Can we treat neurodegenerative diseases by preventing an age-related decline in microRNA expression?
Can we treat neurodegenerative diseases by preventing an age-related decline in microRNA expression?
MicroRNA pathway is down-regulated in aged dopaminergic neurons: Parkinson’s disease (PD) is the most frequent motor neurodegenerative disorder and is morphologically mainly associated with progressive dopaminergic neuronal loss in the ventral midbrain.The cause of this pathology is unknown, but aging is well established as the strongest risk factor, which by far prevails over gender,environmental and genetic factors. In our recent work (Chmielarz et al., 2017), we have demonstrated that the expression of Dicer, a multidomain ribonuclease III and a key endonuclease in microRNA(miRNA) maturation pathway, is significantly down-regulated in aged mouse midbrain. Further, using a laser-assisted microdissection and quantitative PCR profiling techniques, we analyzed microRNAomes of dopaminergic neurons from the mouse substantia nigra and identified a predominant decrease of miRNA expression in aged dopaminergic neurons. Dicer mRNA levels are also reduced in the ventral midbrain of PD patients (Simunovic et al., 2010). Importantly, several miRNAs have been shown to regulate PD-associated genes, suggesting that age-related deregulation of miRNA signaling may contribute to neurodegeneration (Heman-Ackah et al., 2013).
1. Does the miRNA-mediated regulation provide an essential protection mechanism from neurodegeneration?
2. May this protective component be compromised during aging and make the dopamine system more susceptible for other genetic and environmental factors leading to PD?
In our studies, we attempt to resolve these two challenges. Recently, we have found a way to answer the first of these two major questions: miRNA pathway indeed turned out to be cytoprotective for adult dopaminergic neurons (Chmielarz et al., 2017). An increasing number of published and ongoing studies also start to address the second question, identifying neuronal functions- and survival-regulating genes and pathways targeted by miRNA network in the context of neurodegeneration (Briggs et al., 2015).
Factors impacting microRNA biogenesis pathway:MiRNAs are important post-transcriptional regulators of gene expression that destabilize mRNA and/or suppress protein translation via binding to complementary sites in the 3′ untranslated region (UTR) of target mRNA.e sequences of a miRNA and its binding site do not have to be exactly complementary, which gives a potential for each miRNA to regulate hundreds of transcripts, but also makes the prediction of miRNA targets very challenging. MiRNAs may control the activity of about a half of all protein coding genes, mediate a formation of large-scale regulatory networks and enable a crosstalk between different cellular pathways. Throughout the development and aging, miRNAs are expressed in all tissues including the brain where they regulate neuronal differentiation, development, survival,synaptic functions and plasticity as well as neurotrophin and neuropeptide signaling (Vinnikov et al., 2014; Davis et al., 2015). Primary microRNAs (pri-miRNAs) are initially cleaved by Drosha/DiGeorge syndrome critical region gene 8 (DGCR8) protein complex to form pre-miRNA hairpins which are transported to the cytoplasm by Exportin 5-mediated nuclear export. Nuclear pre-miRNAs can also be degraded by the Translin/Trax ribonuclease complex. In the cytoplasm, pre-miRNAs are further processed by a complex containing a double strand RNA-specific nuclease Dicer, Argonaute 2 (Ago2),protein activator of protein kinase R (PACT), and TAR RNA-binding protein 2 (TRBP) resulting in mature ~22 nucleotides-long miRNAs. These mature miRNAs direct RNA-induced silencing complex (RISC) to target mRNAs (Heman-Ackah et al., 2013; Emde and Hornstein, 2014) (Figure 1). Some miRNAs can also be processed by Drosha- or Dicer-independent pathways, however, this is relatively rare, and the majority of miRNAs are cleaved by the “canonical” pathway described above. Indeed, in mouse neurons, the ablation of Dicer disrupts miRNA processing pathway leading to the loss of mature brain-specific miRNAs (Konopka et al., 2010). The activity of Dicer-PACT-TRBP-Ago2 complex is inhibited by cellular stressors, such as reactive oxygen species, hypoxia, ultraviolet (UV)irradiation, endoplasmic reticulum (ER) stress and accumulation of misfolded proteins (Figure 1). Long-term cellular stress may lead to age-related decline in Dicer expression, resulting in diminished processing of pre-miRNAs and age-related down-regulation of mature miRNAs that, in turn, may deregulate stress-response pathways resulting in a vicious stress-amplifying cycle (Emde and Hornstein,2014).e age-related down-regulation of Dicer and attenuation of miRNA signaling (Chmielarz et al., 2017) can lead to deregulation of cellular homeostasis and activation of apoptosis in dopaminergic neurons, compromising their functions and survival and eventually leading to neurodegeneration (Figure 2). To identify pathways affected by aging and compromising viability of dopaminergic neurons, a possible strategy is to combine immunoprecipitation techniques (such as HITS-CLIP/miRAP) or miRNA and mRNA expression profiling with proteomics and target identification approaches,followed by microRNA/target/pathway validation. Similar strategies involving pathway enrichment analyses identified miRNAs and their target genes regulating apoptosis, protein degradation, metabolism and inflammation in a PD-related context (Briggs et al., 2015). On the contrary, stimulation of miRNA biogenesis may reduce stress and promote cell survival. In line with this hypothesis, several studies have shown that various cytoprotective factors, such as caloric restriction, metformin, and interferon β1a can, directly or indirectly,stimulate Dicer activity.
MicroRNAs protect dopaminergic neurons from degeneration:Involvement of Dicer and miRNAs in regulation of cellular stress pathways suggests that deregulation of miRNA biogenesis may also play an important role in degeneration of dopaminergic neurons in PD. The selective loss of dopaminergic neurons during PD is attributed to genetic mutations, mitochondrial dysfunction, endoplasmic reticulum stress and accumulation of insoluble α-synuclein-containing protein aggregates. As discussed above, these stressors may directly or indirectly affect the activity of miRNA biogenesis pathway. Using a mouse line with an inducible deletion of Dicer in adult dopaminergic neurons, we have recently directly proved that Dicer is essential for survival of this neuronal population. Dicer deletion results in a progressive degeneration of axonal projections to the striatum and depletion of striatal dopamine followed by the loss of neuronal bodies, progressive locomotor deficits and classical parkinsonian symptoms including postural instability, slowness, rigidity,and tremor. Interestingly, we have also observed a decrease in striatal dopamine levels in Dicer heterozygous mice, suggesting that even a partial deregulation of microRNA pathway (which we have also found to occur in aged animals) could be sufficient to affect dopaminergic neuron functions prior to the onset of clinical symptoms.Interestingly, this dopamine decline was more profound in males,similar to gender-biased PD pathology (Chmielarz et al., 2017).
Approaches to modulate microRNA pathway activity:We have shown that a stimulation of Dicer activity with enoxacin, a small molecule interacting with TRBP and enhancing pre-miRNA cleavage in the Dicer-PACT-TRBP-Ago2 protein complex, promotes survival of dopaminergic neurons and protects them from ER stress(Chmielarz et al., 2017). However, in addition to its effects on Dicer activity, enoxacin affects several other cellular pathways, that greatly complicates its utilization for dopaminergic neuron protection in vivo. Enoxacin is a broad-spectrum fluoroquinolone antibiotic inhibiting bacterial DNA gyrase and topoisomerase IV, it does not efficiently cross blood-brain barrier, and it also may act as a γ-ami-nobutyric acid (GABA) antagonist promoting seizures.us, novel more efficient and more specific drugs to stimulate Dicer activity are clearly needed. Such Dicer-stimulating compounds can be found by utilizing the cell-based reporter systems for the screening of miRNA processing activators.
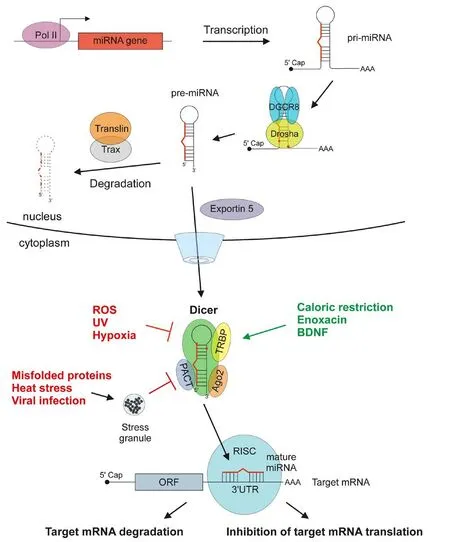
Figure 1 Scheme of microRNA (miRNA) biogenesis pathway and factors affecting miRNA processing activity of the Dicer complex.
Apart from stimulation of Dicer activity, application of either individual miRNAs or combinations of several miRNAs can also be used to promote neuronal survival. For example, miR-301a stimulates Akt/PI3K cell survival pathway by targeting PTEN, miR-223 reduces glutamate toxicity by targeting glutamate receptors, miR-29b has anti-apoptotic activity by targeting BH3-only proteins, and miR-7 and miR-153 directly target α-synuclein and may prevent its aggregation in PD patients (Heman-Ackah et al., 2013). miRNAs can be delivered in vivo in form of miRNA-mimicking oligonucleotides (Vinnikov et al., 2016) or using viral vectors. CRISPR/Cas9 system can also be used to stimulate transcription of individual miRNA genes or inactive natural miRNA inhibitors such as circular RNA.

Figure 2 Effect of aging on miRNA biogenesis.
Challenges in microRNA basic and clinical studies:e research on individual miRNAs and their therapeutic application is hampered by difficulties in predicting miRNA targets and, therefore, a possibility of unforeseen unspecific off-target effects. However, the ability for a given miRNA to target multiple mRNAs can also be beneficial when one miRNA targets several pathways and thus exerts stronger pro-survival effects. For example, a search in the database of experimentally supported miRNA targets (TarBase v7.0) identified several miRNAs targeting both PTEN and Bim, and thus potentially activating Akt/PI3K pathway while inhibiting apoptosis. In the future studies, the experimental approaches should ideally include miRNA and mRNA sequencing and proteomics from the same sample and require thorough bioinformatics analysis to integrate the results.Proteomics studies are important as miRNAs may inhibit translation,but not necessary lead to mRNA degradation. Another problem is the difficulty to obtain a homogenous population of desired cells (for example, dopaminergic neurons) from in vivo source in quantities sufficient for miRNA/mRNA/proteomics studies. This is currently technically extremely difficult, but not impossible, as the field of single cell profiling is evolving very fast.e development of single cell miRNA/mRNA sequencing combined with proteomics and improvement of computational algorithms to predict miRNA targets will undoubtedly lead to deeper understanding of the interactions between miRNAs and their targets in cell-specific and context-specific manner. Notably, in vivo research on rodents requires thorough behavioral phenotyping.erefore, implementation of highly sensitive techniques such as our modified rotarod assay (Domanskyi et al.,2011, 2014; Chmielarz et al., 2017) is crucial for the accurate analysis of progressive locomotor impairments reflecting neurodegeneration of the dopamine system.
②条目频次统计:根据抽取到的字段信息对条目内元素的频次进行统计和降序排列(包括:关键词、作者、期刊名等)。
Outlook:Overall, we hypothesize that certain miRNAs abundant in specific neuronal populations such as dopaminergic neurons,are specifically modulated in response to ER stress, mitochondrial damage, excitotoxicity, prion-like protein-aggregation, environmental, hormonal and nutrient cues. Stress-induced regulation of target genes by such miRNAs can represent an attempt to keep the homeostatic balance of affected neurons. Moreover, some miRNAs loaded into RISC and localized in neuronal terminals can regulate local translation of mRNAs and directly modify synaptic activity and plasticity, also in response to stress. Under certain conditions such as age-associated impairment of autophagy, prion-like protein aggregation, ER stress or reactive oxygen species-induced accumulation of mitochondrial genome mutations, function of miRNAs or miRNA biogenesis machinery components such as Dicer, Ago2,TRBP or PACT can be impaired leading to an altered pattern of expression and thus resulting in reduced neuronal fitness and/or inability to adequately compensate for the intra- and extracellular challenges. Notably, several miRNAs have already been proven to be involved in pathologic conditions while for others, genome-wide association studies (GWAS) have identified mutations in 3′-UTR target sites. Finally, some miRNAs could be subjects to more complex regulation processes such as context-dependent shiin endonuclease cleavage site during miRNA maturation or altered activity of natural miRNA “sponges” such as circular RNAs. Aging and cellular stress can affect multiple steps of miRNA biogenesis process and regulation, compromising miRNA/mRNA networks and cellu-lar homeostasis.ere are examples of synergistic effects of small or large groups of miRNAs regulating cell survival or other aspects of neuronal physiology (Davis et al., 2015) when combinations of several miRNAs can target multiple pathways. Our ongoing Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses on predicted targets using the DIANA miRPath algorithm identified several significantly enriched pro-survival pathways, such as Wnt, neurotrophin, and ErbB signalling targeted by a mixture of the candidate miRNAs capable of alleviating dopamine content and motor function in Dicer-depleted animals, with individual miRNAs targeting components of Akt/PI3K and MAPK pathways. We, and others, have previously demonstrated the importance of PI3K-AktmTOR (Domanskyi et al., 2011) and neurotrophic factors signaling(Domanskyi et al., 2015) for the maintenance of adult dopaminergic neurons. Based on these data and predicted targets, we suggest that combined action of selected candidate miRNAs on neurotrophin signaling and other pathways in dopaminergic neurons could promote their maintenance and function, including sustained or even enhanced dopamine production. However, several obstacles need to be overcome for successful clinical applications of neuroprotective miRNAs for PD treatment. As one miRNA can target many cellular mRNAs and target prediction algorithms are not yet optimal, extensive studies have to be conducted to minimize unexpected off-target effects. One of possible ways to check for such effects is overexpression of particular candidate miRNA in cultured neurons followed by mRNA expression profiling. Once miRNA targets are identified,a direct targeting of the gene product might in some cases be more effective and specific than modulating miRNA expression.e other aspect concerns a targeted delivery to the brain and, specifically,to dopaminergic neurons – a small neuronal population in the ventral midbrain. Ideally, therapeutic miRNA formulation would need to be delivered systemically, for example, as an intravenous injection or intranasal spray. While the stability of delivered miRNA mimics can be enhanced by incorporating specific chemical modifications (Vinnikov et al., 2017), the problems of crossing the blood-brain barrier and specific targeting of dopaminergic neurons are more challenging to address. In this respect, promising strategies may be to encapsulate miRNA mimics into antibody-coated nanoparticles or liposomes modified to efficiently cross blood-brain barrier and recognize specific antigen(s) on dopaminergic neuron surface. Emerging alternatives implementing light- and magnetic field-controlled molecular machines delivering therapeutics to target cells may also become available in the coming years. Alternatively, miRNAs can be delivered in form of adeno-associated virus (AAV)-mediated gene therapy. Different AAV serotypes can preferentially transduce distinct cellular populations while the expression of the gene of interest can be further restricted by using a cell-specific promoter. More studies are clearly needed to identify best possible delivery and targeting methods in respect to efficiency,specificity and safety.
Conclusions:Altogether, the potential to use miRNA biogenesis pathway stimulation or targeting individual miRNAs for PD treatment is not yet fully explored.erefore, it is important to study the roles of miRNAs in protecting adult dopaminergic neurons from stressors and implement the results for future therapeutic strategies.Indeed, we have demonstrated that even partial down-regulation of miRNA pathway in dopaminergic neurons (occurring, for example,during aging or in Dicer heterozygous mice) can compromise the dopamine system and may promote neurodegeneration resulting in characteristic clinical manifestations (Chmielarz et al., 2017). At present, we cannot expect that Dicer stimulation or supplementation of protective miRNAs will result in a stand-alone therapeutic approach to cure PD. However, in combination with neurotrophic factors (Domanskyi et al., 2015) or substances preventing aggregation of prion-like proteins or promoting mitochondrial fitness, drugs stimulating the activity of Dicer and/or individual miRNAs can offer promising therapeutic options.
Ilya A. Vinnikov*, Andrii Domanskyi*
Laboratory of Molecular Neurobiology, Sheng Yushou Center of Cell Biology and Immunology, Department of Genetics and
Developmental Biology, School of Life Science and Biotechnology,Shanghai Jiao Tong University, Shanghai, China (Vinnikov IA)Institute of Biotechnology, HiLIFE, University of Helsinki, Finland(Domanskyi A)
*Correspondence to:Ilya A. Vinnikov, M.D. or Andrii Domanskyi,Ph.D., ilya.vinnikov@gmail.com or andrii.domanskyi@helsinki.fi.
orcid:0000-0003-0488-8484 (Ilya A. Vinnikov)
0000-0002-4755-5981(Andrii Domanskyi)
Accepted: 2017-09-05
How to cite this article:Vinnikov IA, Domanskyi A (2017) Can we treat neurodegenerative diseases by preventing an age-related decline in microRNA expression? Neural Regen Res 12(10):1602-1604.
Plagiarism check:Checked twice by ienticate.
Peer review:Externally peer reviewed.
Open access statement:is is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer reviewer:Lei Xiao, University of Texas Southwestern Medical Center, USA.
Briggs CE, Wang Y, Kong B, Woo TU, Iyer LK, Sonntag KC (2015) Midbrain dopamine neurons in Parkinson’s disease exhibit a dysregulated miRNA and target-gene network. Brain Res 1618:111-121.
Chmielarz P, Konovalova J, Najam SS, Alter H, Piepponen TP, Erfle H, Sonntag KC, Schutz G, Vinnikov IA, Domanskyi A (2017) Dicer and microRNAs protect adult dopamine neurons. Cell Death Dis 8:e2813.
Davis GM, Haas MA, Pocock R (2015) MicroRNAs: Not “Fine-Tuners” but key regulators of neuronal development and function. Front Neurol 6:245.
Domanskyi A, Saarma M, Airavaara M (2015) Prospects of neurotrophic factors for Parkinson’s disease: comparison of protein and gene therapy. Hum Geneer 26:550-559.
Domanskyi A, Alter H, Vogt MA, Gass P, Vinnikov IA (2014) Transcription factors Foxa1 and Foxa2 are required for adult dopamine neurons maintenance. Front Cell Neurosci 8:275.
Domanskyi A, Geissler C, Vinnikov IA, Alter H, Schober A, Vogt MA, Gass P,Parlato R, Schütz G (2011) Pten ablation in adult dopaminergic neurons is neuroprotective in Parkinson’s disease models. FASEB J 25:2898-2910.
Emde A, Hornstein E (2014) miRNAs at the interface of cellular stress and disease. EMBO J 33:1428-1437.
Heman-Ackah SM, Hallegger M, Rao MS, Wood MJ (2013) RISC in PD: the impact of microRNAs in Parkinson’s disease cellular and molecular pathogenesis. Front Mol Neurosci 6:40.
Konopka W, Kiryk A, Novak M, Herwerth M, Parkitna JR, Wawrzyniak M, Kowarsch A, Michaluk P, Dzwonek J, Arnsperger T, Wilczynski G,Merkenschlager M,eis FJ, Köhr G, Kaczmarek L, Schutz G (2010) MicroRNA loss enhances learning and memory in mice. J Neurosci 30:14835-14842.
Simunovic F, Yi M, Wang Y, Stephens R, Sonntag KC (2010) Evidence for gender-specific transcriptional profiles of nigral dopamine neurons in Parkinson disease. PLoS One 5:e8856.
Vinnikov I, Domanskyi A, Konopka W (2016) Continuous Delivery of Oligonucleotides into the Brain. New York: Humana Press.
Vinnikov IA, Hajdukiewicz K, Reymann J, Beneke J, Czajkowski R, Roth LC,Novak M, Roller A, Dorner N, Starkuviene V,eis FJ, Erfle H, Schutz G,Grinevich V, Konopka W (2014) Hypothalamic miR-103 protects from hyperphagic obesity in mice. J Neurosci 34:10659-10674.
10.4103/1673-5374.217328
- 中国神经再生研究(英文版)的其它文章
- Matrix bound vesicles and miRNA cargoes are bioactive factors within extracellular matrix bioscaffolds
- Diffusion tensor tractography studies on mechanisms of recovery of injured fornix
- Using 3D bioprinting to produce mini-brain
- Beta secretase activity in peripheral nerve regeneration
- Embracing oligodendrocyte diversity in the context of perinatal injury
- On the road towards the global analysis of human synapses