Phase equilibria for the pseudo-ternary system(NaCl+Na2SO4+H2O)of coal gasification wastewater at T=(268.15 to 373.15)K☆
Haijiao Lu ,Jingkang Wang ,Jun Yu ,Yuefeng Wu ,Ting Wang ,Ying Bao ,Dou Ma ,Hongxun Hao ,*
1 National Engineering Research Center of Industry Crystallization Technology,School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,China
2 Collaborative Innovation Center of Chemical Science and Engineering,Tianjin 300072,China
3 East China Engineering Science and Technology Co.,Ltd.,Hefei 230024,China
1.Introduction
As the most abundantfossilfuel,coalholds great potentialto remain the cheapest fuel in the foreseeable future.In recent years,interest in coal technology,especially coal gasification,has been revived.Coal gasification is used to improve the efficiency and efficacy of coal and to supply clean energy.Nevertheless,in coalgasification process,itis inevitable to generate lots ofwastewater containing complicated contaminants and toxic compounds,such as high concentrations of phenolic compounds,polycyclic aromatic hydrocarbons,ammonia,cyanide and thiocyanate[1-3].Moreover,coal gasification wastewater(CGW)is rich in NaCl and Na2SO4which mainly come from the salinity produced by adding chemical agents in the coal gasification process.
After phenol extraction and ammonia stripping pre-treatment,the chemical oxygen demand(COD)of coal gasification wastewater(CGW)is stillrelatively high due to the existence ofsome refractory pollutants,resulting in poor biodegradability[4].Hence,at present,CGW is often delivered to evaporation ponds,producing huge amounts of mixed salts with impurities.With high water solubility,mixed salts can lead to secondary pollution with rainfall in filtration and thus are identified as hazardous wastes.To make matters worse,the large yield and high cost cause serious problems in the treatment of mixed salts.
To solve these problems,technology needs to be developed to extract pure salts which can be reused by other industries,such as Chlor-Alkali Industry,from CGW.As we all know,crystallization is a frequently used unitoperation forthe separation of inorganic salts.To separate pure salts from solutions,especially complicated solutions with several kinds of salts such as CGW,it is crucial to know the characteristics of the phase diagrams of the system.Hence,the determination of phase diagrams is of both theoretical and practical value.To achieve the treatment of CGW and the recovery of NaCl and Na2SO4in CGW,the solubility data of NaCl and Na2SO4in CGWatdifferenttemperatures is essential and necessary.Although there are some other impurity solutes in the pseudo-ternary system,such as COD,NO3-,K+,PO43-etc.,compared with the percentages of NaCl and Na2SO4,the contents of other solutes in CGW are pretty low and can be ignored.In this study,the phase equilibria for the pseudo-ternary system of(NaCl+Na2SO4+H2O)were determined at the temperature range ofT=(268.15 to 373.15)K.
2.Experimental Section
2.1.Materials and instruments
Guaranteed reagents are used for phase equilibria(Tianjin Jinke Fine ChemicalResearch Institute,China):NaCl(≥99.8%by mass fraction)and Na2SO4(≥99.5%by mass fraction).Coal gasification wastewater was provided by a gas-making plant in the northwest of China.The characteristics of CGW are shown in Table 1.The reagents used for chemical analysis are of analytical grade:AgNO3(≥99.8%by mass fraction,Tianjin Yuxiang Technology Co.Ltd.,China),Pb(NO3)2(≥99.0%by mass fraction,Tianjin Real&Lead Chemical Technology Co.Ltd.,China)and anhydrous ethanol(≥99.7%by mass fraction,Tianjin Real&Lead Chemical Technology Co.Ltd.,China).Doubly deionized water(electrical conductivity less than 10-4S·m-1)was used for chemical analysis.
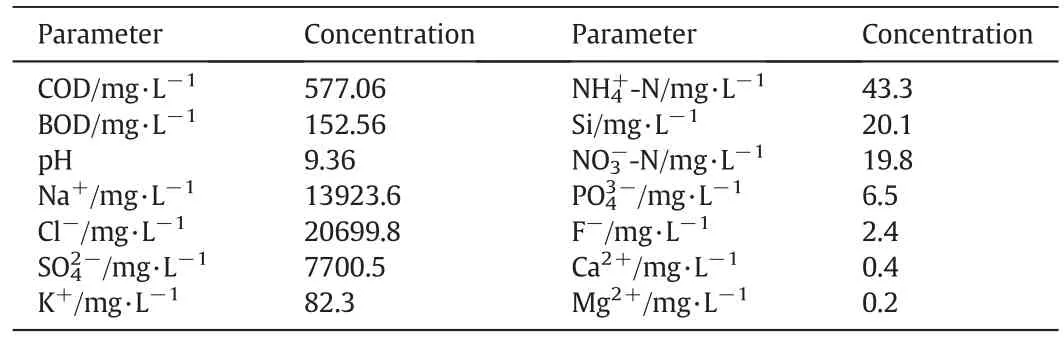
Table 1The characteristics of coal gasification wastewater(BOD:Biochemical oxygen demand)
A WE-1 type constant temperature glycol-water bath oscillator(Tianjin Honour Instrument Co.Ltd.,China)with temperature accuracy of±0.1 K and temperature range from(273.15 to 373.15)K was used for the equilibrium measurements.
An XOYS-2006-II type constant speed temperature controlled thermostat(Nanjing Xianou Instrument Co.Ltd.,China)with temperature accuracy of±0.05 K and temperature range from(153.15 to 473.15)K was used to keep the temperature of the oscillator constant in the experiments.
An EMS-9A type magnetic stirrer(Tianjin Honour Instrument Co.Ltd.,China)was used for the equilibrium measurements.
A WDDY-2008 type automatic potentiometric titrator(Taizhou Datang Analytical Instrument Co.Ltd.,China)was used for chemical analysis.
A standard analytical balance with 220 g capacity and 0.0001 g resolution(AL204-IC,Mettler Toledo Instruments Co.Ltd.,Switzerland)was used to measure the solution densities.
Rigaku D/max-2500 X-ray diffraction(Rigaku,Japan)analyzer was employed for XRD characterizations.
2.2.Experimental methods
The solid-liquid equilibria in this work were studied by the method of isothermal solution saturation[5,6].The samples of pseudo-ternary systems were prepared by gradually adding the second salt to the binary co-saturated points at a certain temperature[7,8].
AtT=(273.15 to 373.15)K,NaCl,Na2SO4and coal gasification wastewater(CGW)were mixed in 50 ml sealed conical flasks.All the conical flasks were placed into an oscillator controlled at constant temperature.At 268.15 K,the solid-liquid equilibrium experiments were conducted in jacketed glass vessels,of which the temperature was controlled by a thermostat.The solution was agitated by a magnetic stirrer.On the basis ofperiodic analysis ofthe clarified solution,12 h was selected to ensure solid-liquid equilibria atalltemperatures.Then,the conical flasks or jacketed glass vessels were kept static for 3 h for the clarification of the solutions.For the liquid phase,2 ml sample was taken out and transferred to a 100 mlvolumetric flask,diluted with doubly deionized water to volume,and mixed to determine the chemical composition of the liquid phase.Meanwhile,about 2 g of wet residues was weighed precisely,dissolved in a 100 ml volumetric flask and used to analyze the composition of wet residues.The solid phase was determined by Schreinemaker's method[9,10]and verified by XRD.
2.3.Analysis
Diluted aqueous samples of the liquid phase and wet residues were added into pure doubly deionized waterorthe mixture ofdoubly deionized water and anhydrous ethanol(1:3 volume ratio)to determine the concentration of NaCl or Na2SO4by potentiometric precipitation titration with silver nitrate or lead nitrate,respectively.The average relative error was less than±0.003 or±0.005,respectively.In addition,the density of the liquid phase was calculated by a 5 ml gravity bottle.The standard uncertainty was estimated to be 0.01 g·cm-3.The mass fraction of water in the pseudo-ternary system was obtained by mass balance,neglecting the low contents of other solutes apart from NaCl and Na2SO4in CGW.
3.Results and Discussion
The binary system equilibrium data of NaCl-H2O and Na2SO4-H2O in pure waterhas been reported in literature[11,12].The reported solubility data for the binary systems are listed in Table 2.In order to test the reliability and the accuracy of the experimental methods used in this work,the experimental equilibrium data measured in this work is also listed in Table 2.It can be seen from Table 2 that the experimental data measured in this work agrees well with the literature data,which can prove that the experimental methods and the quality of the data measured in this work are reliable.
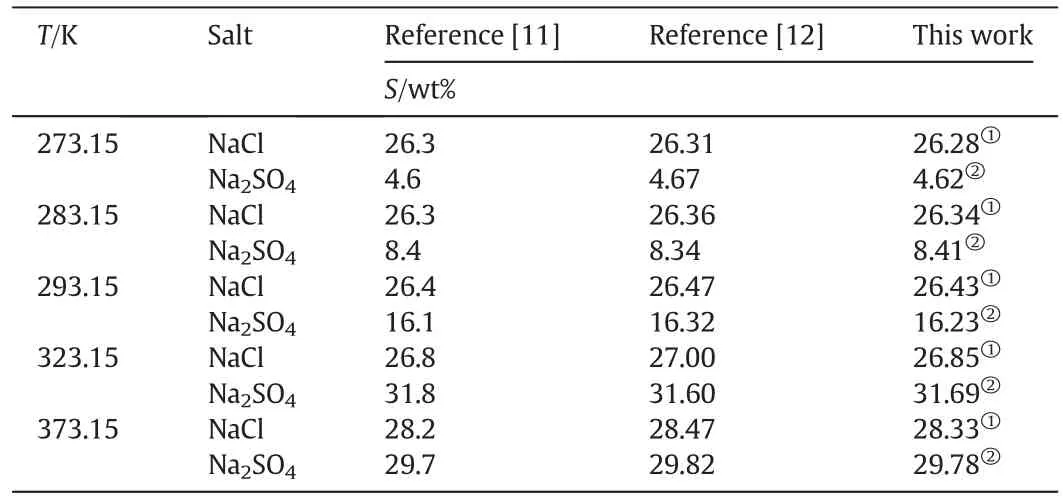
Table 2Solubility(S)in pure water(wt%)at different temperatures,p=101.3 kPa
The measured solubility and the density of the liquid phase for the pseudo-ternary system(NaCl+Na2SO4+H2O)of coal gasification wastewater atT=(268.15 to 373.15)K are shown in Table 3.The compositions of the equilibrium liquid phase and wet residue are expressed in mass fraction.
It can be seen from Table 3 that the solubility of Na2SO4dramatically decreases with decreasing temperature atT<313.15 K and gradually decreases with increasing temperature atT>313.15 K.It indicates that Na2SO4holds the potential for both cooling crystallization at low temperatures and evaporation crystallization at high temperatures.Meanwhile,the solubility ofNaClslightly increases with increasing temperature atT=(268.15 to 373.15)K,which reveals that NaCl should be separated by evaporation crystallization at high temperatures.
3.1.Solid-liquid equilibrium phase diagrams at T=(313.15 to 373.15)K
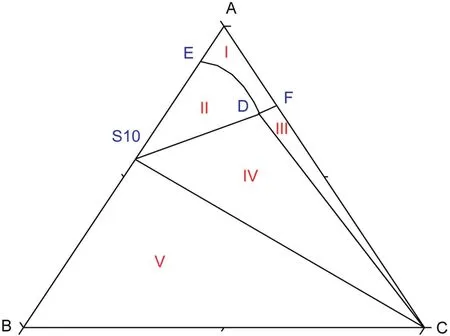
According to the experimental data,the phase diagrams of the pseudo-ternary system atT=(313.15 to 373.15)K are shown in Fig.1.To make a better explanation to the phase diagrams,a schematic diagram is drawn(see Fig.2).
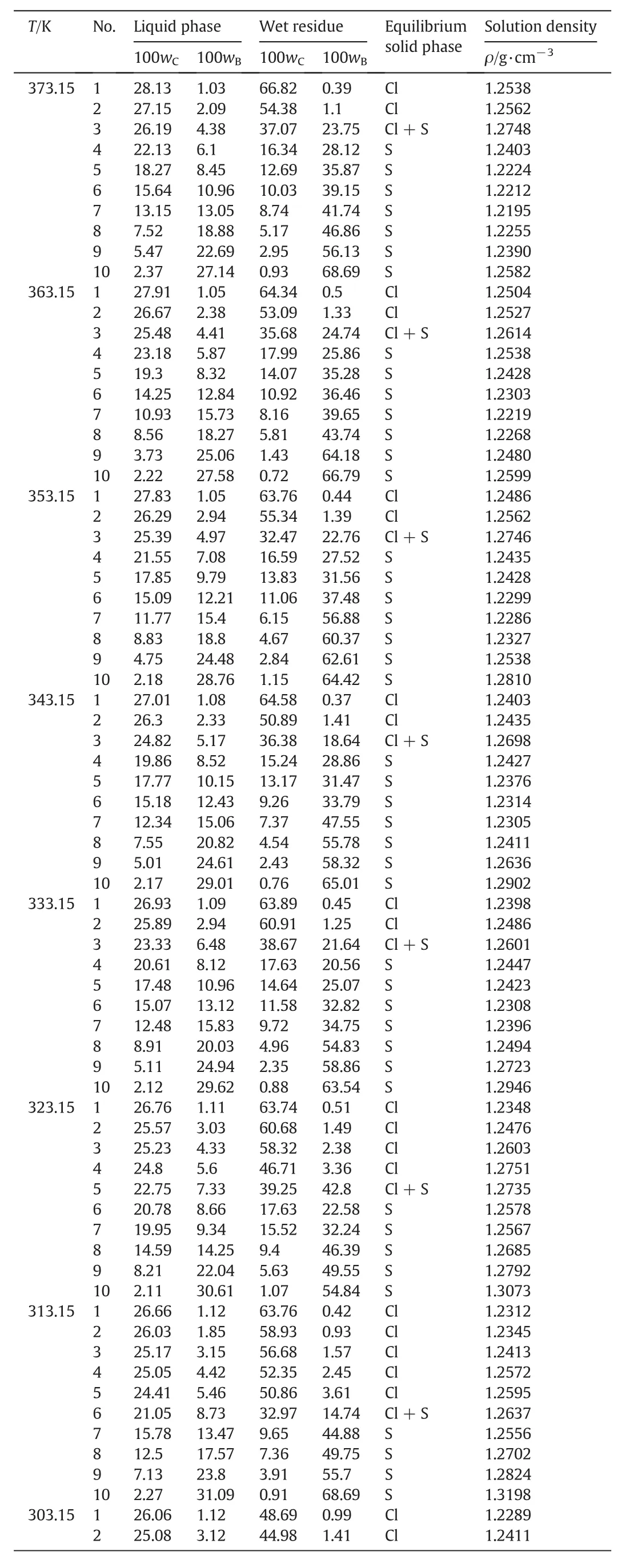
Table 3Solid-liquid equilibrium composition and solution density of the pseudo-ternary system(NaCl+Na2SO4+H2O)of coal gasification wastewater at T=(268.15 to 373.15)K,p=101.3 kPa①
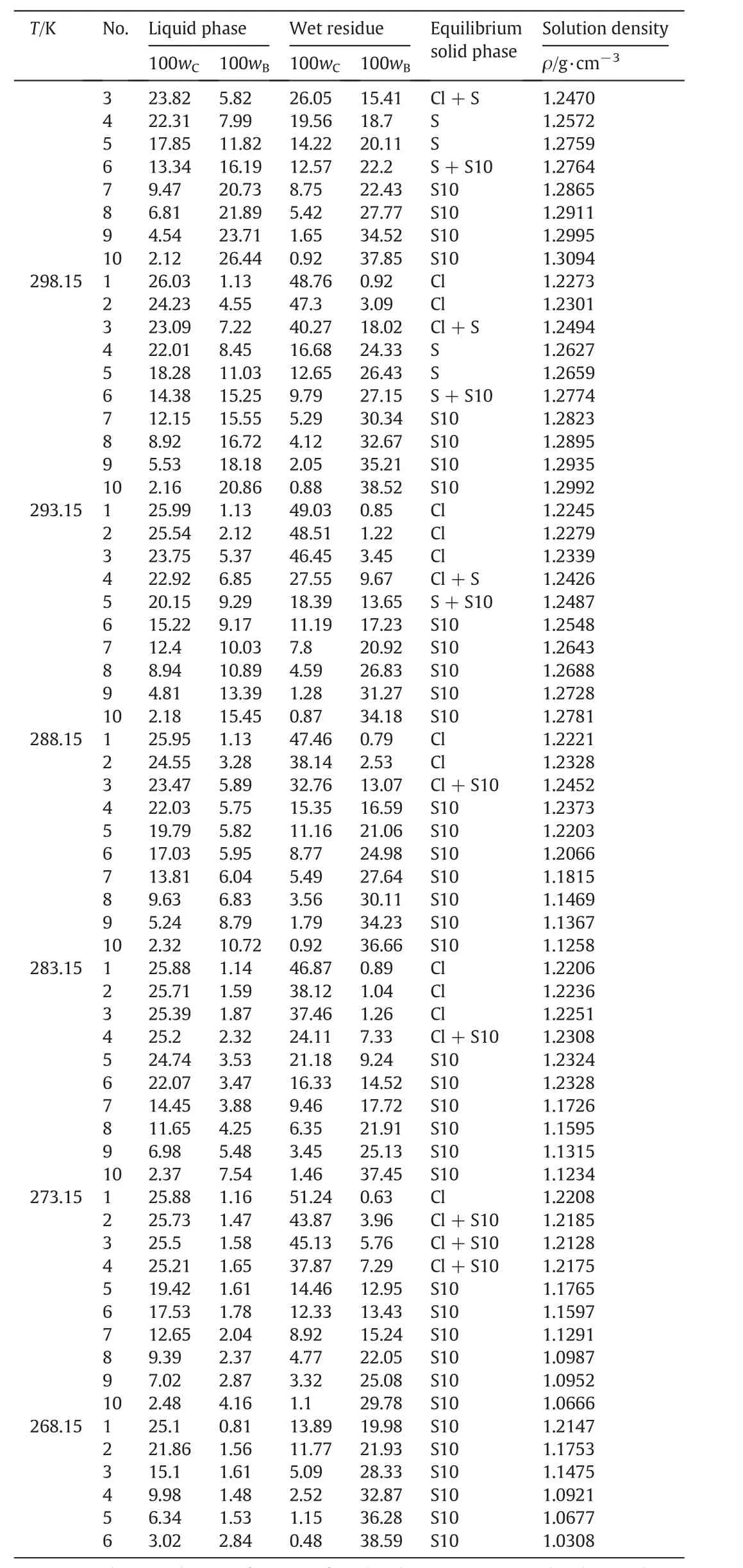
Table 3(continued)
According tofig.1,there is one co-saturated point,two solubility curves,one unsaturated solution region and three crystallization regions in the phase diagrams atT=(313.15 to 373.15)K.As Fig.2 shows,point D is the co-saturated point of two salts,corresponding to the coexistence of solids Na2SO4and NaCl with the saturated solution.And points E and F are the maximum solubility points of Na2SO4and NaCl in the solution,respectively.Thus,DE and DF are the solubility curves of Na2SO4and NaCl,respectively.Itshould be noted that because CGW contains Na2SO4and NaCl already,as shown in Fig.1,DE and DF will not intersect with AB and AC in CGW,respectively.Besides,AEDF(I)is the unsaturated solution region of Na2SO4and NaCl,in which no salt will reach saturation and crystallize outfrom the solution.BDE(II),CDF(III)and BDC(IV)are the crystallization regions of Na2SO4,NaCl and(Na2SO4+NaCl)in CGW,respectively.Apparently,the crystallization region of Na2SO4is larger than that of NaCl and the solubility of Na2SO4always increases with decreasing NaCl concentration atT=(313.15 to 373.15)K.As shown in Eq.(1),the dissolution of Na2SO4in the saturated NaCl solution will lead to an increase in the concentration of Na+.As a consequence of the common ion effect,the dissolution equilibrium in Eq.(2)shifts to the left and results in a decrease of the solubility of NaCl.According to the result,this law also stands for CGW used in this work.
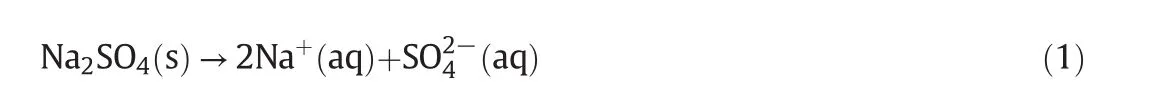
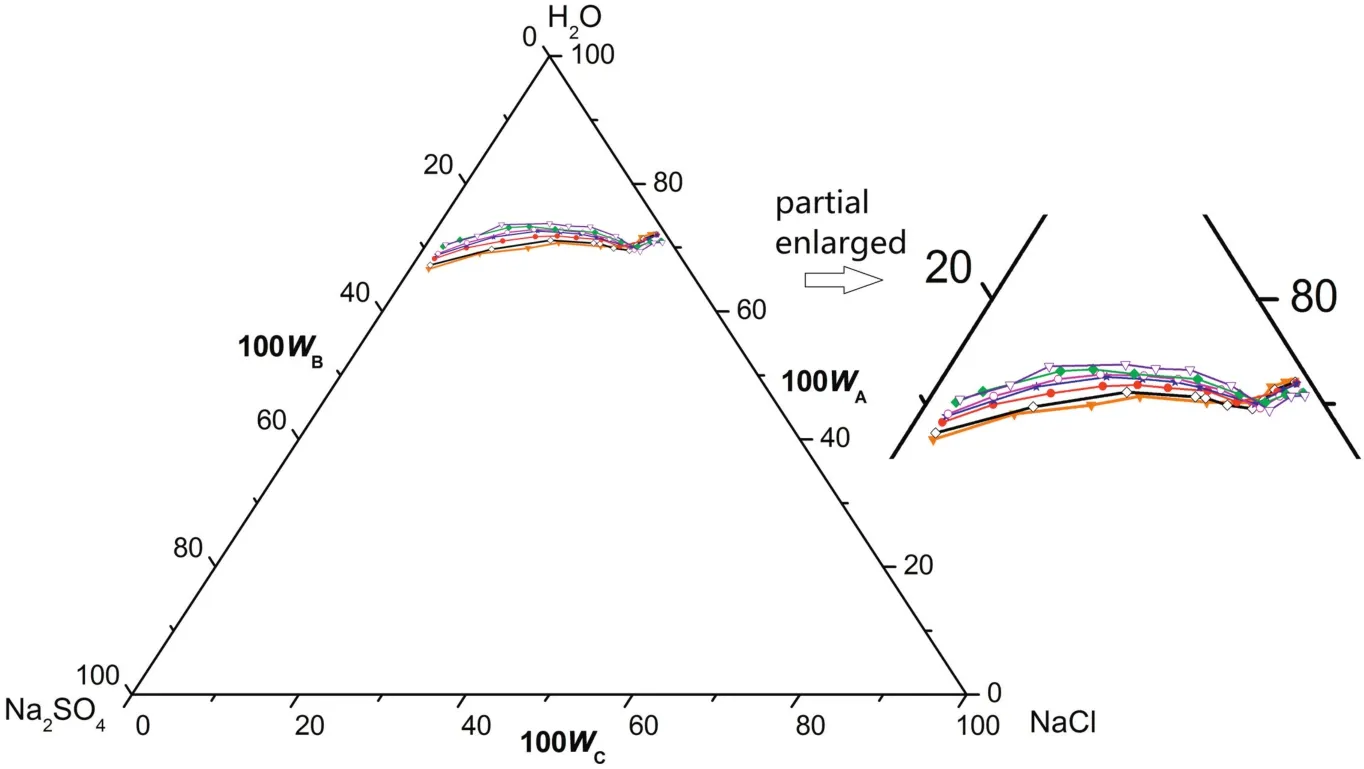
Fig.1.The phase diagrams ofthe pseudo-ternary system(NaCl+Na2SO4+H2O)ofcoalgasification wastewater at T=(313.15 to 373.15)K.w A,w B and w C are the mass fractions of H2O,
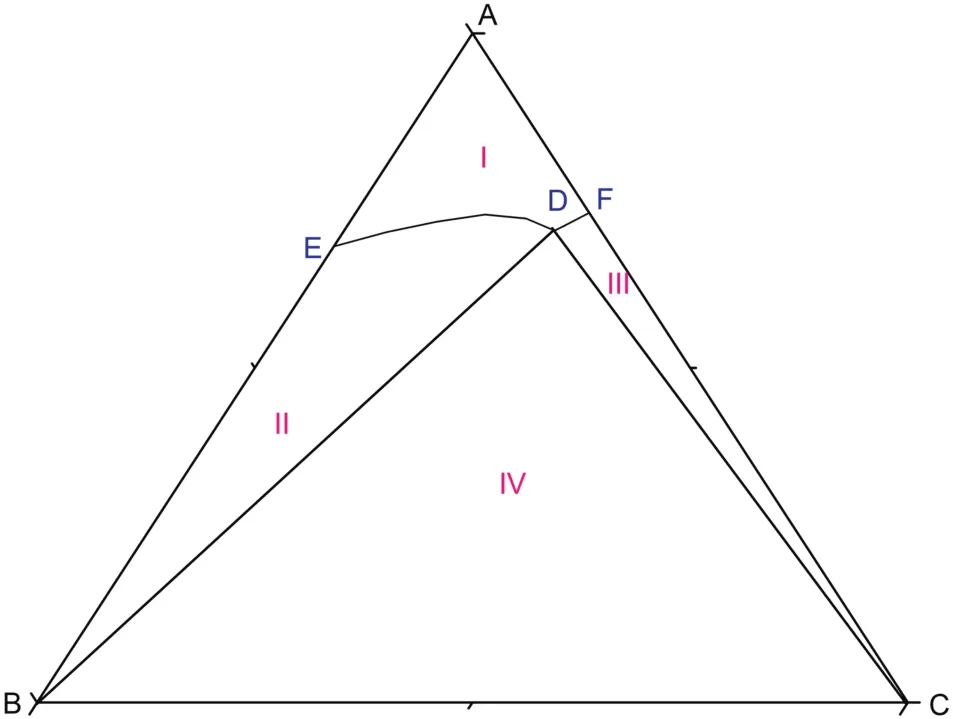
Fig.2.Schematic diagram ofthe ternary system(NaCl+Na2SO4+H2O)at T=(313.15 to 373.15)K.Points:A,H2O;B,Na2SO4;C,NaCl;D,the co-saturated point of(Na2SO4+NaCl);E and F,the saturated points of Na2SO4 and NaCl in the solution,respectively.Curves:DE and DF,the solubility curves of Na2SO4 and NaCl,respectively.Regions:AEDF(I),solution;BDE(II),Na2SO4(s)+solution;CDF(III),NaCl(s)+solution;BDC(IV),Na2SO4(s)+NaCl(s)+solution.
With the temperature increasing from(313.15 to 373.15)K,the solubility curves of Na2SO4and NaCl move upwards and downwards respectively(see Fig.1)and the co-saturated point moves downwards to the right(see Fig.3).Therefore,the crystallization region of Na2SO4becomes larger while the crystallization regions of both NaCl and(Na2SO4+NaCl)become smaller from(313.15 to 373.15)K.As we all know,the solubility of NaCl in pure water is slightly increasing with temperature elevation while that of Na2SO4is gradually decreasing when temperature is above 313.15 K.As the result shows,the rule works in coal gasification wastewater as well.Therefore,Na2SO4shows promise to be separated from the system by high temperature evaporation.
What is more,in comparison with the solubility data of(NaCl+Na2SO4+H2O)in pure water from literature[11](see Table 4),as shown in Fig.4,the solubility data of the system in CGW is consistent with pure water atT=(323.15 and 373.15)K on the NaClrich side.Nevertheless,the solubility curve of Na2SO4in CGW is obviously lower than that of pure water at the same temperature,revealing that the solubility of Na2SO4grows larger in CGW than that in pure water atT=(323.15 and 373.15)K.Besides,the difference is more significant at 323.15 K than 373.15 K.It may demonstrate that the impurities in CGW have a greater impact on the solubility of Na2SO4at low temperatures.
3.2.Solid-liquid equilibrium phase diagrams at T=(293.15 to 303.15)K
The phase diagrams of the pseudo-ternary system atT=(293.15 to 303.15)K are plotted in Fig.5.Meanwhile,a schematic diagram(Fig.6)is provided to give a better understanding of the phase diagrams.
As shown in Fig.5,atT=(293.15 to 303.15)K,there are two cosaturated points and three solubility curves,corresponding to the solid phase Na2SO4,NaCl and a new solid phase Na2SO4·10H2O.According to the literature[11],when sodium sulfate reaches saturation in water,it will crystallize in the form of anhydrous Na2SO4above 305.55 K and Na2SO4·10H2O below 305.55 K.With the concentration of sodium sulfate in the equilibrium liquid phase increasing,Na2SO4·10H2O turns up and anhydrous Na2SO4disappears on the Na2SO4-rich side.Besides,as Fig.6 shows,there are five crystallization regions in the phase diagrams atT=(293.15 to 303.15)K.S10ED1(II),S10BD1(III),BD1D2(IV),BD2C(V)and FD2C(VI)are the crystallization regions of Na2SO4·10H2O,(Na2SO4·10H2O + Na2SO4),Na2SO4,(Na2SO4+NaCl)and NaCl in CGW,respectively.
As shown in Fig.5,the measured solubility data in CGW atT=(293.15 and 298.15)K coincides well with the literature data in pure water(see Table 4)on the NaCl-rich side.However,the solubility curves of both Na2SO4·10H2O and(Na2SO4·10H2O+Na2SO4)shift downwards and the crystallization regions of Na2SO4·10H2O,(Na2SO4·10H2O+Na2SO4),Na2SO4and(Na2SO4+NaCl)become smaller in CGW compared with in pure water.It indicates that the solubility of Na2SO4grows larger in CGW than that in pure water atT=(293.15 to 303.15)K,in compliance with(313.15 to 373.15)K.
3.3.Solid-liquid equilibrium phase diagrams at T=(273.15 to 288.15)K
The phase diagrams of the pseudo-ternary system and a schematic diagram atT=(273.15 to 288.15)K are exhibited in Figs.7 and 8,respectively.
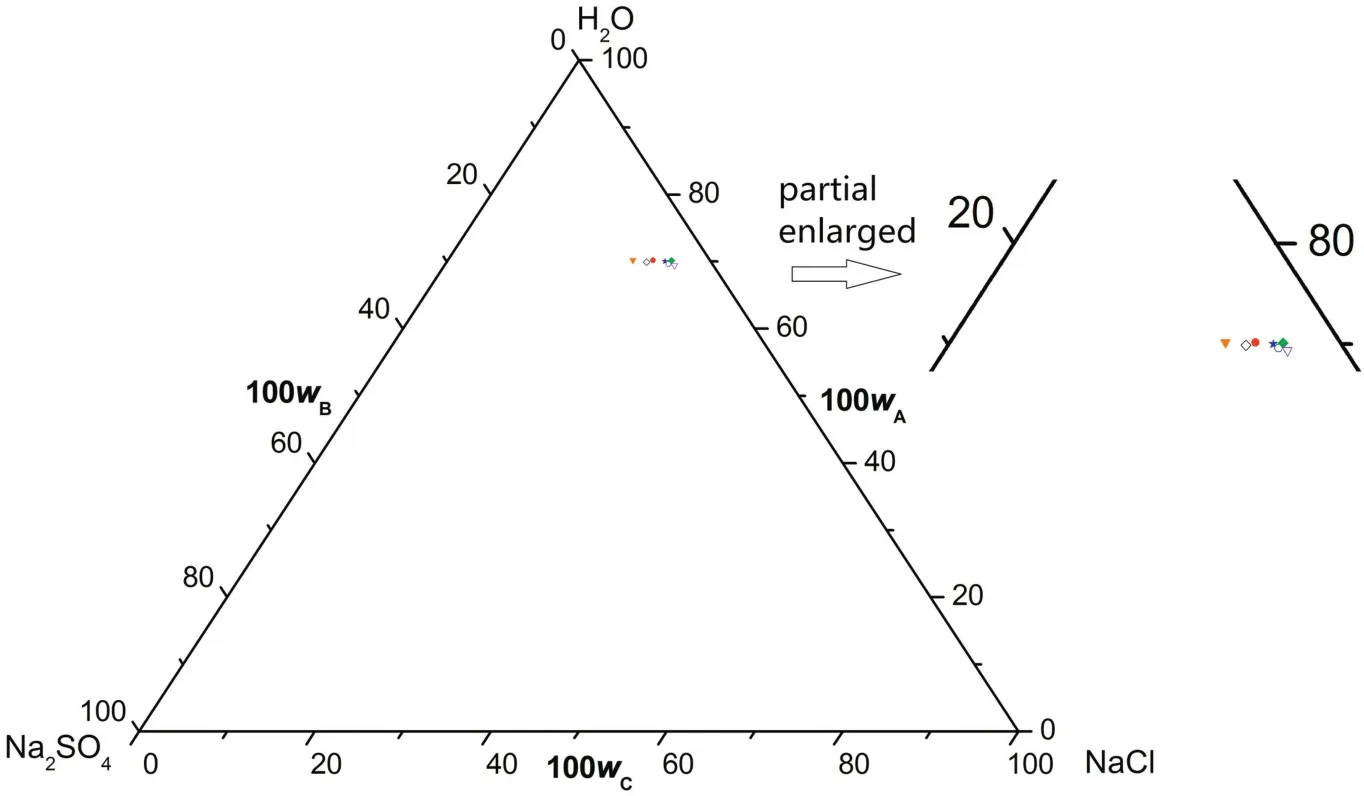
According tofig.7,the phase diagrams have one co-saturated point of Na2SO4·10H2O and NaCl,two solubility curves corresponding to the solid phase Na2SO4·10H2O and NaCl,respectively,and one unsaturated solution region of Na2SO4·10H2O and NaCl atT=(273.15 to 288.15)K.As illustrated in Fig.8,there is one more crystallization region at the temperature range of(273.15 to 288.15)K than(313.15 to 373.15)K,in which Na2SO4·10H2O,Na2SO4and NaCl are in coexistence in solid form.Similarto(313.15 to 373.15)K and(293.15 to 303.15)K,the measured solubility data in CGW atT=(273.15 to 288.15)K coincides well with the literature data in pure water(see Table 4)on the NaCl-rich side.However,the solubility curve of Na2SO4·10H2O shifts downwardsand the crystallization region of Na2SO4·10H2O becomes smaller in CGW compared with in pure water.It demonstrates that the solubility of Na2SO4grows larger in CGW than that in pure water atT=(273.15 to 288.15)K,in accordance with(313.15 to 373.15)K and(293.15 to 303.15)K.
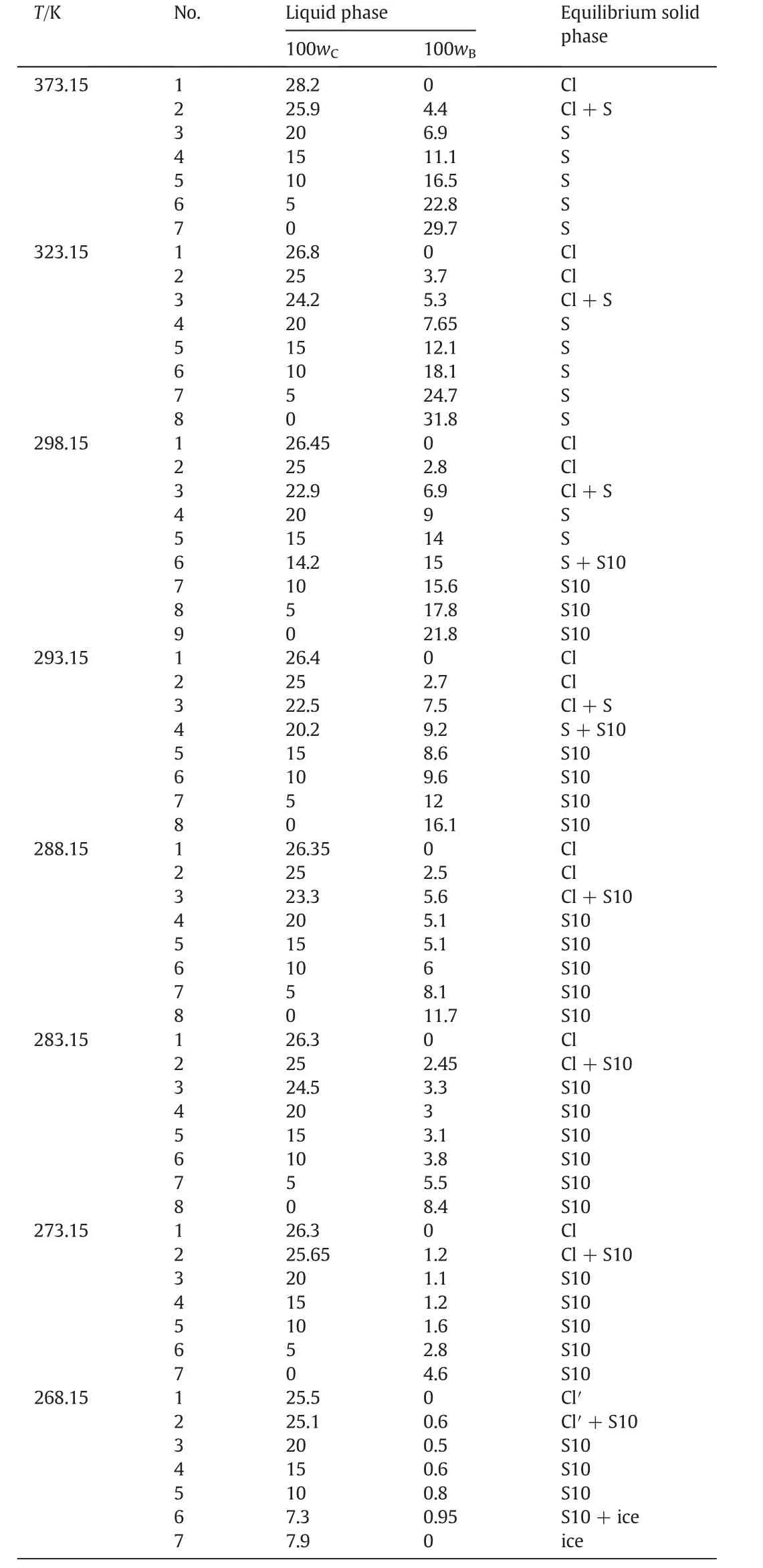
Table 4Solid-liquid equilibrium solubility data of the ternary system(NaCl+Na2SO4+H2O)in pure water taken from literature[11]
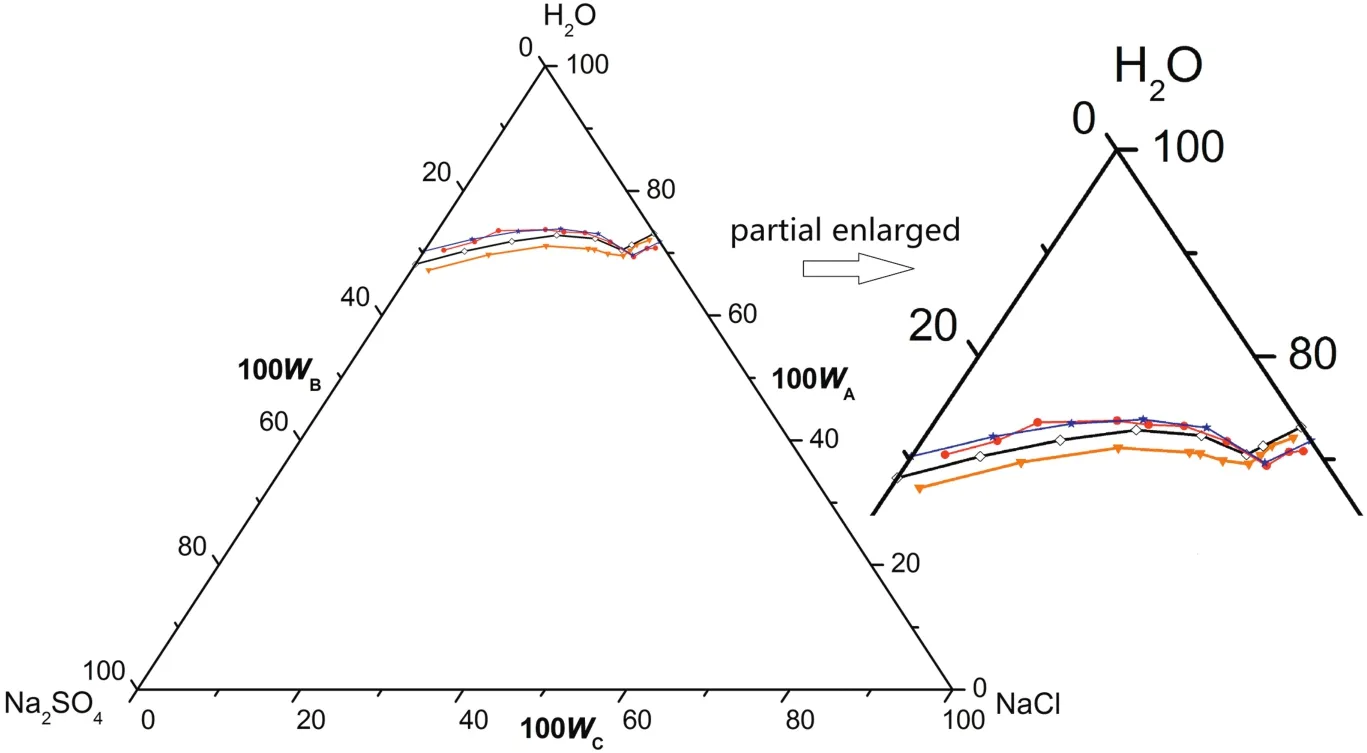
Fig.5.The phase diagrams of the pseudo-ternary system(NaCl+Na2SO4+H2O)of coal gasification wastewater(CGW)at T=(293.15 to 303.15)K and the ternary system(NaCl+Na2SO4+H2O)of pure water(PW)at T=(293.15 and 298.15)K.w A,w B and w C are the mass fractions of H2O,Na2SO4 and NaCl,respectively.(T=303.15 K CGW;◇T=298.15 K CGW;T=298.15 K PW;T=293.15 K CGW;T=293.15 K PW).
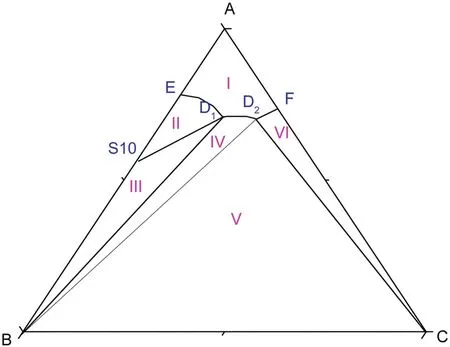
Fig.6.Schematic diagram of the ternary system(NaCl+Na2SO4+H2O)at T=(293.15 to 303.15)K.Points:A,H2O;B,Na2SO4;C,NaCl;S10,Na2SO4·10H2O;D1,the co-saturated point of(Na2SO4·10H2O+Na2SO4);D2,the co-saturated point of(Na2SO4+NaCl);E and F,the saturated points of Na2SO4·10H2O and NaCl in the solution,respectively.Curves:ED1,D1D2 and D2F,the solubility curves of Na2SO4·10H2O,(Na2SO4·10H2O+Na2SO4)and NaCl,respectively.Regions:AED1D2F(I),solution;S10ED1(II),Na2SO4·10H2O(s)+solution;S10BD1(III),Na2SO4·10H2O(s)+Na2SO4(s)+solution;BD1D2(IV),Na2SO4(s)+solution;BD2C(V),Na2SO4(s)+NaCl(s)+solution;FD2C(VI),NaCl(s)+solution.
3.4.Solid-liquid equilibrium phase diagrams at T=268.15 K
Since the solubility of Na2SO4decreases with the decrease of temperature atT=(268.15 to 313.15)K,pure Na2SO4·10H2O can be crystallized out when the system is cooled to 268.15 K.Therefore,cooling crystallization is also a potential method for the separation of Na2SO4from CGW.
In a word,itcan be concluded that,atT=(268.15 to 373.15)K,there is no significant difference between CGW and pure water on the NaClrich side while the solubility of Na2SO4is obviously larger in CGW than that in pure water,and the difference becomes more apparent when the temperature drops.The increase in the solubility of Na2SO4is most likely resulted from the effects of other impurities in CGW.Furthermore,compared with the case of pure water,the impurities in CGW lead to decreasing of the crystallization region of Na2SO4,which is unfavorable for the crystallization of Na2SO4to some extent.
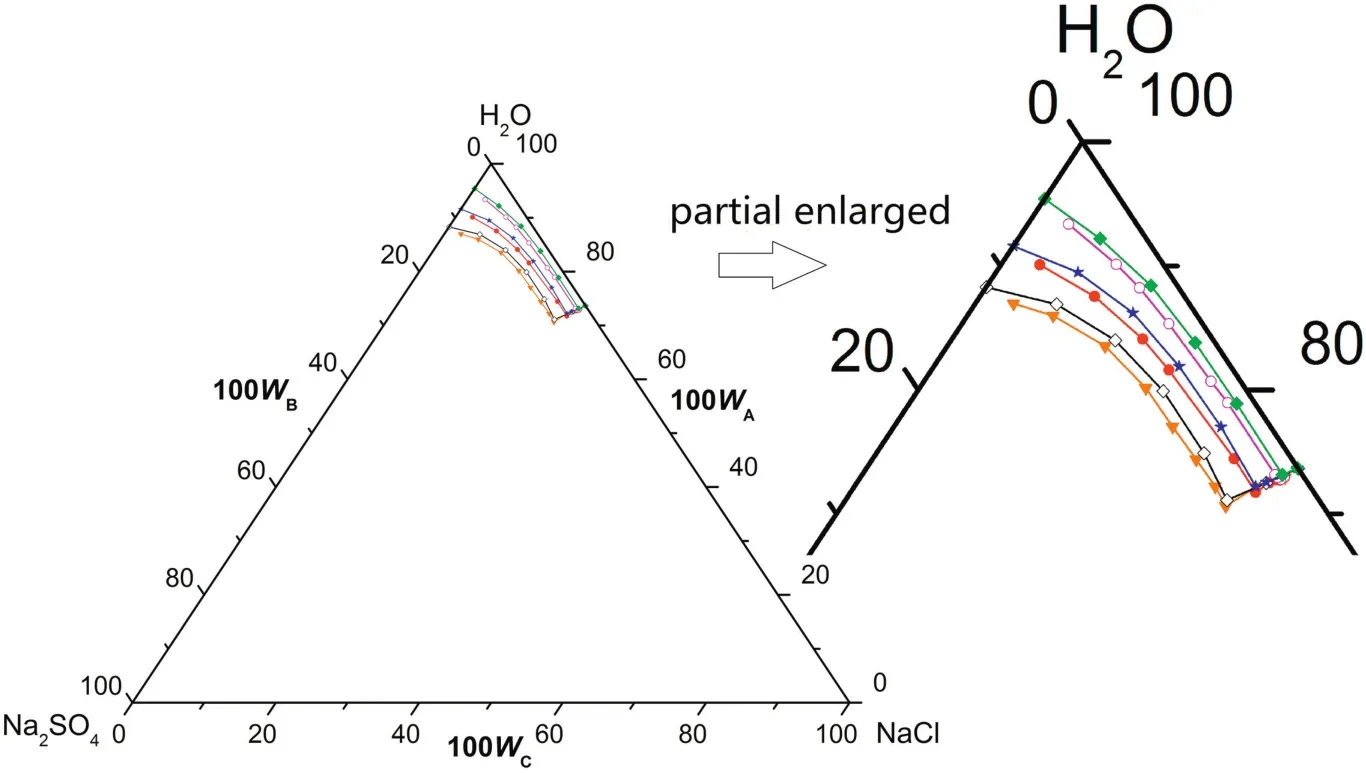
Fig.7.The phase diagrams of the pseudo-ternary system(NaCl+Na2SO4+H2O)of coal gasification wastewater(CGW)and the ternary system(NaCl+Na2SO4+H2O)of pure water(PW)at T=(273.15 to 288.15)K.w A,w B and w C are the mass fractions of H2O,Na2SO4 and NaCl,respectively.(T=288.15 K CGW;◇T=288.15 K PW;T=283.15 K CGW;T=283.15 K PW;T=273.15 K CGW;T=273.15 K PW).
4.Conclusions
The pseudo-ternary system of(NaCl+Na2SO4+H2O)of coal gasification wastewater was studied atT=(268.15 to 373.15)K.According to solid-liquid equilibrium data,the phase diagrams of coal gasification wastewater were determined,analyzed in detail and compared with those of pure water.In summary,there was no significant solubility difference on the NaCl-rich side between coal gasification wastewater and pure water.Nevertheless,the solubility of Na2SO4was obviously larger in coal gasification wastewater than that in pure water,and the difference turned out to be more significant at low temperatures.The increase in the solubility of Na2SO4was most likely due to the effects of other impurities in coal gasification wastewater.The phase diagrams are ofgreatuse for surmounting the intractable problemofsaltrecovery in coal gasification wastewater treatment.
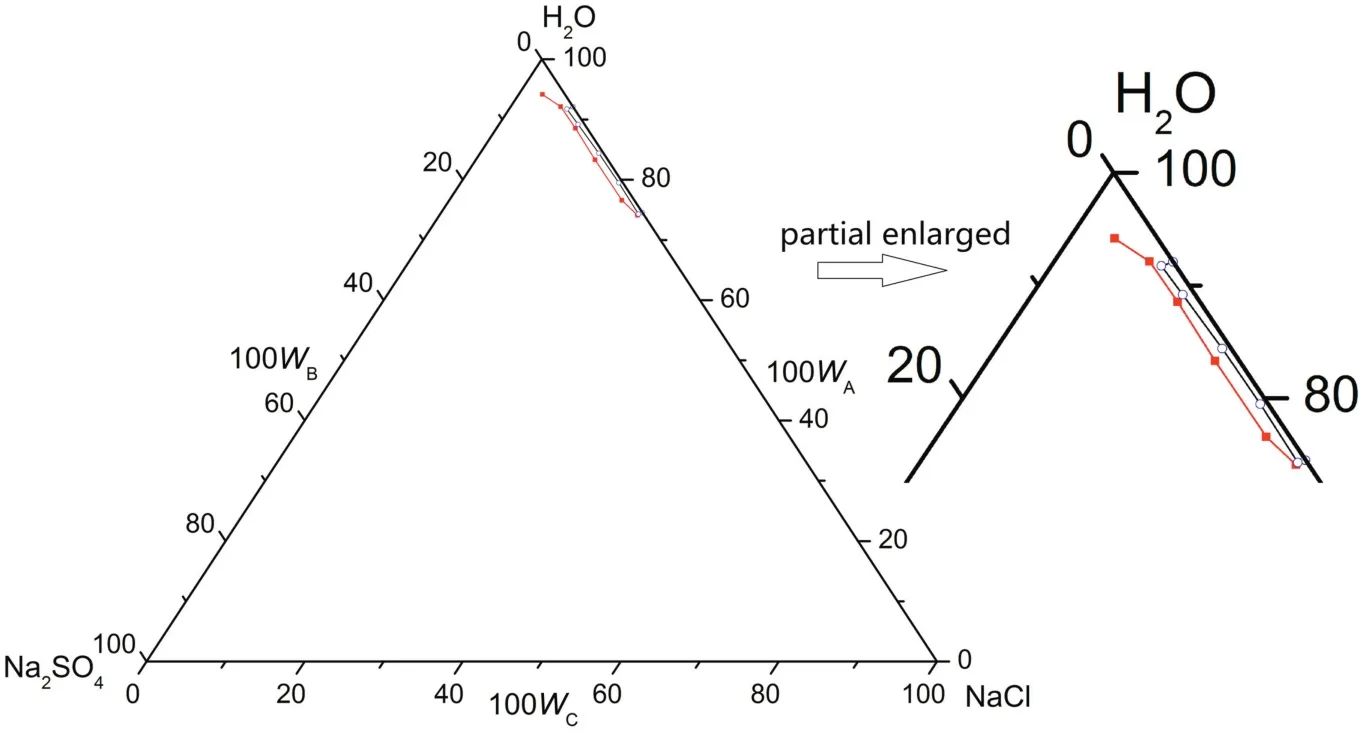
Fig.9.The phase diagrams of the pseudo-ternary system(NaCl+Na2SO4+H2O)of coal gasification wastewater(CGW)and the ternary system(NaCl+Na2SO4+H2O)of pure water(PW)at T=268.15 K.w A,w B and w C are the mass fractions of H2O,Na2SO4 and NaCl,respectively.(T=268.15 K CGW;T=268.15 K PW).
Nomenclature
pPressure
SSolubility
TTemperature
稳定型冠心病的发病机制主要是冠状动脉存在固定狭窄或部分闭塞的基础上发生需氧量的增加。其中冠状动脉痉挛和微循环障碍也参与其中,但稳定型心绞痛在一些诱发因素下可以部分转为不稳定型心绞痛,其主要发病原因是由稳定的斑块发生侵蚀或破裂,继发血小板聚集,因此稳定型心绞痛的抗血小板治疗也是必不可少的,有相关专家就此进行论述。
uStandard uncertainty
u(w) Standard uncertainty of mass fraction
u(T) Standard uncertainty of temperature
u(p) Standard uncertainty of pressure
u(ρ) Standard uncertainty of density
wAThe mass fraction of H2O
wBThe mass fraction of Na2SO4
wCThe mass fraction of NaCl
ρ Solution density
[1]W.Wang,H.Han,M.Yuan,H.Li,F.Fang,K.Wang,Treatment of coal gasification wastewater by a two-continuous UASB system with step-feed for COD and phenols removal,Bioresour.Technol.102(2011)5454-5460.
[2]Z.Wang,X.Xu,Z.Gong,F.Yang,Removal of COD,phenols and ammonium from Lurgi coal gasification wastewater using A2O-MBR system,J.Hazard.Mater.235-236(2012)78-84.
[3]Z.Yu,Y.Chen,D.Feng,Y.Qian,Process development,simulation,and industrial implementation of a new coal-gasification wastewater treatment installation for phenol and ammonia removal,Ind.Eng.Chem.Res.49(2010)2874-2881.
[4]W.Wang,H.Han,M.Yuan,H.Li,Enhanced anaerobic biodegradability of real coal gasification wastewater with methanol addition,J.Environ.Sci.22(2010)1868-1874.
[5]W.Shen,Y.Ren,T.Wang,C.Hai,Stable(solid+liquid)phase equilibrium for the ternary systems(K2SO4+KH2PO4+H2O),(K2SO4+KCl+H2O)atT=313.15 K,J.Chem.Thermodyn.90(2015)15-23.
[6]C.Du,S.Zheng,H.Li,Y.Zhang,Solid-liquid equilibria of K2CO3+K2CrO4+H2O system,J.Chem.Eng.Data51(2006)104-106.
[7]J.Hu,S.Sang,Q.Liu,Phase equilibria in the ternary systems KBr-MgBr2-H2O and NaBr-MgBr2-H2O at 348.15 K,Fluid Phase Equilibr.392(2015)127-131.
[8]S.Sang,Y.Hu,R.Cui,J.Hu,Y.Wang,Measurements of solid-liquid equilibria in the ternary system NaCl-NaBr-H2O at 373 K,Russ.J.Phys.Chem.89(2015)1152-1157.
[9]H.Schott,A mathematicalextrapolation for the method ofwetresidues,J.Chem.Eng.Data3(1961)324.
[10]Y.Su,B.Lu,X.Wang,Phase Diagram Analyses of Inorganic Chemical Production:Basic Theory,Chemical Industry Press,Beijing,1985 136-137.
[11]Z.Niu,F.Cheng,The Phase Diagram of Salt-Water System and its Application,second ed.Tianjin University Press,Tianjin,2002.
[12]H.Stephen,T.Stephen,Solubilities of Inorganic and Organic Compounds,Pergamon Press Ltd.,Oxford,1979.
 Chinese Journal of Chemical Engineering2017年7期
Chinese Journal of Chemical Engineering2017年7期
- Chinese Journal of Chemical Engineering的其它文章
- Suppression of gold nanoparticle agglomeration and its separation via nylon membranes☆
- Effects of solubility parameter differences among PEG,PVP and CA on the preparation of ultra filtration membranes:Impacts of solvents and additives on morphology,permeability and fouling performances
- Experimental detection of bubble-wall interactions in a vertical gas-liquid flow☆
- Horizontal gas mixing in rectangular fluidized bed:A novel method for gas dispersion coefficients in various conditions and distributor designs
- Synthesis of clay-supported nanoscale zero-valent iron using green tea extract for the removal of phosphorus from aqueous solutions
- Hydrodynamic dispersion ofreactive solute in a Hagen-Poiseuille flow of a layered liquid
