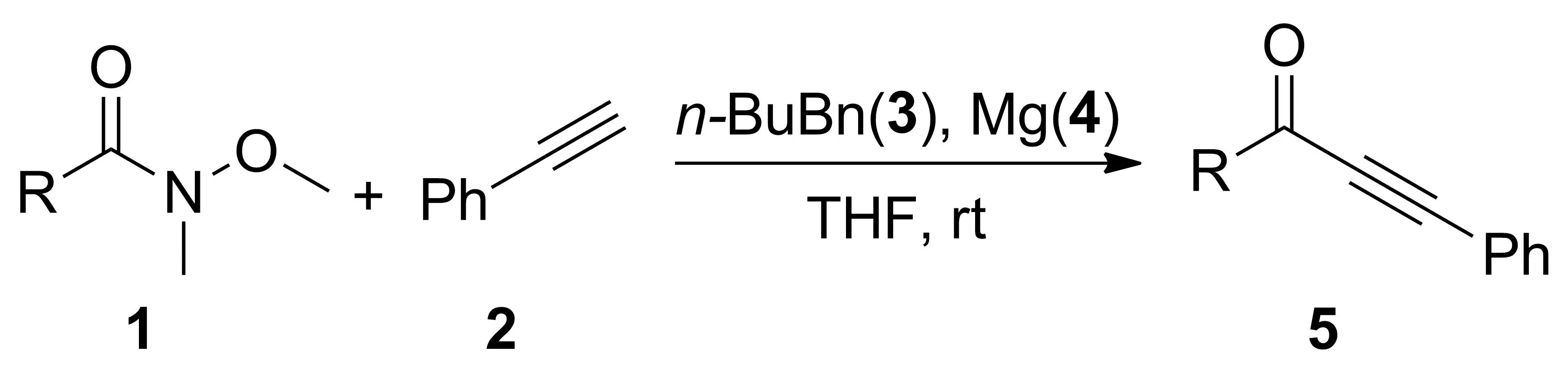
Scheme 1
1 实验部分
1.1 仪器与试剂
X-4B型显微熔点仪(温度未校正);Varian Mercury 400 plus型核磁共振仪和Agilent DD2600型核磁共振仪(CDCl3为溶剂,TMS为内标)。
石油醚(沸程60~90 ℃)和乙酸乙酯,工业级;1按文献[46]方法合成;其余所用试剂均为分析纯,THF使用前经除水除氧处理。
1.2 5的合成通法
氩气保护下,在干燥的两口瓶中加入4 0.03 g(1.25 mmol)和THF 1 mL,于室温搅拌10 min;滴加3 0.15 g(1.1 mmol),滴毕,加热至微沸(约2 min),冷却至室温,搅拌2 h;缓慢滴加2 0.07 g(0.75 mmol)的THF(2 mL)溶液,滴毕,搅拌1 h;滴加1(0.5 mmol),滴毕,于室温反应至终点(TLC检测)。加入饱和氯化铵溶液10 mL淬灭反应,用乙酸乙酯(3×10 mL)萃取,合并有机相,用无水硫酸镁干燥,减压蒸出乙酸乙酯,残余物经硅胶柱层析[洗脱剂:V(石油醚) ∶V(乙酸乙酯)=30 ∶1]纯化得5。
1,3-二苯基-2-丙炔-1-酮(5a)[24]: 黄色液体,产率86%;1H NMRδ: 8.23~8.22(m, 2H), 7.69~7.68(m, 2H), 7.63(t,J=6.0 Hz, 1H), 7.53~7.47(m, 3H), 7.42(t,J=6.0 Hz, 2H);13C NMRδ: 178.0, 136.9, 134.1, 133.0, 130.8, 129.5, 128.6, 128.5, 120.1, 93.1, 86.9。
1-苯基-3-(4-甲苯基)-2-丙炔-1-酮(5b): 淡黄色固体,产率82%, m.p.64~66 ℃(68~70 ℃[28]);1H NMRδ: 8.11(d,J=6.0 Hz, 2H), 7.66(d,J=6.0 Hz, 2H), 7.45(t,J=6.0 Hz, 1H), 7.39(t,J=9.0 Hz, 2H), 7.29(d,J=6.0 Hz, 2H), 2.41(s, 3H);13C NMRδ: 177.5, 145.1, 134.5, 132.9, 130.6, 129.5, 129.2, 128.5, 120.1, 92.5, 86.9, 21.7。
1-苯基-3-(3-甲苯基)-2-丙炔-1-酮(5c)[28]: 黄色液体,产率80%;1H NMRδ: 8.03(d,J=6.0 Hz, 1H), 7.99(s, 1H), 7.66(d,J=6.0 Hz, 2H), 7.45(t,J=6.0 Hz, 1H), 7.41~7.37(m, 4H), 2.42(s, 3H);13C NMRδ: 178.0, 138.3, 136.7, 134.8, 132.9, 130.6, 129.6, 128.5, 128.4, 126.9, 120.0, 92.7, 86.9, 21.2。
1-苯基-3-(2-甲苯基)-2-丙炔-1-酮(5d)[28]: 黄色液体,产率74%;1H NMRδ: 8.30(d,J=6.0 Hz, 1H), 7.65(d,J=12.0 Hz, 2H), 7.45(t,J=6.0 Hz, 2H), 7.40~7.34(m, 3H), 7.27(d,J=12.0 Hz, 1H), 2.67(s, 3H);13C NMRδ: 179.7, 140.4, 135.6, 133.1, 132.8, 132.1, 130.5, 128.6, 125.8, 120.3, 91.8, 88.3, 21.9。
1-苯基-3-(4-氯苯基)-2-丙炔-1-酮(5e): 白色固体,产率71%, m.p.96~98 ℃(104~105 ℃[28]);1H NMRδ: 8.13(d,J=6.0 Hz, 2H), 7.66(d,J=6.0 Hz, 2H), 7.47~7.45(m, 3H), 7.41~7.39(m, 2H);13C NMRδ: 176.4, 140.6, 135.2, 133.0, 130.9, 130.7, 128.9, 128.6, 119.7, 93.5, 86.5。
1-苯基-3-(3-氯苯基)-2-丙炔-1-酮(5f): 白色固体,产率67%, m.p.93~95 ℃(86~90 ℃[16]);1H NMRδ: 8.15(s, 1H), 8.09(d,J=12.0 Hz, 1H), 7.67(d,J=12.0 Hz, 2H), 7.58(d,J=6.0 Hz, 1H), 7.49~7.40(m, 4H);13C NMRδ: 176.3, 138.3, 134.8, 133.9, 133.1, 131.0, 130.0, 129.2, 128.7, 127.6, 119.7, 93.9, 86.4。
1-苯基-3-(2-氯苯基)-2-丙炔-1-酮(5g)[47]: 橘红色液体,产率45%;1H NMRδ: 8.09(d,J=6.0 Hz, 1H), 7.65(d,J=6.0 Hz, 2H), 7.48~7.47(m, 3H), 7.42~7.39(m, 3H);13C NMRδ: 176.7, 135.8, 133.5, 133.3, 133.1, 132.5, 131.5, 130.9, 128.7, 126.8, 120.0, 93.9, 88.3。
1-苯基-3-(4-甲氧基苯基)-2-丙炔-1-酮(5h): 白色固体,产率82%, m.p.93~95 ℃(98~99 ℃[28]);1H NMRδ: 8.18(d,J=6.0 Hz, 2H), 7.65(d,J=6.0 Hz, 2H), 7.44(t,J=9.0 Hz, 1H), 7.39(t,J=9.0 Hz, 2H), 6.97(d,J=6.0 Hz, 2H), 3.85(s, 3H);13C NMRδ: 176.4, 164.3, 132.7, 131.7, 130.4, 130.1, 128.5, 120.1, 113.7, 92.1, 86.8, 55.4。
1-苯基-3-(4-氟苯基)-2-丙炔-1-酮(5i): 白色固体,产率73%, m.p.48~50 ℃(47~49 ℃[29]);1H NMRδ: 8.24~8.22(m, 2H), 7.66(d,J=12.0 Hz, 2H), 7.47(t,J=9.0 Hz, 1H), 7.41(t,J=6.0 Hz, 2H), 7.17(t,J=9.0 Hz, 2H);13C NMRδ: 176.2, 166.3(d,JC-F=255.0 Hz), 133.3(d,JC-F=3.0 Hz), 133.0, 132.1(d,JC-F=10.5 Hz), 130.8, 128.6, 119.8, 115.7 (d,JC-F=22.5 Hz), 93.2, 86.5;19F NMRδ: -63.54。
1-苯基-3-(4-三氟甲基苯基)-2-丙炔-1-酮(5j): 淡黄色固体,产率68%, m.p.65~67 ℃(70~73 ℃[28]);1H NMRδ: 8.30(d,J=6.0 Hz, 2H), 7.76(d,J=6.0 Hz, 2H), 7.68(d,J=6.0 Hz, 2H), 7.49~7.47(m, 1H), 7.41(t,J=9.0 Hz, 2H);13C NMRδ: 176.5, 139.3, 135.0 (q,JC-F=31.5 Hz), 133.1, 131.1, 129.6, 128.7, 125.5(q,J=4.5 Hz), 123.5(q,JC-F=271.5 Hz), 119.5, 94.3, 86.5;19F NMRδ: -63.53。
1-苯基-3-(3,5-二氯苯基)-2-丙炔-1-酮(5l): 黄色固体,产率55%, m.p.80~82 ℃;1H NMRδ: 8.02~8.01(m, 2H), 7.68(d,J=6.0 Hz, 2H), 7.56~7.55(m, 1H), 7.52~7.49(m, 1H), 7.43(t,J=6.0 Hz, 2H);13C NMRδ: 174.9, 139.1, 135.6, 133.5, 133.2, 131.2, 128.7, 127.6, 119.3, 94.7, 86.1; HR-MS(ESI)m/z: Calcd for C15H8OCl2{[M+H]+}275.002 5, found 275.002 2。
3-苯基-1-(2-萘基)-2-丙炔-1-酮(5m): 白色固体,产率77%, m.p.82~84 ℃(81~83 ℃[29]);1H NMRδ: 8.71(s, 1H), 8.17(d,J=12.0 Hz, 1H), 7.95(d,J=12.0 Hz, 1H), 7.85~7.81(m, 2H), 7.68(t,J=6.0 Hz, 2H), 7.57~7.50(m, 2H), 7.45~7.37(m, 3H);13C NMRδ: 177.6, 135.9, 134.2, 132.9, 132.4, 132.2, 130.6, 129.7, 128.8, 128.5, 128.3, 127.7, 126.8, 123.7, 120.0, 92.9, 87.0。
3-苯基-1-(1-萘基)-2-丙炔-1-酮(5n): 淡黄色固体,产率64%, m.p.85~87 ℃( 92~94 ℃[28]);1H NMRδ: 9.25(d,J=6.0 Hz, 1H), 8.63 (d,J=12.0 Hz, 1H), 8.05(d,J=6.0 Hz, 1H), 7.88(d,J=12.0 Hz, 1H), 7.66(d,J=9.0 Hz, 3H), 7.57~7.53(m, 2H), 745~7.37(m, 3H);13C NMRδ: 179.6, 135.0, 134.5, 133.8, 132.8, 130.6, 130.5, 128.9, 128.6, 128.5, 126.7, 126.0, 124.4, 120.2, 91.6, 88.4。
1,4-二苯基-3-丁炔-2-酮(5p)[22]: 黄色液体,产率53%;1H NMRδ: 7.45(d,J=6.0 Hz, 2H), 7.42(d,J=6.0 Hz, 1H), 7.37(t,J=9.0 Hz, 2H), 7.34(s, 1H), 7.33~7.29(m, 4H), 3.92(s, 2H);13C NMRδ: 185.2, 133.2, 133.1, 130.8, 129.8, 128.7, 128.5, 127.4, 119.8, 92.9, 87.7, 52.1。
1,5-二苯基-1-戊烯-4-炔-3-酮(5q)[17]: 黄色液体,产率49%;1H NMRδ: 7.91(d,J=16.2 Hz, 1H), 7.65(d,J=7.2 Hz, 2H), 7.59(t,J=3.9 Hz, 2H), 7.47~7.35(m, 6H), 6.86(d,J=16.2 Hz, 1H);13C NMRδ: 178.1, 148.2, 134.0, 132.8, 131.1, 130.5, 129.0, 128.6, 128.6, 128.5, 120.1, 91.5, 86.6。
1-苯基-4-己烯-1-炔-3-酮(5r)[29]: 黄色液体,产率48%;1H NMRδ: 7.60(d,J=8.4 Hz, 2H), 7.45(t,J=7.8 Hz, 1H), 7.39(t,J=7.8 Hz, 2H), 7.32~7.26(m, 1H), 6.26(d,J=15.6 Hz, 1H), 2.03(d,J=6.6 Hz, 3H);13C NMRδ: 178.3, 149.4, 134.0, 132.8, 130.5, 128.6, 120.2, 91.0, 86.2, 18.4。
2 结果与讨论
2.1 5a的合成条件优化
以5a的合成(Scheme 2)为例,研究了物料比r[n(1a) ∶n(2) ∶n(3) ∶n(4)]和反应溶剂对5a产率的影响,结果见表1。
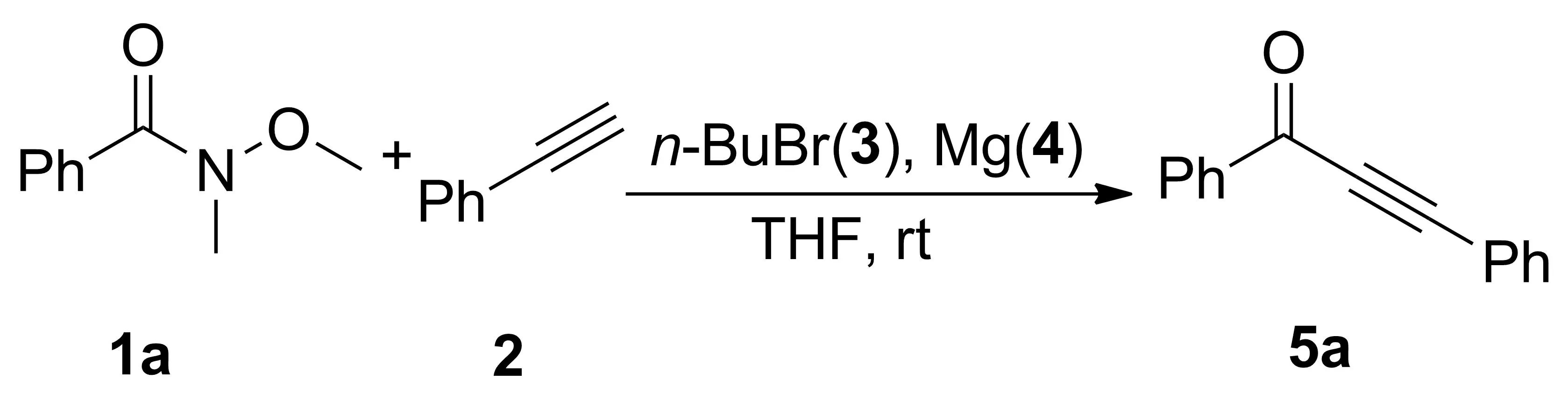
Scheme 2表1 反应条件筛选和优化aTable 1 Optimization of the synthesis conditions for 5a
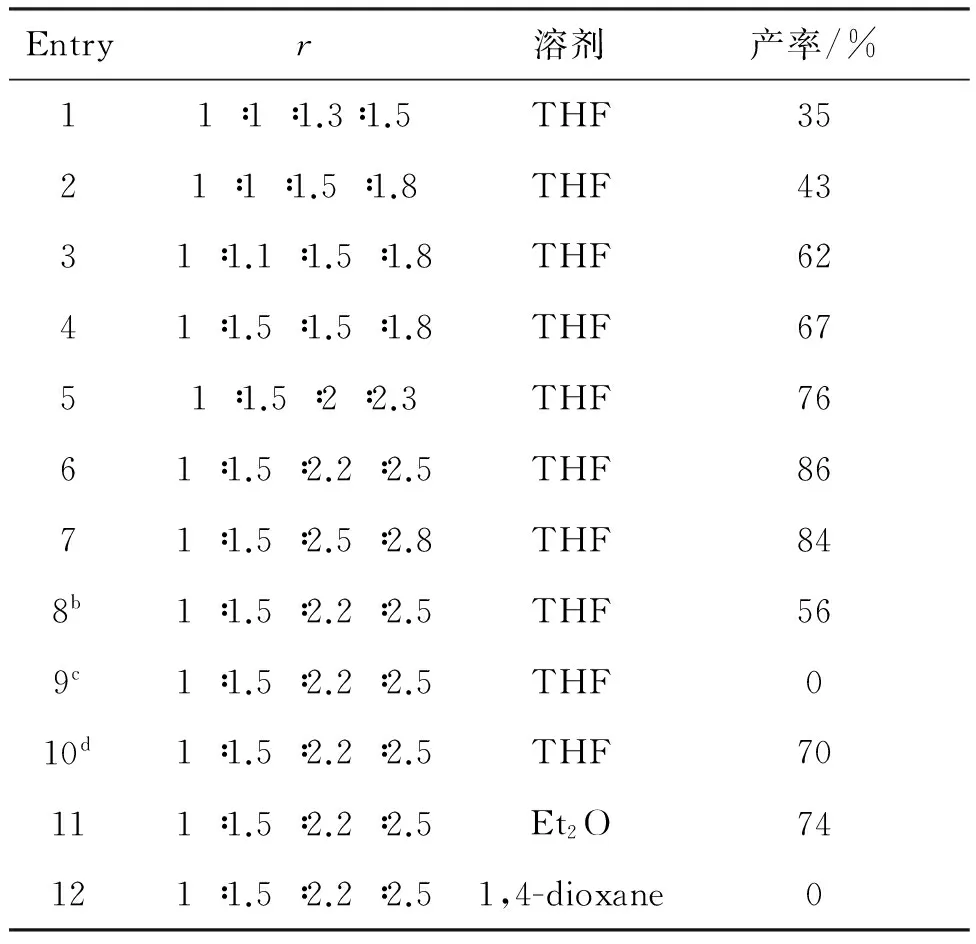
Entryr溶剂产率/%11∶1∶1.3∶1.5THF3521∶1∶1.5∶1.8THF4331∶1.1∶1.5∶1.8THF6241∶1.5∶1.5∶1.8THF6751∶1.5∶2∶2.3THF7661∶1.5∶2.2∶2.5THF8671∶1.5∶2.5∶2.8THF848b1∶1.5∶2.2∶2.5THF569c1∶1.5∶2.2∶2.5THF010d1∶1.5∶2.2∶2.5THF70111∶1.5∶2.2∶2.5Et2O74121∶1.5∶2.2∶2.51,4-dioxane0
a反应条件同1.2;b氩气氛下,2, 3和4于室温搅拌1 h;加入1a 0.5 mmol;c氩气氛下,在THF中滴加3 7~8滴,滴毕,加入4,反应10 min;缓慢加入剩余的3, 2和1a 0.5 mmol;d氩气氛下,先加入3和4,于室温搅拌1 h,缓慢加入2和1a 0.5 mmol的混合溶液。
由表1可以看出,Entry 1~7为r对5a产率的影响,随着2, 3和4的用量增大,产率逐渐升高,当r=1 ∶1.5 ∶2.2 ∶2.5时,产率最高(86%)。 Entry 7~10为加料顺序对反应的影响,先将2, 3和4混合,于室温搅拌反应1 h后再加入1a,产率仅56%。将少量3和4加入体系中引发反应,然后加入剩余的3, 2和1a,反应不能发生。将3和4先于室温搅拌反应1 h;然后缓慢加入2和1a,产率也较低(70%)。最后,我们研究了反应溶剂对产率的影响(Entry 6, 11, 12),乙醚为溶剂,反应能够发生,但产率较低(74%);1,4-二氧六环为溶剂,反应不能进行。
综上所述,合成5a的最佳条件为:THF为溶剂,3 1.1 mmol, 4 1.25 mmol,混拌2 h;加入2 0.75 mmol, 搅拌1 h;加入1a 0.5 mmol,于室温反应。
2.2 反应普适性
在最优反应条件下,我们换用其他Weinreb酰胺进行反应(Scheme 3),研究该合成方法的普适性,结果见表2。
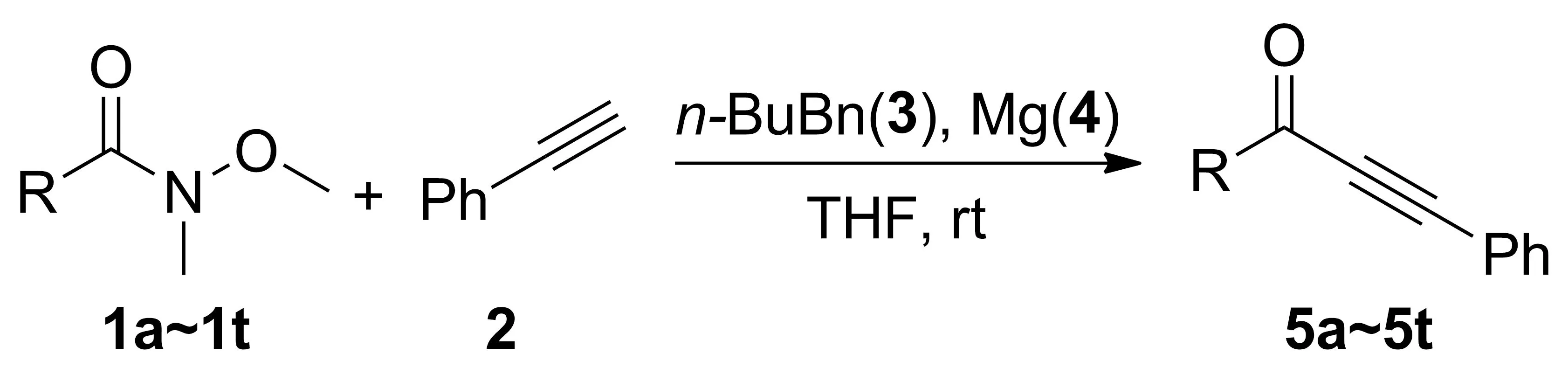
Scheme 3表2 镁粉促进Weinreb酰胺和苯乙炔的 “一锅法”反应研究aTable 2 Study on the “one-pot” reaction of Weinreb amines with phenylacetylene promoted by magnesium
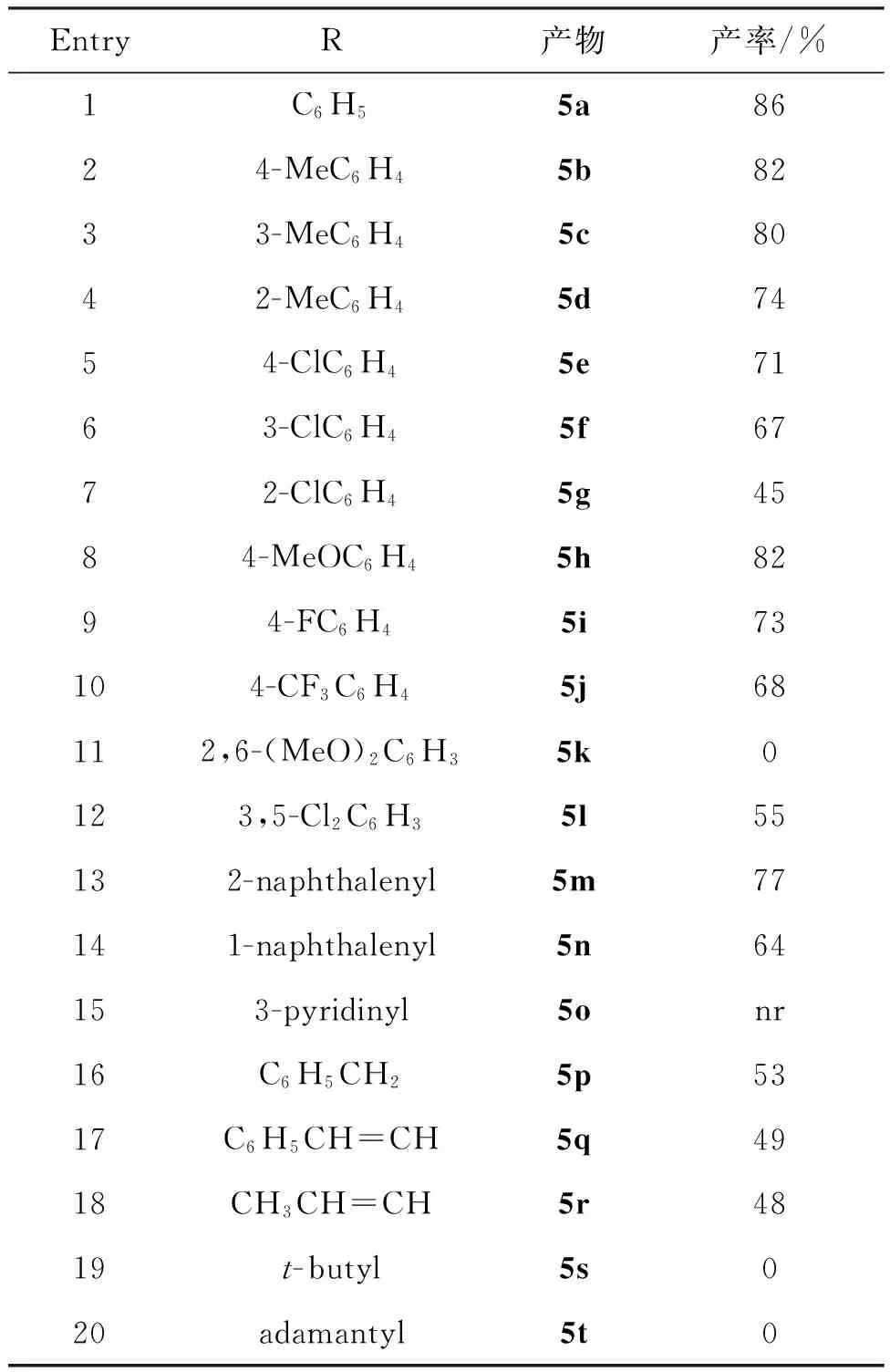
EntryR产物产率/%1C6H55a8624-MeC6H45b8233-MeC6H45c8042-MeC6H45d7454-ClC6H45e7163-ClC6H45f6772-ClC6H45g4584-MeOC6H45h8294-FC6H45i73104-CF3C6H45j68112,6-(MeO)2C6H35k0123,5-Cl2C6H35l55132-naphthalenyl5m77141-naphthalenyl5n64153-pyridinyl5onr16C6H5CH25p5317C6H5CH=CH5q4918CH3CH=CH5r4819t-butyl5s020adamantyl5t0
a反应条件同表1中加料方式c。
由表2可见,在镁粉促进下,大多数芳香族Weinreb酰胺都能和苯乙炔,正丁基溴发生“一锅法”反应得到α,β-炔酮类化合物(Entry 1~10, 12~14)。 Weinreb酰胺苯环上的取代基对产率有较大影响,苯环上连有供电子基,产率比吸电子基高,但当苯环上连有两个取代基时,情况有所不同。如2,6-二甲氧基芳香Weinreb酰胺不能发生反应(Entry 11), 3,5-二氯芳香Weinreb酰胺可以反应,产率55%(Entry 12)。此外,取代基在苯环上的位置对反应也有影响,无论取代基是吸电子基还是供电子基,取代基位于苯环对位的Weinreb酰胺的产率均高于取代基在邻位和间位的(Entry 2~4和Entry 5~7)。当Weinreb酰胺的取代基为萘环时,反应可以进行(Entry 13~14),但是杂环Weinreb酰胺不能发生反应(Entry 15)。对于侧链有苯环的Weinreb酰胺, 如取代基是苄基,肉桂基和巴豆基时,反应可以进行,但产率降低(Entry 16~18)。空间位阻较大(叔丁基和金刚烷基)的脂肪族Weinreb酰胺作底物,没有合成预计的目标产物(Entry 19~20)。
报道了一种镁粉促进“一锅法”合成α,β-炔基酮类化合物的方法。该方法具有操作简便、条件温和和反应时间短等优点,为α,β-炔基酮类化合物的合成提供了一定参考。
[2] Shankar R, Chakravarti B, Singh U S,etal. Synthesis and biological evaluation of 3,4,6-triaryl-2-pyranones as a potential new class of anti-breast cancer agents[J].Bioorg Med Chem,2009,17:3847-3856.
[3] Fuchs F C, Eller G A, Holzer W. Heterocyclic analogs of thioflavones:Synthesis and NMR spectroscopic investigations[J].Molecules,2009,14:3814-3832.
[4] Forsyth C J, Xu J, Nguyen S T,etal. Antibodies with broad specificity to azaspiracids by use of synthetic haptens[J].J Am Chem Soc,2006,128:15114-15116.
[5] Marco C J, Opazo E. Synthesis of enantiomerically pure,highly functionalized,medium-sized carbocycles from carbohydrates:Formal total synthesis of (+)-Calystegine B2[J].J Org Chem,2002,67:3705-3717.
[6] Jiang H, Pan X, Huang L,etal. Synthesis of 4H-cyclopenta[c]furans via cooperative PdCl2-FeCl2catalyzed cascade cyclization reaction involving a novel acyl rearrangement process[J].Chem Commun,2012,48:4698-4700.
[7] Kirkham J D, Edeson S J, Stokes S,etal. Synthesis of ynone trifluoroborates toward functionalized pyrazoles[J].Org Lett,2012,14:5354-5357.
[8] Bannwarth P, Valleix A, Grée D,etal. Flexible synthesis of pyrimidines with chiral monofluorinated and difluoromethyl side chains[J].J Org Chem,2009,74:4646-4649.
[9] Liu H L, Jiang H F, Zhang M,etal. One-pot three-component synthesis of pyrazoles through a tandem coupling-cyclocondensation sequence[J].Tetrahedron Lett,2008,49:3805-3809.
[10] Arcadi A, Aschi M, Marinelli F,etal. Pd-catalyzed regioselective hydroarylation ofα-(2-aminoaryl)-α,β-ynones with organoboron derivatives as a tool for the synthesis of quinolines:Experimental evidence and quantum-chemical calculations[J].Tetrahedron,2008,64:5354-5361.
[11] Lee K Y, Lee M J, Kim J N. Facile synthesis ofα,β-acetylenic ketones and 2,5-disubstituted furans:Consecutive activation of triple and double bond with ZnBr2toward the synthesis of furan ring[J].Tetrahedron,2005,61:8705-8710.
[12] Karpov A S, Merkul E, Rominger F,etal. Concise syntheses of meridianins by carbonylative alkynylation and a four component pyrimidine synthesis[J].Angew Chem Int Ed,2005,44:6951-6956.
[13] Kel’in A V, Sromek A W, Gevorgyan V. A novel Cu-sssisted cycloisomerization of alkynyl imines:Efficient synthesis of pyrroles and pyrrole-containing heterocycles[J].J Am Chem Soc,2001,123:2074-2075.
[14] Arcadi A, Marinelli F, Rossi E. Synthesis of functionalised quinolines through tandem addition/annulation reactions ofβ-(2-aminophenyl)-α,β-ynones[J].Tetrahedron,1999,55:13233-13250.
[15] Yuan J W, Wang J, Zhang G H,etal. Bimetallic zinc complex-active species in coupling of terminal alkynes with aldehydes via nucleophilic addition/oppenauer oxidation[J].Chem Commun,2015,576-581.
[16] Ogiwara Y, Kubota M, Kurogi K,etal. Oxidative coupling of terminal alkynes with aldehydes leading to alkynyl ketones by using indium(III) bromide[J].Chem Eur J,2015,21:18598-18600.
[17] Heffernan S J, Tellam J P, Queru M E,etal. Double gold-catalysed annulation of indoles by enynones[J].Adv Synth Catal,2013,355:1149-1159.
[18] Pu L. Asymmetric alkynylzinc additions to aldehydes and ketones[J].Tetrahedron,2003,59:9873-9886.
[19] Chavan S P, Varadwaj G B B, Parida K,etal. Palladium anchored on amine-functionalized K10 as an efficient, heterogeneous and reusable catalyst for carbonylative Sonogashira reaction[J].Applied Cata A:General,2015,506:237-245.
[20] Zhao H, Cheng M Z, Zhang J T,etal. Recyclable and reusable PdCl2(PPh3)2/PEG-2000/H2O system for the carbonylative Sonogashira coupling reaction of aryl iodides with alkynes[J].Green Chem,2014,16:2515-2522.
[21] Neumann K T, Laursen S R, Lindhardt A T,etal. Palladium-catalyzed carbonylative sonogashira coupling of aryl bromides using near stoichiometric carbon monoxide[J].Org Lett,2014,16:2216-2219.
[22] Feng X J, Song J L, Liu H S,etal. Palladium-catalyzed carbonylative coupling of (chloromethyl)arenes with terminal arylalkynes to produce 1,4-diaryl-3-butyn-2-ones[J].RSC Advances,2013,3:18985-18991.
[23] Wang Y, Liu J H, Xia C G. Cross-linked polymer supported palladium catalyzed carbonylative Sonogashira coupling reaction in water[J].Tetrahedron Lett,2011,52:1587-1591.
[24] Wu X F, Neumann H, Beller M. A general and convenient palladium-catalyzed carbonylative Sonogashira coupling of aryl bromides[J].Chem Eur J,2010,16:12104-12107.
[25] Ahmed M S M, Mori A. Carbonylative Sonogashira coupling of terminal alkynes with aqueous ammonia[J].Org Lett,2003,5:3057-3060.
[26] Sun W J, Wang Y, Wu X,etal. Palladium-,ligand-,and solvent-free synthesis of ynones by the coupling of acyl chlorides and terminal alkynes in the presence of a reusable copper nanoparticle catalyst[J].Green Chem,2013,15:2356-2361.
[27] Boersch C, Merkul E, Müller T J J. Catalytic syntheses ofN-heterocyclic ynones and ynediones byinsituactivation of carboxylic acids with oxalyl chloride[J].Angew Chem Int Ed,2011,50:10448-10452.
[28] Park A, Park K, Kim Y,etal. Pd-catalyzed carbonylative reactions of aryl iodides and alkynyl carboxylic acids via decarboxylative couplings[J].Org Lett,2011,13:944-947.
[29] Santra S, Dhara K, Ranjan P,etal. A supported palladium nanocatalyst for copper free acyl sonogashira reactions:One-pot multicomponent synthesis ofN-containing heterocycles[J].Green Chem,2011,11:3238-3247.
[30] D’Souza D M, Müller T J J. Catalytic alkynone generation by Sonogashira reaction and its application in three-component pyrimidine synthesis[J].J Nat Protoc,2008,3:1660-1665.
[31] Karpov A S, Müller T J J. New entry to a three-component pyrimidine synthesis by TMS-ynones via Sonogashira coupling[J].Org Lett,2003,5:3451-3454.
[32] Karpov A S, Muller T J J. Straight forward novel one-pot enaminone and pyrimidine syntheses by coupling-addition-cyclocondensation sequences[J].Synthesis,2003,35:2815-2826.
[33] Lei A W, Wu Y X, Tang H Y,etal. Rh(III)- or Ir(III)-catalyzed ynone synthesis from aldehydes via chelation-assisted C—H bond activation[J].Chem Commun, 2015,7871-7875.
[34] Zhang R Y, Xi L Y, Zhang L,etal. Metal-free synthesis of ynones via direct C—H alkynylation of aldehydes with ethynylbenziodoxolones[J].Tetrahedron,2015,71:6176-6173
[35] Huang H C, Zhang G J , Chen Y Y. Dual hypervalent iodine(III) reagents and photoredox catalysis enable decarboxylative ynonylation under mild conditions[J].Angew Chem Int Ed,2015,54:7872-7876.
[36] Tang T, Fei X D, Ge Z Y,etal. Palladium-catalyzed carbonylative Sonogashira coupling of aryl bromides via tert-butyl isocyanide insertion[J].J Org Chem,2013,78:3170 - 3175.
[37] 赵蔚,刘伟. Weinreb 酰胺在有机合成中的应用进展[J].有机化学,2015,35:55-69.
[38] Singh J, Satyamurthi N, Aidhen I S. The growing synthetic utility of Weinreb’s amide[J].J Prakt Chem/Chem-Ztg,2000,342:340-354.
[39] Sibi M P. Chemistry ofN-methoxy-N-methylsmides:Applications in synthesis a review[J].Org Prep Proced Int,1993,25:15-40.
[40] Pérez M, Pozo C D, Reyes F,etal. Total synthesis of natural myriaporones[J].Angew Chem Int Ed, 2004,43:1724-1727.
[41] Lygo B, Bhatias M, Cooke J W B,etal. Synthesis of (±)-solanapyrones A and B[J].Tetrahedron Lett,2003,44:2529-2532.
[42] Dias L C, Sousa M A D. Synthetic studies directed toward the total synthesis of dolabriferol[J].Tetrahedron Lett,2003,44:5625-5628.
[43] Shimizu T, Kusada J, Ishiyama H,etal. Efficient synthesis of the 6,6-spiroacetal of spirofungin A[J].Tetrahedron Lett,2003,44:4965-4968.
[44] Taillier C, Bellosta V, Meyer C,etal. Synthesis ofω-hydroxy ketones fromω-benzyloxy weinreb amides by using a chemoselective nucleophilic addition/Birch reduction process[J].Org Lett,2004,6:2145-2147.
[45] 王凤娇,陆爱玲,黄丹凤,等. 镁粉促进 Weinreb 酰胺和卤代烃的“一锅法”制备酮类反应研究[J].有机化学,2015,35:1046-1051.
[46] Niu T, Wang K H, Huang D,etal. One-pot transition-metal-free synthesis of Weinreb amides directly from carboxylic acids[J].Synthesis,2014,46:320-330.
[47] Maeda Y, Kakiuchi N, Matsumura S,etal. Oxovanadium complex-catalyzed aerobic oxidation of propargylic alcohols[J].J Org Chem,2002,67:6718-6724.
“One-Pot” Synthesis ofα,β-Alkynones Promoted by Magnesium Powder
YANG Tian-yu, HUANG Dan-feng, WANG Ke-hu, SU Ying-peng, HU Yu-lai*
(College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou 730070, China)
“One-pot” synthesis ofα,β-alkynones(5a~5j, 5l~5n, 5p~5r) from Weinreb amides(1a~1j, 1l~1n, 1p~1r) and phenylacetylene(2) promoted by magnesium powder(4) in the presence ofn-butyl bromide(3) was reported. The results indicated that under the optimum reaction conditions(THF as solvent, 3 1.1 mmol, 4 1.25 mmol, stirring for 2 h; then add 2 0.75 mmol, stirring for 1 h; add 1 0.5 mmol, reaction at rt), the yield of 5a~5j, 5l~5n, 5p~5r were 45%~86%. The structures were confirmed by1H NMR and13C NMR.
one-pot synthesis; magnesium powder promotion; Weinreb amide; phenylacetylene;α,β-alkynone
2016-10-21;
2017-01-05
国家自然科学基金资助项目(21262031, 21462037)
杨天宇(1990-),男,回族,辽宁沈阳人,硕士研究生,主要从事有机合成的研究。 E-mail: maxli101@sina.com
胡雨来,教授,博士生导师, E-mail: huyl@nwnu.edu.cn
O622.4
A
10.15952/j.cnki.cjsc.1005-1511.2017.02.16264

