非诺贝特在非酒精性脂肪性肝病大鼠中的作用探讨
李 丹,李异玲
非诺贝特在非酒精性脂肪性肝病大鼠中的作用探讨
李 丹,李异玲*
目的 高脂饮食构建NAFLD大鼠模型,观察非诺贝特对NFALD大鼠血清学肝功、血脂及肝脏病理的影响。方法 将27只雄性SD大鼠随机分为正常组(N组)、病理组(B组)及非诺贝特组(F组),每组9只,分别予正常饮食及高脂饮食建立NAFLD模型。于4、8、12周后,每组各处死大鼠3只,称体重、肝湿重,计算肝指数;8周后测定血清天冬氨酸氨基转移酶(AST)、丙氨酸氨基转移酶(ALT)、三酰甘油(TG)、总胆固醇(TC);肝脏病理切片行HE染色,观察病理改变。F组第5周开始药物干预。结果 8周时B组大鼠体重及肝指数高于N组,差异有统计学意义(P<0.05),F组肝指数低于B组,差异有统计学意义(P<0.05);12周时N组与F组大鼠肝指数组间比较差异无统计学意义(P>0.05),B组大鼠体重和肝指数大于N组,差异有统计学意义(P<0.05)。血清学指标:8周时N组与F组AST、ALT、TG、TC比较差异无统计学意义(P>0.05),B组AST、ALT、TG、TC明显高于N组,且差异有统计学意义(P<0.05)。12周时B组AST、ALT、TG、TC明显高于N组,差异有统计学意义(P<0.05),B组ALT、TG高于F组,差异有统计学意义(P<0.05),F组与N组AST、ALT、TC、TG差异无统计学意义(P>0.05)。结论 改良法高脂饮食诱导SD大鼠非酒精性脂肪性肝病,造模成功;非诺贝特对NAFLD大鼠的肝脏酶学、血脂及病理学均有一定改善。
非酒精性脂肪性肝病;SD大鼠;非诺贝特
0 引言
近年来,非酒精性脂肪性肝病(Nonalcoholic fatty liver disease,NAFLD)在世界范围内发病率逐年上升,包括单纯性脂肪肝(NASFL)、脂肪性肝炎(NASH)、脂肪性肝纤维化、脂肪性肝硬化以及相关的肝细胞癌[1-2]。高血脂是NAFLD的危险因素,长期持续高脂饮食后,肝细胞合成三酰甘油(Triglyceride,TG)增多,肝细胞内脂肪沉积,出现脂肪变性,逐渐形成脂肪肝[3]。
目前治疗NAFLD的常用药物主要有抗炎保肝药、胰岛素增敏剂如二甲双胍、抗氧化剂、降脂药等。其中降血脂药逐渐吸引广大研究者的注意,能否用降脂药来治疗NAFLD逐渐被重视[4-5]。非诺贝特属于第二代贝特酸类衍生物,通过激活过氧化物酶体增殖物激活受体(PPARα)、抑制肝脏炎症反应,起到治疗NAFLD的作用[5-6]。虽然大量研究提示非诺贝特作为降血脂的重要药物,治疗NAFLD有效,但也有文献报道对于治疗伴有不同程度肝功能异常的NAFLD患者,非诺贝特具有肝损害的潜在危险性[7-8]。本文探讨非诺贝特对非酒精性脂肪性肝病大鼠肝功、血脂及肝脏病理的影响,进一步为非诺贝特对非酒精性脂肪性肝病的治疗提供依据。
1 材料与方法
1.1 实验动物与分组 健康雄性SD大鼠(SPF级)27只,体重(260±20)g,周龄:8周,由中国医科大学动物部提供。饲养温度为(20±2)℃,湿度为50%±5%,将大鼠于实验开始前1周置于实验环境中,自由进食进水,自然昼夜节律周期。分为正常组(N)、病理组(B)及非诺贝特组(F),每组9只,所有大鼠均在中国医科大学动物部饲养。
1.2 NAFLD动物造模的制备 高脂饲料:10%猪油,2%胆固醇,5%蛋黄粉,0.5%胆酸钠及82.5%基础饲料,由中国医科大学动物实验中心提供。N组予自由进食基础饲料及饮用水;B组大鼠高脂饲料持续饲养及自由进食饮用水;F组高脂饮食喂养,于第5周开始灌胃给予非诺贝特,进行药物干预。分别于造模第4周、8周、12周处死大鼠,测量肝重、体重,8周时腹主动脉穿刺取血,留取肝脏组织,行病理检查。
1.3 检测指标及标本留取 ①实验前及实验后4周、8周、12周各称体重1次,观察大鼠食欲、活动、精神、毛发情况;测定大鼠肝湿重,肝重/体重。②血清学检测:腹主动脉取血后,将装有5~10 mL的全血试管置入离心机中,3 000 r/min、4 ℃离心20 min后,留取上清液,置入EP管中,送至中国医科大学附属第一医院检验科,通过生化自动分析仪(Olympus)测定血清学ALT、AST、TG、TC。③肝脏病理:大鼠处死后,将整个肝脏组织游离后,清洗,称重,取固定部位肝(肝右叶,1 cm×1 cm×1 cm),用10%中性福尔马林溶液固定48 h,HE染色,石蜡包埋,单盲情况下送病理室,由病理医生协助阅片。
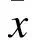
2 结果
2.1 三组大鼠一般特征、体重及肝指数比较 实验期间N组大鼠活动、饮食正常,皮毛发亮发白,整洁,体重持续增长;B组及F组喂养4周后,逐渐出现不同程度的精神萎靡,活动减少,食量下降,皮毛凌乱、发黄,F组经非诺贝特药物干预后,8周及12周大鼠活动、精神状态、食量及皮毛颜色较N组差,较B组好。三组体重及肝指数见表1。
2.2 血清学指标 见表2。

表1 三组体重及肝指数比较
注:与N组比较,*P<0.05;与B组比较,#P<0.05。三组间比较,4、8、12周时P体重=0.997、0.026、0.052,P肝指数=0.578、0.034、0.019
2.3 大体及病理 4~12周时,N组大鼠肝脏外观均正常,颜色鲜红,边缘锐利,光镜下肝小叶结构正常,肝组织结构完整,肝细胞排列成肝索,在中央静脉周围成放射状,细胞呈多边形;B组大鼠肝脏逐渐增大,光镜下脂肪变逐渐加重,至12周明显肿大,黄白色,油腻,边缘钝,光镜下肝索放射状排列不明显,大部分细胞肿胀,大小不均,细胞轮廓模糊,胞核消失或被脂肪空泡挤向一侧,肝细胞以气球样变为主;F组大鼠肝脏外观及病理学改变介于二者之间,肝脏增大,色黄,镜下可见肝索排列,但不清晰,肝细胞体积增大,胞浆内充满脂肪空泡,可见核被挤向一侧,病变以肝小叶交界处明显。各组病理改变见图1。

表2 三组血清学指标比较
注:与N组比较,*P<0.05,**P<0.01;与B组比较,#P<0.05,##P<0.01。三组间比较,8、12周时PALT=0.002、0.011,PAST=0.004、0.017,PTG=0.030、0.021,PTC=0.008、0.076

图1 三组大鼠肝脏HE染色病理图(×200)
3 讨论
NAFLD包含NASFL、NASH、脂肪性肝纤维化及脂肪性肝硬化以及相关的肝细胞癌。是由环境-遗传-代谢应激等多种因素共同作用,导致肝脏细胞内脂肪过度沉积而发展起来的[9]。高脂饮食诱导脂肪肝是目前较为常用的模型。外源性摄入的TG形成乳糜微粒,在脂蛋白脂肪酶(Lippoprotein,LPL)调节下,生成游离脂肪酸(Free fatty acids,FFA),过多的摄入高脂食物,脂肪被酯化成TG或FFA,导致FFA水平升高。过多的FFA通过肝细胞吸收,使过量的TG在肝细胞内沉积,导致肝脏脂肪变性,形成非酒精性脂肪性肝病[10-15]。本实验主要通过高脂饮食诱导大鼠非酒精性脂肪性肝病的发生,造模4周,肝脏发生脂肪变性,到12周时,脂肪肝改变已明显,提示造模成功。本研究显示,开始喂养到12周结束,B组大鼠ALT、AST、TC及TG逐渐升高;同一时间点,B组大鼠血清学改变较N组大鼠明显升高,提示高脂饮食对大鼠肝功及血脂有影响。NAFLD的发病机制复杂,且目前的机制学说多数通过动物实验,虽然国内主要通过高脂饮食大鼠造模,但这些模型可比性较低,结果不尽一致,与人类NAFLD发病机制的相符程度仍不十分确定。期待今后能在动物模型稳定的基础上,进一步研究NAFLD的发病机制。
目前NAFLD的治疗药物主要包括抗炎保肝药、胰岛素增敏剂、抗氧化剂、减肥药、调脂药等[16-18]。高脂血症是NAFLD发生的重要血清学基础,因此,改善血脂异常成为NAFLD治疗的重要课题。目前多数研究提示,他汀类及贝特类降脂药在NAFLD中均有治疗作用,也有研究者将该两种药物进行对比得出,贝特类药物在治疗伴有高脂血症的NAFLD患者中疗效较好。非诺贝特作为贝特类的代表药,通过激活PPAR-α起到改善胰岛素抵抗、抗炎及调节脂质作用[9,19-21]。脂肪三酰甘油酶(Adipose triglyceride lipase,ATGL)是一种可特异性脂解TG为二酰甘油的脂肪酶。有报道,非诺贝特可能增加ATGL酶活性,并增强TG水解活性,从而起到治疗非酒精性脂肪性肝病的作用[19,22]。有研究表明,非诺贝特可抑制CCl4诱导的小鼠肝纤维化程度,其机制可能与上调肝脏中PPARα表达,抑制炎症反应,升高SOD发挥抗氧化作用有关[23]。目前,关于非诺贝特是否可以影响胰岛素敏感性存在争议。Black等[24]研究发现,非诺贝特对胰岛素敏感性没有影响,其结果仍需大量的临床及模型试验进一步证实。本研究结果提示,F组大鼠在大体及肝脏外观方面较B组改善,而体重、肝指数、血清学TC及TG较B组好转。但有研究指出,贝特类药物治疗与AST或ALT升高有关。通过血清学改变推断,大鼠的转氨酶升高主要与疾病本身相关。贝特类药物使氨酶升高可能与药物应用的剂量有关,目前尚无大量实验研究得出非诺贝特治疗NAFLD的最佳剂量,期待今后在此方面开展更多的研究,为临床上治疗NAFLD提供帮助。
[1]Nanji AA.Animal models of nonalcoholic fatty liver disease and steatohepatitis[J].Clin Liver Dis,2004,8(3):559-574.
[2]Dowman JK,Tomlinson JW,Newsome PN.Pathogenesis of non-alcoholic fatty liver disease[J].QJM,2010,103(2):71-83.
[3]Jou J,Choi SS,Diehl AM.Mechanisms of disease progression in nonalcoholic fatty liver disease[J].Semin Liver Dis,2008,28:370-379.
[4]Orime K,Shirakawa J,Togashi Y,et al.Lipid-lowering agents inhibit hepatic steatosis in a non-alcoholic steatohepatitis-derived hepatocellular carcinoma mouse model[J].Eur J Pharmacol,2016,772:22-32.
[5]Kostapanos MS,Kei A,Elisaf MS.Current role of fenofibrate in the prevention and management of non-alcoholic fatty liver disease[J].World J Hepatol,2013,5(9):470-478.
[6]Zhang N,Lu Y,Shen X,et al.Fenofibrate treatment attenuated chronic endoplasmic reticulum stress in the liver of nonalcoholic fatty liver disease mice[J].Pharmacology,2015,95(3-4):173-180.
[7]Gandhi N,Lenton R,Bhartia M,et al.Effect of fibrate treatment on liver function tests in patients with the metabolic syndrome[J].Springerplus,2014,3:14.
[8]Kobayashi A,Suzuki Y,Kuno H,et al.Effects of fenofibrate on plasma and hepatic transaminase activities and hepatic transaminase gene expression in rats[J].J Toxicol Sci,2009,34(4):377-387.
[9]朱月永,吴佳蓉,郑琦,等.非诺贝特对非酒精性脂肪性肝病大鼠肝细胞凋亡的影响[J].中华肝脏病杂志,2015,23(9):688-693.
[10]Bloomgarden ZT.Second world congress on the insulin resistance syndrome:insulin resistance syndrome and nonalcoholic fatty liver disease[J].Diabetes Care,2005,28(6):1518-1523.
[11]Park SH,Kim BI,Kim SK,et al.Body fat distribution and insulin resistance:beyond obesity in nonalcoholic fatty liver disease among overweight men[J].Am Coil Nutr,2007,26(4):321-326.
[12]Mcculloush AJ.Pathophysiology of nonalcoholic steatohepatitis[J].J Clin Gastroenterol,2006,40(3Suppl1):S17-S29.
[13]季光,张莉.非酒精性脂肪性肝病发病机制进展[J].中国医师进修杂志,2010,33(1):1-3.
[14]梁淑文,王晓英,屈昌民,等.非酒精性脂肪性肝病患者胰岛素抵抗与肠道菌群失调相关性研究[J].中国医药,2016,11(1):83-86.
[15]Liu Q,Bengmark S,Qu S.The role of hepatic fat accumulation in pathogenesis of non-alcoholic fatty liver disease(NAFLD)[J].Lipids Health Dis,2010,9:42.
[16]朱雨霏,黄滨,郭南京,等.艾塞那肽治疗2型糖尿病合并非酒精性脂肪肝临床疗效[J].实用医学杂志,2016,32(6):991-993.
[17]刘莹,项莹.胰高血糖素样肽1治疗2型糖尿病合并非酒精性脂肪性肝病的研究进展[J].中国医药,2016,11(8):1260-1264.
[18]Nakajima K.Multidisciplinary pharmacotherapeutic options for nonalcoholic fatty liver disease[J].Int J Hepatol,2012,2012:950693.
[19]沈俊飞,代芳,王长江,等.非诺贝特和TNF对3T3-L1脂肪细胞内脂素表达的影响[J].山东医药,2008,48(19):28-30.
[20]Day CP,James OFW.Steatohepatitis:a tale of two “hits”[J].Gastroenterology,1998,144:842-845.
[21]Kondo K,Sugioka T,Tsukada K,et al.Fenofibrate,a peroxisome proliferator-activated receptor alpha agonist,improves hepatic microcirculatory patency and oxygen availability in a high-fat-diet-induced fatty liver in mice[J].Adv Exp Med Biol,2010,662:77-82.
[22]南京,杨水祥.非他汀类调脂药物的研究进展[J].中华老年心脑血管病杂志,2016,18(6):655-658.
[23]Xie C,Li L,Xu YP,et al.Anti-fibrosis of fenofibrate in mice with hepatic fibrosis[J].Zhonghua Gan Zang Bing Za Zhi,2013,21(12):914-919.
[24]Black RN,Ennis CN,Young IS,et al.The peroxisome proliferator-activated receptor alpha agonist fenofibrate has no effect on insulin sensitivity compared to atorvastatin in type 2 diabetes mellitus:a randomised,double-blind controlled trial[J].J Diabetes Complications,2014,28(3):323-327.
Mechanism of fenofibrate in rat model of non-alcoholic fatty liver disease
LI Dan,LI Yi-ling*
(Department of Gastroenterology,the First Affiliated Hospital of China Medical University,Shenyang 110001,China)
Objective To establish a rat model for non-alcoholic fatty liver disease (NAFLD) and evaluate the effectiveness of fenofibrate treatment on NAFLD,based on liver enzyme tests,blood lipid levels and liver pathology of the NAFLD rats after treatment with fenofibrate.Methods A total of 27 male SD rats were randomly divided into 3 groups,with 9 rats in each group,which were respectively assigned as normal group (group N),pathological group (group B) and fenofibrate group (group F).Group N was given a normal diet,while both group B and group F were fed with a high fat diet to create the NAFLD model.Three rats from each group were sacrificed after 4 weeks,8 weeks and 12 weeks,respectively.The body mass,liver weight and liver index were recorded.The serum levels of aspartate aminotransferase (AST),alanine aminotransferase (ALT),triglycerides (TG) and total cholesterol (TC) were determined after 8 weeks.HE staining was done on liver tissue samples to observe for pathological changes.Group F was treated with fenofibrate on the fifth week.Results After 8 weeks,a significantly higher body mass and liver index were observed in rats of group B compared to group N (P<0.05).The average liver index of rats in group F was lower than group B (P<0.05).At the 12th week,the liver indexes of rats from both group N and group F were similar with no significant difference (P>0.05),while group B had a significantly higher liver index and body mass index compared to group N (P<0.05).Serological indicators:measurements of AST,ALT,TG and TC taken at the 8th week were comparable between group F and group N (P>0.05).After 8 and 12 weeks,group B showed remarkably higher levels of AST,ALT,TG and TC compared to group N (P<0.05).At the 12th week,the levels of ALT and TG in group F were significantly lower than group B (P<0.05).At the same time,there was no statistical difference in levels of AST,ALT,TG and TC between group F and group N (P>0.05).Conclusion An improved rat model of NAFLD is successfully established by administration of high-fat diet to SD rats.Serological indicators,including levels of ALT,AST,TG and TC,as well as pathological findings,show that fenofibrate is an effective treatment for NAFLD.
Non-alcoholic fatty liver disease;SD rats;Fenofibrate
2016-05-29
中国医科大学附属第一医院消化内科,沈阳 110001
辽宁省科技厅基金资助项目(2011225015);沈阳市科技厅基金资助项目(F13-220-9-61)
10.14053/j.cnki.ppcr.201702006
*通信作者

