Synthesis of Cu/ZnO Flower-like Hierarchical Porous Structures and Investigation of Their Catalytic Performance for Dimethyl Oxalate Hydrogenation
San Xiaoguang; Zhao Guodong; Wang Guosheng; Qi Jian; Jin Quan; Meng Dan
(1. College of Chemical Engineering, Shenyang University of Chemical Technology, Shenyang 110142; 2. State Key Laboratory of Biochemical Engineering, CAS Center for Excellence in Nanoscience, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190)
Synthesis of Cu/ZnO Flower-like Hierarchical Porous Structures and Investigation of Their Catalytic Performance for Dimethyl Oxalate Hydrogenation
San Xiaoguang1; Zhao Guodong1; Wang Guosheng1; Qi Jian2; Jin Quan2; Meng Dan1
(1. College of Chemical Engineering, Shenyang University of Chemical Technology, Shenyang 110142; 2. State Key Laboratory of Biochemical Engineering, CAS Center for Excellence in Nanoscience, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190)
The Cu/ZnO flower-like hierarchical porous structures were successfully synthesized via the cetyltrimethyl ammonium bromide (CTAB) assisted hydrothermal method. The morphology and structure as well as the catalytic performance for dimethyl oxalate (DMO) hydrogenation to ethylene glycol (EG) were investigated. Through annealing the zinc copper hydroxide carbonate (ZCHC) precursors, the Cu/ZnO fower-like hierarchical porous structures were obtained, which were assembled by a number of porous nanosheets. The catalyst made of these well-defned fower-like hierarchical porous structures with large specifc surface area and effective gas diffusion path via the well-aligned porous structures showed higher EG selectivity and yield as compared to the Cu/ZnO catalyst obtained by conventional co-precipitation technique. The results indicated that the Cu/ZnO flower-like hierarchical porous structures have excellent potential application for manufacture of high performance catalysts.
hierarchical porous structure; Cu/ZnO catalyst; dimethyl oxalate; hydrogenation
1 Introduction
Ethylene glycol (EG) is an important commodity chemical widely used as antifreeze, solvent, starting material for manufacture of polyester and many other chemical products[1-2]. The synthesis of EG from cheap syngas is attracting great interest owing to its economical and environmentally friendly nature[3]. This route includes a two-step process, viz.: the coupling of CO with nitrite esters to form dimethyl oxalate (DMO) and the subsequent hydrogenolysis of DMO to EG. Insuffcient catalytic activity and stability are key barriers to the commercial development of the vapour-phase hydrogenation of DMO to EG.
The Cu/ZnO composites have been widely applied in catalytic hydrogenation of esters and aldehydes[4-6]. According to the reports[7-10]it is known that catalysis is controlled not only by the chemical composition and size of the catalyst but also by the type of active sites on the catalyst surface. Therefore, the design and synthesis of ZnO with different morphology to interact with Cu species have been attracting considerable attention. In recent years, the Cu/ZnO catalyst with different ZnO morphology has been synthesized, with its catalytic properties investigated. For example, Lei, et al.[11]reported that the Cu/ZnO catalyst with flamentlike ZnO structures exhibited excellent performance for hydrogenation of CO2to methanol. Zhang, et al.[5]designed Cu-ZnO catalyst with controlled structures, which showed that the synergistic effect between Cu0ZnO and Cu+could lead to higher selectivity, efficiency and stability of γ-valerolactone obtained from hydrogenation of levulinic acid. Judging from the viewpoint of catalytic reaction, porous materials with higher specific surface area and effective gas diffusion path via the well-aligned porous structures can provide abundant adsorption sites and enhance the diffusion of reactingmedia and products, which can significantly improve the catalytic performance[12-14]. Great efforts have been made to explore new synthesis methods and control the formation of porous structures to satisfy the needs of specific applications. However, there is little report about the Cu/ZnO catalyst with porous hierarchical architecture, although it has been widely investigated in many other application fields[15-16]. In addition, the controlled fabrication of ordered hierarchical porous structure while governing their shape at the microscopic level is still a signifcantly challenging issue for material research scientists. Therefore, it is of great significance to synthesize the advanced Cu/ZnO catalyst with hierarchical porous structures in a feasible and economic manner allowing for systematically investigating their catalytic properties.
In this work, we reported a simple method for hydrothermal synthesis of Cu/ZnO catalysts with fowerlike hierarchical porous structures, using cetyltrimethyl ammonium bromide (CTAB) as the surfactant. The morphology and structure of Cu/ZnO catalysts as well as their catalytic performance in DMO hydrogenation were investigated to find the optimized reaction conditions. For comparison purpose, the Cu/ZnO catalysts were also prepared by a conventional co-precipitation technique. The results showed that the catalysts manufactured from the well-defned Cu/ZnO fower-like hierarchical porous structures exhibited excellent catalytic performance, demonstrating the potentials of this unique hierarchical architecture for DMO hydrogenation application.
2 Experimental
2.1 Catalyst preparation
The Cu/ZnO flower-like hierarchical porous structures were synthesized by a simple hydrothermal method using CTAB as the surfactant. In a typical experiment, 1.78 g of Zn(NO3)2·6H2O and 0.97 g of Cu(NO3)2·3H2O were dissolved in 80 mL of deionized water under magnetic stirring to obtain a clear solution. Subsequently, the obtained mixture solution and 80 mL of aqueous urea solution (0.15 mol/L) were mixed under magnetic stirring. Then, 2.19 g of CTAB were added into the above solution. After stirring for 30 min, the mixture was transferred into a Teflon-lined stainless-steel autoclave, in which about 80% of the whole volume was filled with the mixture and the distilled water. The autoclave was maintained at 120oC for 12 h and then was cooled down to room temperature naturally. The blue precipitate (zinc copper hydroxide carbonate, ZCHC) was fltered and washed with distilled water and ethanol for three times, respectively. The obtained solid was dried at 80oC for 12 h and then was calcined at 450oC for 4 h in air, followed by being pelletized and screened to the size of 20-40 meshes. Thereafter, the obtained precursor was reduced by a flow of pure hydrogen (at a flow rate of 150 mL/min) at 300oC for 10 h, and then was passivated by 1% of oxygen diluted with nitrogen stream (at a flow rate of 30 mL/ min) at room temperature for 1 h. The resulted Cu/ZnO catalyst was designated as the CTAB-Cu/ZnO catalyst. For comparison, a reference Cu/ZnO catalyst (CC-Cu/ ZnO) was prepared by the conventional co-precipitation method. In brief, 7.5 g of Cu(NO3)2·3H2O and 9.3 g of Zn(NO3)2·6H2O were dissolved in 300 mL of distilled water, and then the obtained mixture solution and 300 mL of aqueous Na2CO3solution (1.0 mol/L) were mixed synchronously to maintain a constant pH value of 8.5 at 50oC under continuous stirring to form a blue color slurry. After aging for 10 h, the precipitate were washed thoroughly with deionized water and dried at 80oC for 12 h, followed by calcination at 450oC for 4 h in air.
2.2 Catalyst characterization
The BET surface area and the pore size distribution were determined by the N2physisorption method using an automatic gas adsorption system (Micromeritics SSA-4000). Before the measurements, samples were degassed under vacuum at 25oC for 3 h.
The powder X-ray diffraction (XRD) patterns of the passivated samples were collected using a Rigaku RINT 2400 X-ray diffractometer with Cu-Kα radiation (λ=0.154 nm). The X-ray tube was operated at 40 kV and 40 mA. The H2-TPR was carried out on a Micromeritics AutoChemⅡ. The sample was heated in helium atmosphere at 400oC for 60 min, followed by cooling to room temperature. The temperature was then increased under a 10% H2/Ar fow (at a fow rate of 50 mL/min) at a temperature increase rate of 10oC/min until the specifed temperature of 550oC was reached. The H2consumptionwas detected using a TCD.
The metallic copper dispersion in the reduced catalyst was determined by the method of N2O oxidation, followed by H2titration. The Cu surface area was measured by pulse titration of the Cu surface atoms in the pre-reduced samples with N2O. 30 mg of Cu/ZnO catalyst were reduced in a flow of 5% hydrogen diluted by nitrogen stream at 220oC for 1 h. After cooling the catalyst to 90oC with the helium stream, N2O was injected as pulses through a six-port valve. The amount of N2O consumed was monitored by a thermal conductivity detector (TCD). The dissociative adsorption proceeds according to the reaction shown below:
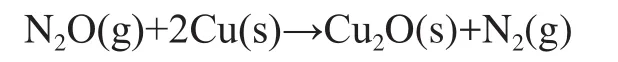
The Cu surface area of the catalyst was determined by the amount of N2O consumed.

The reduction behavior of Cu catalysts was studied using the temperature-programmed reduction (TPR) technique. The temperature of samples in the reactor was increased from 130oC to 427oC at a temperature increase rate of 5oC/min. The reducing gas consisting of 5% H2in N2stream (at a fow rate of 50 mL/min) was introduced into the reactor. The 5A molecular sieve at the outlet of reactor absorbed the water formed in the course of reduction before entering the TCD.
The morphology and dimension of the samples were investigated using a ZEISS Ultra Plus field emission scanning electron microscope (FE-SEM). The TEM images were obtained on a JEOL EM002B transmission electron microscope (TEM) with an accelerating voltage of 200 kV.
2.3 Catalyst testing
The catalytic reaction of DMO hydrogenation to EG was conducted in a packed-bed stainless-steel reactor (9.5 mm OD). 1.0 g of catalyst particles were loaded into the reactor as mentioned above. Before the reaction, the catalyst samples were reduced by a flow of pure hydrogen at 300oC for 2 h. After cooling to the reaction temperature, the reactant (15% of DMO in methanol solution) was injected from the top of the reactor through a high-pressure pump. H2(H2/DMO molar ratio=80) was introduced and the reaction pressure was maintained at 2.0 MPa. The liquid hourly space velocity (LHSV) of DMO was 0.5 h-1. The reaction temperature was varied from 180oC to 230oC. The products were collected in an ice-water trap and analyzed by a flame-ionization detector (FID, Shimadzu GC 2014) equipped with a HP-5 capillary column (Hewlett-Packard Company, 30 m × 0.32 mm×0.5 μm). All liquid products were confrmed by GC-MS (Shimadzu GCMS 1600). All of the selectivity values in the fgures and the tables mentioned here were calculated in molecular selectivity.
3 Results and Discussion
3.1 Structure of the catalysts
The microstructure and morphology of the ZCHC precursors and the corresponding Cu/ZnO products, which were calcined at 450oC for 4 h in air, were investigated by SEM, TEM and XRD techniques with the results shown in Figure 1. It can be seen from the SEM images (Figure 1(a)-(b)) that the ZCHC precursors were composed of fower-like structures with a diameter ranging from 1 μm to 5 μm. These fower-like structures were constructed of many nanosheets with smooth surface, and some of them were densely packed to form a multilayered structure. It is interesting to know that the fower-like structures were successfully retained after calcination (Figure 1(b)). In addition, it should be noted that the diameter of these flower-like structures did not change after calcination. It is also found out that the surface of these nanosheets was rough with a lot of pores, which should be attributed to removal of water and release of gas molecules during the pyrolysis of the precursor. Further observation of TEM images (Figure 1(c)) also demonstrates that Cu/ ZnO flower-like structures were constructed of porous nanosheets. This kind of structures with many petals, pores and intervals should play an important role in enhancing the catalytic performance of Cu/ZnO catalyst. It can be clearly seen from the XRD pattern (Figure 1(d)) that all the diffraction peaks of the ZCHC precursors could be indexed to monoclinic Zn3Cu2(OH)6(CO3)2, which were in good agreement with the standard JCPDS card No. 82-1253. No impurity peaks were found inthe XRD patterns, indicating to a high purity of the asobtained Zn3Cu2(OH)6(CO3)2. After the ZCHC precursors were heat-treated at 450oC for 4 h in air, the characteristic peaks of hexagonal wurtzite ZnO (JCPDS 36-1451) and monoclinic-phased CuO (JCPDS card No. 89-5898) were observed. The XRD peaks were quite different from those of precursor before calcination, indicating that Zn3Cu2(OH)6(CO3)2was decomposed to hexagonalphased ZnO and monoclinic-phased CuO at 450oC.

Figure 1 (a) SEM image of the ZCHC precursors, (b) SEM image of Cu/ZnO products, (c) TEM images of Cu/ZnO products, (d) XRD pattern of the ZCHC precursors and the corresponding Cu/ZnO products
The SEM image of the Cu/ZnO product prepared by conventional co-precipitation method is shown in Figure 2(a). Nanoparticles with a diameter ranging from 100 nm to 300 nm were obtained, and some of them aggregated together to form large particles. No uniform and hierarchical porous structures were obtained, because thermal decomposition of basic carbonate salt could yield metal oxides without morphological deformation[17]. The XRD patterns of the products obtained by the coprecipitation method are shown in Figure 2(b). Before calcination, all the diffraction peaks of the products could be indexed to monoclinic Zn3Cu2(OH)6(CO3)2, which were in good agreement with the standard JCPDS card No. 82-1253. It is found that after calcination all the diffraction peaks could be indexed to the hexagonal wurtzite ZnO (JCPDS 36-1451) and the monoclinicphased CuO (JCPDS card No. 89-5898). The product exhibited strong and sharp diffraction peaks, indicating to its good crystallinity.
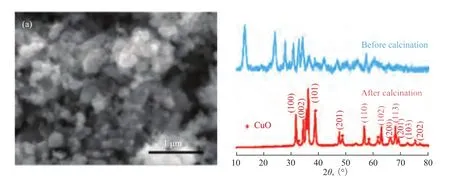
Figure 2 (a) FESEM image and (b) XRD pattern of the products obtained by conventional co-precipitation technique
3.2 The properties of catalysts
The H2-TPR measurements were carried out to investigate the reducibility of the copper species in the CC-Cu/ZnO catalyst obtained by the conventional co-precipitation method and in the CTAB-Cu/ZnO catalyst obtained by the hydrothermal method. The reduction profle is shown in Figure 3. It is found that the reduction profles of both two catalysts exhibited one broad peak at below 300oC. In order to ensure the sufficient reduction of the Cu/ ZnO catalysts, the reduction of the catalyst was then conducted at 300oC. It is also found that in comparison with the CC-Cu/ZnO catalyst, the CTAB-Cu/ZnO catalyst exhibited a broader peak region and a lower reduction temperature.
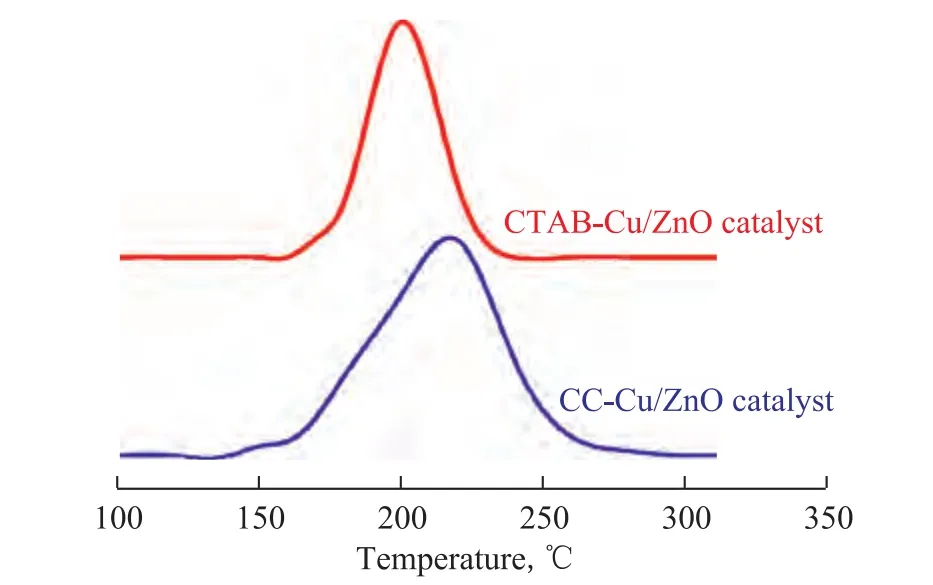
Figure 3 H2-TPR patterns of CC-Cu/ZnO catalyst obtained by conventional co-precipitation technique and CTAB-Cu/ ZnO catalyst obtained by hydrothermal method
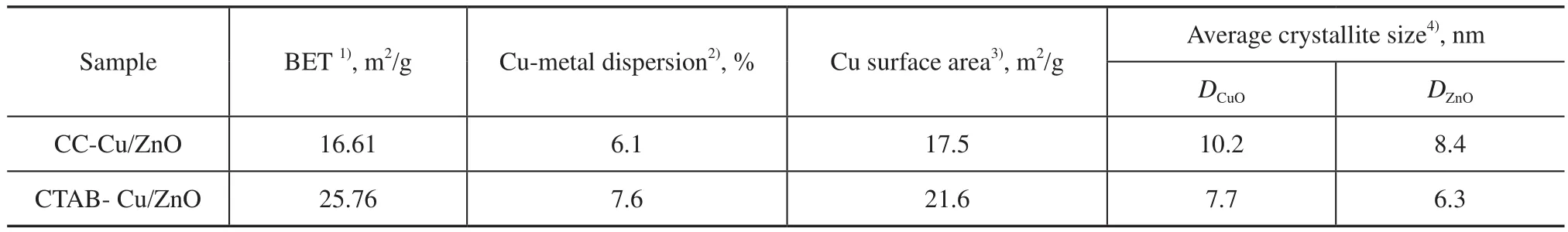
Table 1 Physicochemical properties of the Cu/ZnO catalysts
3.3 Catalytic performance
The catalytic properties of the CTAB-Cu/ZnO catalyst and CC-Cu/ZnO catalyst for DMO hydrogenation to EG were tested. It is well-known that hydrogenation of DMO proceeds via the intermediate methyl glycolate (MG) to EG, while EG can be dehydrated further to form ethanol[18-19]. The products will react with each other to produce other byproducts at high temperature such as the reaction between EG and ethanol on basic sites which can yield 1,2-butanediol (BDO). DMO hydrogenation to EG involves the following processes:
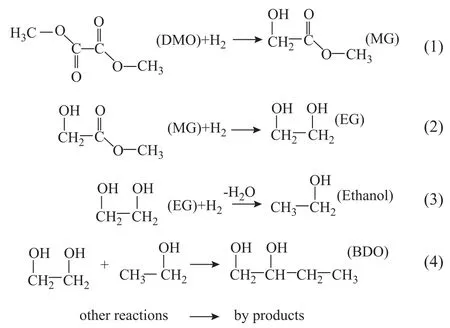
The major product was EG, and the side products included MG, ethanol (EtOH), and other impurities such as 1,2-butandiol (BDO), etc. The conversion of DMO on Cu/ZnO catalysts is shown in Figure 4. The CC-Cu/ZnO catalyst displayed poor catalytic activity in the hydrogenation of DMO, and the conversion of DMO was increased from 33.96% to 42.25%, when the reaction temperature increased from 180oC to 230oC. In comparison with the CC-Cu/ZnO catalyst, the performance of the CTAB-Cu/ZnO catalyst was improved significantly. When the reaction temperature increased from 180oC to 230oC, the conversion of DMO increased from 66.35% to 73.31%, which was obviously higher than that achieved by the CC-Cu/ZnO catalyst.

Figure 4 DMO conversion at different reaction temperatures
The selectivity achieved by the Cu/ZnO catalysts at different reaction temperatures is shown in Figure 5(a)and (b). As regards the CTAB-Cu/ZnO catalyst, when the reaction temperature increased from 180oC to 220oC, the selectivity of MG dropped from 34.58% to 15.78% and the selectivity of EG increased from 64.45% to 82.42%. These results showed that higher temperature was favourable to hydrogenation of MG to EG. Whereas, when the temperature was above 220oC, the selectivity of EG began to decrease because overheating tended to promote side reactions. Therefore, the selectivity of side products such as ethanol and other impurities increased obviously when the temperature was higher than 220oC (insert of Figure 5 (a) and (b)). For both of two catalysts studied, the selectivity of products showed the same tendency of variation, when the reaction temperature increased from 180oC to 230oC. Moreover, the CTAB-Cu/ZnO catalyst showed a relatively high EG selectivity than the CCCu/ZnO catalyst in the range of temperature studied. The yield of EG as a function of reaction temperature is presented in Figure 5(c). It is noted that the yield of EG increased to a maximum value at 220oC and then dropped with a further increase in reaction temperature for both catalysts. The highest yield of EG was 59.52% for the CTAB-Cu/ZnO catalyst, while it was 11.38% for the CC-Cu/ZnO catalyst. Briefy, the optimized catalytic performance of CTAB-Cu/ZnO catalyst was achieved at 220oC, which made the selectivity and yield of EG reach the maximum value of 82.42% and 59.52%, respectively. In addition, the CTAB-Cu/ZnO catalyst showed a relatively higher selectivity and yield of EG than those achieved by the CC-Cu/ZnO catalyst at all temperature range tested. The results indicated that the fower-like porous CTAB-Cu/ZnO catalyst owing to its large surface areas coupled with great surface activity could increase the conversion of DMO and exhibit higher activity for DMO hydrogenation as compared to the CC-Cu/ZnO catalyst.
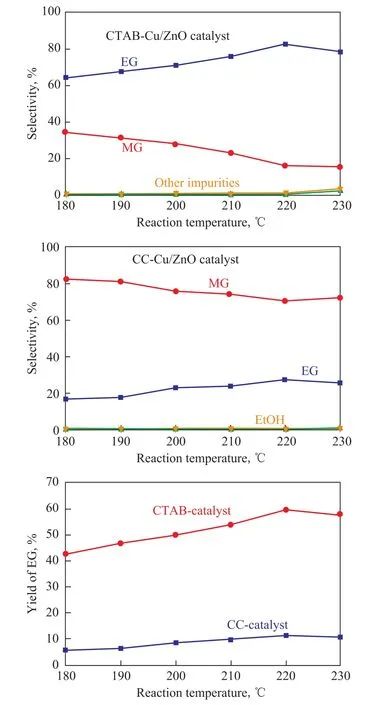
Figure 5 (a) and (b):Selectivity on Cu/ZnO catalysts at different reaction temperatures; (c): Yield of EG as a function of reaction temperature
Upon taking into account the catalyst characterization and catalytic activity results, it can be inferred that the copper particle size, the dispersion of Cu species and the morphology play an important role in improving the catalytic activity of the Cu/ZnO catalyst. It can be easily expected that the Cu/ZnO catalysts with small copper particle size and fine dispersion of Cu species can be favourable to DMO hydrogenation reaction to exhibit a high catalytic activity. Furthermore, the catalyst with a large surface area and an effective gas diffusion path is expected to result in a high catalytic performance[13-14]. Hereby, the flower-like porous-structured CTAB-Cu/ ZnO catalyst was assembled by the interconnected porous nanosheets, which could promote the activation of DMO by the abundant active sites and the Cu phase at the interface assisted by molecular rearrangement (CH3O−, CH3OOC-CO−and−OC-CO−etc.) and hydrogenation to obtain methanol, MG and EG, respectively. In addition, the high porosity of the hierarchical porous structures could provide excellent channels and “surfaceaccessibility” for the mass transportation of target gases. Therefore, the hierarchical porous-structured CTAB-Cu/ ZnO catalyst did exhibit excellent catalytic performance as compared to the CC-Cu/ZnO catalyst, indicating that the unique fower-like hierarchical porous structures with many petals, pores and intervals were a good candidate for catalyst.
4 Conclusions
In summary, the high-performance Cu/ZnO flowerlike hierarchical porous structures were synthesized by hydrothermal process using CTAB as the surfactant. The morphology and structures as well as their catalytic performance for DMO hydrogenation were investigated. For comparison, the Cu/ZnO catalysts were also obtained by the conventional co-precipitation technique. It is found that the Cu/ZnO flower-like hierarchical porous structures were constructed of a number of porous nanosheets. The catalyst made of the well-defned Cu/ZnO fower-like porous structures with large special surface area and effective gas diffusion path via the well-aligned porous structures showed higher EG selectivity and yield at 220oC as compared to the catalyst obtained by the co-precipitation technique. The results suggested that the Cu/ZnO flower-like porous structures had excellent potential application for manufacture of high performance catalysts.
Acknowledgments: The authors gratefully acknowledge the financial support of the National Science Foundation of China (No. 21503137 and 61403263), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, the State Education Ministry (No. 20141685), and the Liaoning Educational Department Foundation (No. L2015425), and the Chinese Scholarship Council (No. 201604910230).
[1] Gong J, Yue H, Zhao Y, et al. Synthesis of ethanol via syngas on Cu/SiO2catalysts with balanced CuO–Cu+sites [J]. Journal of the American Chemical Society, 2012, 134(34): 13922–13925
在现有价格下,联通和移动各自获得的支付都是50。当联通选择涨价时,如果移动采取跟随策略同时涨价,则两家公司的利润都会提高,都可以获得更大的支付70。联通涨价时如果移动采取不涨价策略,则会因为有部分联通宽带用户转而使用移动宽带获得额外支付变为90,移动则会损失市场份额导致获得的支付减少降为10。反之亦然。
[2] Liu P, Hensen E J M. Highly efficient and robust Au/ MgCuCr2O4catalyst for gas-phase oxidation of ethanol to acetaldehyde [J]. Journal of the American Chemical Society, 2013, 135(38): 14032–14035
[3] Kerr R A, Service R F. What can replace cheap oil--and when? [J]. Science, 2005, 309 (5731): 101
[4] Tan Z Y, Wei D, Yong Y, Zhang Z H, et al. Nanostructured Cu/ZnO coupled composites: Toward tunable Cu nanoparticle sizes and plasmon absorption [J]. The Journal of Physical Chemistry C, 2013, 117(20): 10780–10787
[5] Zhang B, Chen Y, Li J W, et al. High effciency Cu-ZnO hydrogenation catalyst: The tailoring of Cu-ZnO interface sites by molecular layer deposition [J]. ACS Catalysis, 2015, 5(9): 5567–5573
[6] Wang D, Yang G H, Ma Q X, et al. Facile solid-state synthesis of Cu–Zn–O catalysts for novel ethanol synthesis from dimethyl ether (DME) and syngas (CO+H2) [J]. Fuel, 2013, 109: 4–6
[7] Narayanan R, El-Sayed M A. Catalysis with transition metal nanoparticles in colloidal solution: nanoparticle shape dependence and stability [J]. The Journal of Physical Chemistry B, 2005, 109: 12663–12676
[8] Liao F L, Huang Y Q, Ge J W, et al. Morphology dependent interactions of ZnO with Cu nanoparticles at the materials’ interface in selective hydrogenation of CO2to CH3OH [J]. Angewandte Chemie International Edition, 2011, 9(50): 2162–2213
[9] Tao K, Ma Q, Tsubaki N, et al. Molybdenum containing cage-like mesoporous KIT-5 for enhanced catalytic conversion of 1-butene and ethylene to propene [J]. Journal of Molecular Catalysis A: Chemical, 2016, 416: 39–46
[10] Ma Q X, Zhao T S, Wang D, et al. Synthesis of dipropyl carbonate over calcined hydrotalcite-like compounds containing La [J]. Applied Catalysis A: General, 2013, 464–465: 142–148
[11] Lei H, Nie R F, Wu G Q, et al. Hydrogenation of CO2to CH3OH over Cu/ZnO catalysts with different ZnO morphology [J]. Fuel, 2015, 154: 161–166
[12] Pritchard J, Filonenko G A, Putten R, et al. Heterogeneous and homogeneous catalysis for the hydrogenation of carboxylic acid derivatives: History, advances and future directions [J]. Chemical Society Reviews, 2015, 44: 3808–3833
[13] Parlett C M A, Wilson K, Lee A F. Hierarchical porous materials: Catalytic applications [J]. Chemical Society Reviews, 2013, 42: 3876–3893
[14] Linares N, Silvestre-Albero A M, Serrano E, et al. Mesoporous materials for clean energy technologies [J]. Chemical Society Reviews, 2014, 43: 7681–7717
[15] Huang J R, Dai Y J, Gu C P, et al. Preparation of porousflower-like CuO/ZnO nanostructures and analysis of their gas-sensing property [J]. Journal of Alloys and Compounds, 2013, 575: 155–122
[16] Ma J, Liu C T, Chen K Z. Assembling non-ferromagnetic materials to ferromagnetic architectures using metalsemiconductor interfaces [J]. Scientifc Reports, 2016, 6: Article number: 34404
[17] Tao K, Zhang Y, Yoneyama Y, et al. Chemical and spatial promotional effects of bimodal pore catalysts for methane dry reforming [J]. Chemical Engineering Journal, 2011, 170: 258–263
[18] Lin J D, Zhao X Q, Cui Y H, et al. Effect of feedstock solvent on the stability of Cu/SiO2catalyst for vapor-phase hydrogenation of dimethyloxalate to ethylene glycol [J]. Chemical Communications, 2012, 48: 1177–1179
[19] Chen L F, Guo P J, Qiao M H, et al. Cu/SiO2catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol [J]. Journal of Catalysis, 2008, 257: 172–180
Zeolite Membrane Reactor for DME Synthesis Developed by Ningbo Institute of Materials Technology and Engineering
The inorganic membrane separation and catalysis research team of the Ningbo Institute of Materials Technology and Engineering (NIMTE) under CAS has successfully constructed a FAU-LTA zeolite membrane reactor with a sandwich-composite structured reaction and separation double functions by regulating and controlling the microscopic structure of the zeolite membrane, while taking into account the specific features of reaction for dimethyl ether (DME) synthesis via catalytic conversion of methanol in compliance with the product separation needs.
The LTA zeolite membrane in the reactor can uninterruptedly separate the byproduct steam from the reaction system to break away from the existing chemical reaction equilibrium to shift the reaction towards the production of DME in order to increase the conversion rate and product selectivity as well as to suppress side reactions. Furthermore, the LTA zeolite membrane through selectively separating the byproduct steam, which functions as a catalytic poison, can increase the reaction rate and extend the catalyst service life. Research work has shown that manufacture of DME via catalytic reaction on the zeolite membrane can result in a methanol conversion of 90.9% at 310oC, with the DME selectivity reaching 100%, which is much higher than the indicators achieved by the fixed-bed reactors to assume a good application prospects.
Achievement of Key Technology for Methanol-Based Manufacture of Ethanol Passed Appraisal
The key technology “The pilot scale study on heterogeneous carbonylation of methanol to methyl acetate” jointly developed by the CAS Dalian Institute of Chemical Physics and the Shandong Union Chemical Industry Limited Liability Company has passed in Beijing the appraisal of research achievements sponsored by the China Petroleum and Chemical Industry Association. The experts attending the appraisal meeting unanimously recognized that this technology is innovative in nature and will bring about good economic and social benefits, with its technical indicators reaching the internationally advanced level.
The research team has developed the Ir-La based catalyst for heterogeneous carbonylation of methanol to methyl acetate featuring high dispersion, high activity, high selectivity and high stability of catalyst that can effectively reduce the cost for separating the products of carbonylation and avoid the metal loss. The application of Ir-La based catalyst and control over the methanol conversion coupled with the adoption of Hastelloy alloy tubing have successfully reduced the project investment cost and production cost, with the overall hourly space velocity reaching 3—5 kg/ kg of catalyst•hour.
date: 2016-11-29; Accepted date: 2017-03-18.
Associate Professor Meng Dan, Telephone: +86-24-89386342; E-mail: mengdan0610@hotmail. com.
- 中国炼油与石油化工的其它文章
- Correlation of Deactivation of Ni-Mo-W/Al2O3during Ultra-Low-Sulfur Diesel Production with Surface Carbon Species
- Effect of Lithium Nitrate on the Structure and Property of α-Al2O3Platelets Prepared via Solid-state Reaction
- Effect of Preparation Process on Compressive Strength and Hydrogenation Performance of Raney-Ni/Al2O3Catalyst
- Corrosion Inhibition by Co-Immobilized Lysozyme and Lipase in Circulating Cooling Water System
- Preliminary Analysis of Diesel-Degrading Bacteria Immobilized on Organic Carriers in Seawater
- Facile Synthesis of Cu/Al2O3with High Copper Dispersion for Direct Synthesis of Dimethyl Ether from Syngas

