Expression of NG2 and platelet-derived growth factor receptor alpha in the developing neonatal rat brain
Ping Li, Heng-xi Li, Hong-yan Jiang, Lie Zhu, Hai-ying Wu, Jin-tao Li, Jiang-hua Lai
1 College of Forensic Science, Health Science Center, Xi’an Jiaotong University, Xi’an, Shaanxi Province, China
2 Department of Anatomy and Histology & Embryology, Faculty of Basic Medical Sciences, Kunming Medical University, Kunming, Yunnan Province, China
3 Department of Plastic Surgery, Changzheng Hospital, Shanghai, China
4 Department of Emergency and Intensive Care Unit, First Affiliated Hospital, Kunming Medical University, Kunming, Yunnan Province, China
5 Neuroscience Institute, Faculty of Basic Medical Sciences, Kunming Medical University, Kunming, Yunnan Province, China
How to cite this article:Li P, Li HX, Jiang HY, Zhu L, Wu HY, Li JT, Lai JH (2017) Expression of NG2 and platelet-derived growth factor receptor alpha in the developing neonatal rat brain. Neural Regen Res 12(11):1843-1852.
Funding: This study was supported by grants from the National Natural Science Foundation of China, No. 31100769.
Introduction
The cellular elements of the mammalian central nervous system include 10% neurons and 90% glial cells. The glial cells are divided into astrocytes, oligodendrocytes and microglia, including NG2 expressing cells (Chang et al., 2000;Yu et al., 2013). NG2 was initially considered a new type of glial cell but is now considered as a marker of oligodendrocyte precursor cells (OPCs); however, it is not clear what the differences are between NG2 and the widely accepted OPC marker, platelet-derived growth factor receptor α (PDGFRα).
NG2, a chondroitin sulfate proteoglycan (CSPG), was shown to be expressed in neural cell lines in the late 1970s(Wilson et al., 1981). It was recognized in a rat neural cell line as an immunogen which reacted with some neuronal(N) cell lines as well as with glial (G) cell lines; hence, it was named NG2 (Arner and Stallcup, 1981). NG2 was shown to be localized in the central nervous system on the surface of multiprocess-bearing cells in the adult cerebellum that resembled stellate interneurons, but their identity was not ascertained (Stallcup et al., 1983). Groups of cells marked with NG2 are widely distributed in gray matter and white matter,and NG2-labeled cells account for approximately 2–9% of the total cells in the brains of normal adult rodents (Dawson et al., 2003). They do not express other stable markers of mature glial cells, such as the glial fibrillary acidic protein of astrocytes, the myelin basic protein of myelin-producing oligodendrocytes, or the OX-42 of microglia (Levine et al.,1993; Reynolds and Hardy, 1997; Butt et al., 1999), indicating that NG2 antibody positive cells (NG2+cells) are distinct from the classically defined types of neuroglial cells, as well as neurons (Nishiyama et al., 1999; Levine et al., 2001). This makes them distinguishable from the classical glial cell types and identifies them as the fourth major glial population in the central nervous system (Nishiyama, 2007).
In general, the morphology of NG2+cells is stellate,with multiple branched processes (Levine and Card, 1987;Nishiyama et al., 1996b, 1997, 1999; Ong and Levine, 1999).Recently, the multiprocessed NG2+cells have been named polydendrocytes because of their morphological features and lineage relationship to oligodendrocytes. Some evidence supports the notion that the processes of NG2+cells that are radially oriented in the gray matter are different from those of NG2+cells that are usually parallel with axons in the white matter (Staugaitis and Trapp, 2009). However, few studies have investigated if such a configuration is uniform across the different layers of the cerebral cortex, which is a typical representation of the gray matter, nor whether NG2+cells in the corpus callosum (CC), which is a typical representation of the white matter, show similar phenotypic features.
NG2+cells were characterized by using glial cell cultures derived from perinatal cerebellum and rat optic nerve(Levine and Stallcup, 1987; Stallcup and Beasley, 1987). The NG2 proteoglycan was found on the surface of OPCs that could be identified by expression of the ganglioside A2B5(Raffet al., 1983), but was lost when they gradually differentiated into oligodendrocytes that could be identified by galactocerebroside (Hall et al., 1996). It has been demonstrated that the NG2 proteoglycan antibody labels a large population of OPCs in the central nervous system of developing and adult rats, bothin vitro(cell cultures) andin vivo(tissue sections) (Stallcup and Beasley, 1987; Nishiyama et al., 1996b; Keirstead et al., 1998; Ong and Levine, 1999). Just like neural stem cells, NG2 proteoglycan-expressing OPCs are long-lived and slowly proliferate and differentiate into oligodendrocytes throughout the adult central nervous system (Ffrench-Constant and Raf f, 1986; Wolswijk and Noble,1989). Besides NG2, numerous previous studies have indicated that PDGFRα is a marker of OPCs (Yeh et al., 1993;Ataliotis and Mercola, 1997; Heldin and Westermark, 1999;Li et al., 2000; Berry et al., 2002; Greenwood and Butt, 2003;Kantor et al., 2004). Therefore, it is now widely accepted that PDGFRα+cells are OPCs. Indeed, NG2 proteoglycan and PDGFRα are co-expressed in some OPCs in rodent and human brains (Wilson et al., 2006).
Although NG2 and PDGFRα are considered as markers of OPCs in the central nervous system, differences in their distribution and morphology have not been directly examined. The identification of the developmental stages of OPCs with the two markers remains to be elucidated. Based on this, we investigated the expression and relationship of NG2 and PDGFRα in the developing brain of neonatal rats and explored the alteration of expression of these two proteins under the adverse condition of hypoxic insult. Our results will aid further understanding of the functional roles of these proteins in the central nervous system.
Materials and Methods
Animals and hypoxia intervention
Twenty-five 1-day-old neonatal Wistar rats, of both sexes,were obtained from the Laboratory Animals Centre, National University of Singapore, Singapore. All rats were exposed to hypoxia following protocols as previously described (Li et al., 2008, 2009). The neonatal rats were placed in a chamber(Model: MCO 18M, Sanyo Biomedical Electrical Co., Ltd.,Tokyo, Japan) filled with 5% oxygen and 95% nitrogen for 2 hours. After hypoxia, they were put in normoxic conditions to recover for 3 and 24 hours, and 3, 7, or 14 days before sacrifice (n= 5 for each time point). Another group of 25 neonatal Wistar rats were kept outside the chamber in normoxic conditions and were used as age-matched controls.All experiments were carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23). The project was approved by the Institutional Animal Care and Use Committee, National University of Singapore (IACUC number 122/08). All efforts were made to reduce the number of rats and their suffering.
Immunohistochemistry
The rats were anesthetized with sodium pentobarbital(60 mg/kg) and then perfused transcardially with saline,followed by fixation with 2% paraformaldehyde in 0.1 M phosphate buffer at 4°C. The brains were removed, postfixed in the same fixative for 3 hours, and then in 15% su-crose in 0.1 M phosphate buffer at 4°C overnight. Coronal frozen sections of the forebrain at 20 μm thickness were cut and mounted onto gelatin-coated microscope slides and stored at −20°C until use. The brain sections were washed 3 × 10 minutes in 0.1% Triton X-100 in 0.1 M phosphate buffered saline (PBS) at pH 7.4, and then preincubated with 5% normal donkey serum and 0.1% Triton X-100 in 0.1 M PBS for 1 hour at room temperature. This was followed by incubation overnight with antibodies against NG2 (rabbit polyclonal IgG, 1:200; Chemicon International, Temecula, CA, USA) and PDGFRα (goat polyclonal IgG, 1:200; a marker for OPCs (Jones et al., 2002; Nishiyama et al., 2002),Cell Signaling Technology Inc., Danvers, MA, USA). The secondary antibody (biotin-conjugated donkey anti-rabbit IgG, 1:100 or biotin-conjugated donkey anti-goat IgG, 1:100;Vector Laboratories, Burlingame, CA, USA) was applied for 1 hour at room temperature, followed by avidin-biotinylated horseradish peroxidase (Vectastain ABC kit, 1:100; Vector Laboratories) for 1 hour. Peroxidase reaction products were visualized using 3,3′-diaminobenzidine tetrahydrochloride.The tissue sections were counterstained with hematoxylin and mounted with Permount after dehydration and clearing.For controls, the tissue sections were incubated in the incubation medium without the primary antibody or the medium was replaced with normal serum. All images were captured under a microscope (BH-2; Olympus, Tokyo, Japan).
Double immunofluorescence labeling and quantitative analysis
For double immunofluorescence labeling, tissue sections at different time points (n= 5 for each time point) were washed 3 × 10 minutes in 0.1 M PBS of pH 7.4. They were preincubated with 5 % normal donkey serum in 0.1 M PBS for 1 hour at room temperature. For the double immunofluorescence labeling of NG2 and PDGFRα, some sections were incubated overnight with NG2 (rabbit polyclonal IgG,1:500) at 4°C and then incubated in secondary antibody Cy3-conjugated donkey anti-rabbit IgG (1:100; Sigma-Aldrich, St. Louis, MO, USA) for 2 hours at room temperature,and then incubated in PDGFRα (goat polyclonal IgG, 1:200)for 4 hours at room temperature, followed by incubation in secondary antibody FITC-conjugated donkey anti-goat IgG(1:100; Sigma-Aldrich) for 2 hours at room temperature.For the double immunofluorescence labeling of NG2 and OX-42, PDGFαR and OX-42, some sections were incubated overnight in NG2 (1:500) or PDGFRα (1:200) premixed with OX-42 (Mouse monoclonal IgG, 1:400, Chemicon International, Temecula, CA, USA), a specific marker of microglia (Cameron and Landreth, 2010). NG2 and OX-42 expression was then localized with a secondary antibody mixture of Cy3-conjugated donkey anti-rabbit IgG (1:100)and FITC conjugated donkey anti-mouse IgG (1:100) for 2 hours at room temperature. PDGFRα and OX-42 expression was localized with a secondary antibody mixture of Cy3-conjugated donkey anti-goat IgG (1:100) and FITC conjugated donkey anti-mouse IgG (1:100) for 2 hours at room temperature. After counterstaining of the cell nuclei with 4′,6-diamidino-2-phenylindole (DAPI) (1:50,000;Vector Laboratories, Burlingame, CA, USA), fluorescence mounting medium (Dako Cytomation, Glostrop, Denmark)was used to mount the sections. All images were captured under a confocal microscope (LSM FV 1000; Olympus).
Quantitative analysis of the cell number of NG2+cells,PDGFRα+cells and NG2+/PDGFRα+cells in the CC1 area,which is part of the corpus callosum above the lateral ventricles, was carried out by counting labeled cells in five randomly selected fields on each section with a 40× objective, by a blinded observer. The double-labeled cells refer to NG2+cells with red fluorescence that overlapped with green PDGFRα+cells, while those cells emitting only red or green fluorescence were counted as NG2+cells or PDGFRα+cells,respectively. The number of cells with positive expression for the respective antibodies was calculated and averaged.
Statistical analysis
The data are presented as the mean ± SD. All data were analyzed using SPSS 16.0 software (SPSS Inc., Chicago, IL,USA). Statistical significance was evaluated by Student’s pairedt-test. A value ofP< 0.05 was considered statistically significant.
Results
Localization of NG2 in different brain areas of normal postnatal rats
By observing the developmental stages of normal postnatal rats from 3 hours to 14 days old, we found that NG2 immunoreactivity was detected in the cortex, corpus callosum(CC1 and CC2), external capsule, medial septal nucleus and piriform cortex (Figure 1A). NG2+cells were undetected in the caudate putamen (Figure 1B6). At higher magnification in the CC1 (Figure 1B1) and the external capsule (Figure 1B3), NG2+cells showed a stellate cell body from which numerous primary processes with profuse ramifications extended radially in multiple directions. In the CC2, piriform cortex and medial septal nucleus, NG2+cells were often polarized, with processes that were preferentially directed along axons, while others appeared to project haphazardly(Figure 1B2, B4, B5).
NG2 immunoreactivity in the cerebral cortex of normal postnatal rats
By observing the developmental stages of normal postnatal rats from 3 hours to 14 days old (Figure 2A), we found that the NG2+cells were distributed in the different layers of the cerebral cortex, except for the second layer, the external granule cell layer (Figure 2B2) and the fourth layer, the internal granule cell layer (Figure 2B4). At a higher magnification in the molecular layer (layer 1), the NG2+cells showed a polygonal cell body with long, slender and tortuous processes that projected in different directions. The primary processes were thick, but tapered offdistally (Figure 2B1). In the third layer,the external pyramidal layer, the NG2+cells were flattened with fewer processes that projected from both poles of the cell body (Figure 2B3). In the fifth layer, the internal pyramidal layer (Figure 2B5) and the sixth layer, the polymorphic layer(Figure 2B6), the NG2+cells typically had a stellate cell body with numerous processes that extended in different directions(Figure 2B5, 6). In the polymorphic layer (layer 6), the NG2+cells showed a spherical cell body with profuse branching processes covering wide territories that overlapped with those of neighboring NG2+cells (Figure 2B6).

Figure 1 Immunohistochemical localization of NG2+ cells in different brain areas of normal postnatal rats.(A) The immunohistochemical localization of NG2+ cells in the cerebral cortex CC, EC, MS and Pir in 24-hour-old normal postnatal rats. (B) Enlarged views of immunolabeled cells in the different brain areas. In the CC1 (B1) and EC (B3), NG2+ cells are stellate, with a central cell body and numerous primary processes extending in multiple directions. In the CC2, Pir and MS, NG2+cells are often polarized with processes that are preferentially horizontal in the CC2 (B2), dorsoventral in the Pir (B4), and vertical in the MS (B5). NG2+cells are absent from the CPU (B6). C: Control; CC: corpus callosum; EC: external capsule; MS: medial septal nucleus; Pir: piriform cortex; CPU: caudate putamen; LV: lateral ventricle; h: hours. Scale bars: 200 μm (A); 20 μm (B1–6).

Figure 2 NG2 immunoreactivity in the cerebral cortex of normal postnatal rats.(A) The immunohistochemical localization of NG2+ cells in the cerebral cortex of 24-hour-old normal postnatal rats. They are distributed in the first layer (molecular layer), the third layer (external pyramidal layer), the fifth layer (internal pyramidal layer), and the sixth layer (polymorphic layer),but not the the second layer (external granular layer) or the fourth layer.(B) Enlarged view of immunolabeled cells in the different layers. The shape of the numerous NG2+ cells is stellate with a centrally placed cell body and radiating primary processes. In layer 1, the NG2 immunoreactivity was more intense than for cells in other layers; the processes are shorter and appear to taper off gradually. C: Control; h: hours. Scale bars: 200 μm (A); 20 μm (B1–6).

Figure 3 NG2-immunoreactive cells in the CC1 of normal postnatal rats during development from 3 hours to 14 days.Enlarged images of NG2+ cells (black arrows) in (Ab-Eb) are seen in (Ac–Ec). NG2+ cells show a polygonal cell body bearing long extended wavy processes in multiple directions. The processes appear to make contact with processes emanating from neighboring cells. The number and length of processes gradually reduced with brain maturation. Scale bars: 200 μm (first column); 50 μm (second column); 20 μm (third column). C: Control; CC1:corpus callosum 1; h: hours; d: days.

Figure 4 NG2-immunoreactive cells in the CC2 of normal postnatal rats during development from 3 hours to 14 days.Enlarged images of NG2+ cells (black arrows) in (Ab–Eb) are seen in (Ac–Ec). NG2+ cells are more intensely stained in C 3 h. They are aligned along the callosal fibers, with elongated cell bodies. The length and number of processes appear to gradually decrease with brain development. Scale bars:200 μm (first column); 50 μm (second column); 20 μm (third column). C:Control; CC2: corpus callosum 2; h: hours; d: days.

Figure 5 PDGFRα-immunoreactive cells in CC1 of normal postnatal rats during development from 3 hours to 14 days.Enlarged images of PDGFRα+ cells (black arrows) in Ab–Eb are seen in Ac–Ec. PDGFRα+ cells are polygonal bearing long, tortuous processes extending in different directions. The processes appear to make contact with processes emanating from neighboring cells. The processes gradually reduced in numbers with brain maturation. Scale bars: 200 μm (first column); 50 μm (second column); 20 μm (third column). C: Control;PDGFRα: platelet-derived growth factor receptor α; CC1: corpus callosum 1; h: hours; d: days.

Figure 6 PDGFRα-immunoreactive cells in the CC2 of normal postnatal rats during development from 3 hours to 14 days.Enlarged images of PDGFRα+ cells (black arrows) in Ab–Eb are seen in Ac–Ec. The PDGFRα+ cells are flattened or rod shaped with primary processes extending from both poles of the cell body. The number and length of processes gradually decreased with brain maturation. Scale bars: 200 μm (first column); 50 μm (second column); 20 μm (third column). C: Control; PDGFRα: platelet-derived growth factor receptor α;CC2: corpus callosum 2; h: hours; d: days.

Figure 8 NG2+, PDGFRα+ and NG2+/PDGFRα+ cells in the CC1 of postnatal rats under normal and hypoxic conditions.(A) Percentage of NG2 cells double-labeled with PDGFRα compared with the total number of NG2+ cells in the CC1 area of normal and hypoxic postnatal rats at different time points; *P < 0.01. (B) Cell counts of NG2+, PDGFRα+ and NG2+/PDGFRα+ in the CC1 of normal and hypoxic postnatal rats; *P < 0.01. Data are expressed as the mean ± SD and were analyzed by Student’s paired t-test. PDGFRα: Platelet-derived growth factor receptor α; CC1: corpus callosum 1.
NG2+ cells in the corpus callosum of normal postnatal ratsCC1 is part of the corpus callosum above the lateral ventricles. In the CC1 area, NG2+cells were polygonal and extended long processes with ramifications in multiple directions covering an extensive area of the white matter. The territorial areas between adjacent cells overlapped with each other.The number and length of the cell projections gradually decreased with development from 3 hours to 14 days (Figure 3).
CC2 is the long narrow region of the corpus callosum packed with many callosal axons. NG2+cells were mainly bipolar with an oval cell body and the processes tended to extend horizontally. The processes of the NG2+cells contact-ed each other. With brain development from 3 hours to 14 days, the processes became reduced in number (Figure 4).
PDGFRα+ cells in brain areas of normal postnatal rats
In normal postnatal rats from 3 hours to 14 days old, just like NG2+cells, PDGFRα+cells were distributed in the cerebral cortex, corpus callosum, external capsule, piriform cortex, and medial septal nucleus. The morphology of these PDGFRα+cells resembled the NG2+cells. In the cerebral cortex, external capsule and CC1, PDGFRα+cells were polygonal with profuse ramifications compared with the fusiform cells in the CC2, piriform cortex, and medial septal nucleus. The processes appeared to make contacts with processes emanating from neighboring cells in CC1 and CC2.The ramifications appeared to decrease with brain development from 3 hours to 14 days. The pictures of CC1 (Figure 5)and CC2 (Figure 6) regions represent the two typical morphologies of PDGFRα+cells.
NG2 and PDGFRα cells in the CC1 of postnatal rats under normal and hypoxic conditions
Double immunofluorescence labeling showed the existence of three kinds of labeled cells in the CC1 region (amoeboid microglial cells are present mainly in the CC1 region) of neonatal rats from 3 hours to 14 days under normal and hypoxic conditions (Figure 7). Colocalization of NG2 and PDGFRα was detected in both cell bodies and processes, and their numbers and length gradually decreased with brain development. NG2+cells in the CC1 following hypoxic exposure were noticeably more intense than in the corresponding controls.
The percentage of NG2+cells double-labeled with PDGFRα compared with the total number of NG2+cells was obtained in the CC1 area of postnatal rats under normal and hypoxic conditions at different time points (Figure 8A). At 3 hours, 46.06% of NG2+cells were co-labeled with PDGFRα, peaking at 65.47% at 3 days and declining by 14 days to 14.25% of NG2+cells. At 14 days, only 14.25% of NG2+cells co-expressed PDGFRα in hypoxic rats, similar to the matching control. Note that the frequency of double-labeled cells declined at 3 days and thereafter.
Cell counts of NG2+, PDGFRα+and NG2+/PDGFRα+cells in the CC1 of normal and hypoxic postnatal rats were compared (Figure 8B). In the control group, the numbers of NG2+cells increased and reached a peak at 14 days, but that of PDGFRα+cells and double-labeled cells decreased from 3 to 14 days. Beginning at 3 hours, the number of NG2+cells increased significantly after hypoxic insult when compared with the corresponding controls. With prolonged time after hypoxic exposure, the count of NG2+cells increased gradually, reaching a peak at 14 days. There was no significant change in the number of PDGFRα+cells or double-labeled cells.
Co-expression of NG2, PDGFRα and OX-42 in the CC1 of postnatal rats under normal and hypoxic conditions
In the CC1 (amoeboid microglial cells are present mainly in the CC1 region of neonatal rats) of the control rats, NG2+cells possessed a satellite cell body, while OX-42+microglial cells were amoeboid. At different time points (3 and 24 hours, 3, 7, and 14 days), co-expression of NG2 and amoeboid microglial cells was not detected in the CC1. However,NG2 expression was specifically detected in some amoeboid microglial cells in the CC1 after hypoxic insult at different time points (Figure 9).
In the CC1 of the control rats, the morphology of PDGFRα+cells was bipolar while the OX-42+cells were amoeboid. Co-expression of PDGFRα and OX-42 was not detected in the CC1 at different time points (3 and 24 hours, 3, 7,and 14 days) after hypoxia (Figure 10).
Discussion
It is well known that numerous NG2+cells are not only widely distributed in the gray matter and white matter(Trotter et al., 2010; Zhu et al., 2010), but also exhibit heterogeneous phenotypes (Komitova et al., 2009) in the normal central nervous system of rodents (Bu et al., 2001; Rivers et al., 2008) and humans (Chang et al., 2000; Nishiyama et al., 2002; Staugaitis and Trapp, 2009). In this study, we found that NG2+cells were widely distributed in the molecular layer (the first layer), external pyramidal layer (the third layer), internal pyramidal layer (the fifth layer), and polymorphic layer (the sixth layer) of the cerebral cortex, which is a typical representation of the gray matter, and the corpus callosum, which is a typical representation of the white matter (CC1 and CC2), external capsule, medial septal nucleus and piriform cortex. However, NG2+cells were absent from the external granule cell layer (the second layer) and internal granule cell layer (the fourth layer) of the cerebral cortex,and caudate putamen. These findings have revealed more detailed features with regard to the distribution of NG2 cells in the postnatal rat brain; indeed, the localization of NG2 cells in different layers of cerebral cortex has been systematically mapped out in our study, which will aid better understanding of the roles of NG2 cells in the cerebrum. NG2+cells were also present in the molecular layer and external pyramidal layer of the cerebral cortex. These sites are strongly associated with incoming nerve impulses. Furthermore,they were also found in the polymorphic and internal pyramidal layers, which are related to outgoing nerve impulses.Therefore, we suggest that NG2 cells may be responsive to neuronal impulses in these regions of the normal postnatal cerebral cortex. It is also clear that the heterogeneity of NG2+cells across the various cortical layers may reflect NG2 cells in different functional states in different brain areas(Chittajallu et al., 2004; Staugaitis and Trapp, 2009). NG2+cells were reported to be pleomorphic in the cerebral cortex of normal developing rats, especially in the molecular layer and deeper layers. These cells were characterized by their polygonal cell body bearing extended long and tortuous processes in a random fashion (Nishiyama et al., 1996b).The present study has further extended the morphological characterization of NG2+cells in different brain areas and every layer of the cerebral cortex. We show here that NG2+cells were mainly stellate and multipolar cells distributed in the cerebral cortex, external capsule, and CC1. NG2+cells were darkly stained and they possessed a polygonal cell body and multiple fine processes radiating in all directions from the cell body. Some NG2+cells in the piriform cortex, medial septal nucleus and CC2 were bipolar. The outline of most cell bodies was elongated and oval in shape with their long axis parallel to the nerve fiber bundles (Horner et al., 2002;Chittajallu et al., 2004). The fine processes extended primarily in the direction of the axons.
Previous studies have shown that in the developing corpus callosum (white matter), NG2 cells exhibited a bipolar shape(Levine et al., 1993; Nishiyama et al. 1996a, b). However, it is worth noting that the NG2+cells in the developing corpus callosum showed two different phenotypes in the present study.For example, in the CC1 area above the lateral ventricles,NG2+cells were stellate, while those in the CC2 area located in the middle were predominantly bipolar. Interestingly, the processes of NG2+cells gradually decreased and shortened over the time of brain development; nonetheless, the processes of adjacent NG2+cells appear to overlap in territorial domains. NG2 cells have processes that contact at the nodes of Ranvier and are associated with synapses (Butt et al., 1999;Ong and Levine, 1999). It is thought that such a configuration of cell processes of NG2+cells may facilitate cell signaling transfer among brain cells (Psachoulia et al., 2009).
In the present study, as for NG2+cells, many PDGFRα+cells were found in the cerebral cortex, corpus callosum,external capsule, piriform cortex and medial septal nucleus.The morphology of these PDGFRα+cells resembled that of NG2+cells. In the cerebral cortex, external capsule and CC1,PDGFRα+cells were stellate, while those in the CC2, piriform cortex and medial septal nucleus were mainly fusiform or rod-shaped. Along with the maturation of the brain, the PDGFRα+cells underwent morphological transformation just like the NG2+cells.
Revealed by double immunofluorescence labeling, colocalization of NG2 and PDGFRα was found in cell bodies and processes of either stellate or flattened cells. Very strikingly, we noted that not all the NG2+cells expressed PDGFRα (Nishiyama et al., 1996a; He et al., 2009). For example, in the CC1 area, 46.06% of NG2+cells in the 3 hour group were double-labeled with PDGFRα. Double-labeled cells peaked at 65.47%at 3 days but declined to 14.25% at 14 days. At 14 days, NG2+cells that lacked the PDGFRα reached their peak number. It is suggested from this study that PDGFRα is a transient marker only for early OPCs, while NG2 is a marker for more mature OPCs. In other words, PDGFRα and NG2 represent different sensitive markers for OPCs at two different developmental stages. Our finding is the first evidence showing that PDGFRα and NG2 are specific markers at two different developmental stages of OPCs. Of course, the analysis is only a preliminary exploration of the difference between the two types of OPC markers. The specific time points of each marker should be further confirmed and the developmental time of the research needs to be further expanded. Previous studies suggested that NG2 cooperates with PDGFRα to regulate OPC proliferation(Nishiyama et al., 1996a). Subsequent studies in NG2/CSPG4-gene knockout mice have identified a role for NG2/CSPG4 in promoting the proliferation of OPCs at postnatal stages(Kucharova and Stallcup, 2010).
Jakovcevski and Zecevic (2005) quantified the percentage of cells double-labeled with NG2 and PDGFRα in various brain areas, with almost 100% double-labeled cells in the subventricular zone. The count of double-labeled cells reduced in the white matter with distance further away from the subventricular zone, and about 50% of the NG2+cells were found to co-express PDGFRα in the cortical plate. In the first week after birth, the extent of co-localization of NG2 and PDGFRα in the same cells was maximal (Nishiyama et al., 1996b). This finding is ample evidence supporting the notion that NG2 expression overlaps with PDGFRα at postnatal day 3 in the striatum, and therefore, NG2 and PDGFRα can serve as reliable markers for O2A progenitor cells (Levine et al., 1993; Trapp et al., 1997). Interestingly,in the early postnatal stage, the numbers of NG2 and PDGFRα co-expressing cells gradually increased and peaked at 3 days, then gradually decreased until 14 days, when they were much lower than at 3 hours. Notably, from 3 hours to 14 days, another two types of cells emerged, namely NG2+/PDGFRα–cells and NG2–/PDGFRα+cells. These findings are consistent with a previous study (Nishiyama et al., 1996b)that reported that considerable numbers of NG2+cells did not co-express PDGFRα. Occasionally, a few cells expressed only PDGFRα, without detectable levels of NG2 in the developing rat brain (Wilson et al., 2006; Staugaitis and Trapp,2009). In other words, NG2 and PDGFRα co-expression predominantly occurs in the early postnatal stage, while during the whole development period including early, middle and advanced stages from 3 hours to 14 days, there are three types of immunophenotypic coding, namely NG2+/PDGFRα+, NG2+/PDGFRα–, and NG2–/PDGFRα+.
Amoeboid microglial cells are present mainly in the CC1 region of neonatal rats (Ling et al., 2001) and our study demonstrated that few NG2+cells were OX-42+amoeboid microglia in the CC1 region. There has been no evidence of co-expression of OX-42 and NG2 in the central nervous system (Sedgwick et al., 1991; Nishiyama et al., 1997; Bu et al.,2001), indicating that they represent two separate cellular entities. However, following hypoxic exposure, some amoeboid microglial cells were induced to express NG2 protein from 3 hours to 14 days in the CC1 region. NG2 expression induced by hypoxia in activated microglia was found in the cell membrane and cytoplasm and was absent from the nucleus. It is therefore suggested that NG2 is a protein whose function may be associated with inflammation in the neonatal rats exposed to hypoxia. It has been reported that a number of NG2+cells also expressed OX-42 in the brains of rat pups that received lipopolysaccharide injection (Gao et al., 2010). It is reasonable to speculate that activated microglial cells can result in neurotoxicity by releasing a variety of inflammatory mediators, including proinflammatory cytokines, chemokines, and reactive oxygen species (Gao et al.,2010). Our results demonstrate that activated microglia in postnatal rats can synthesize the proteoglycan (Gehrmann et al., 1995) under hypoxic conditions. It therefore indicates that amoeboid microglia and NG2 protein are sensitive to hypoxia (Dheen et al., 2007; Zhong and Bellamkonda, 2007).The results suggest that NG2 cells may play important roles other than serving as OPCs. Recent studies have reported an upregulation of NG2 immunoreactivity following different insults to the central nervous system, including physical,viral, excitotoxic and inflammatory insults (Dawson et al.,2000; Fiedorowicz et al., 2008). Although the regulatory mechanisms linking NG2 and proinflammatory cytokines are not clear, the relevance of NG2 for inflammation has been confirmed (Gao et al., 2010; Zhu et al., 2010). Conversely, amoeboid microglia did not express the PDGFRα protein either under normal or hypoxic conditions. These findings suggest that there are differences in the response to hypoxia between NG2 and PDGFRα and NG2 protein may be involved in the inflammatory and pathological processes of amoeboid microglial cells. These similarities and differences between the NG2 and PDGFRα are the factors that need to be considered in neurobiological studies that use them as OPC markers, in terms of analyzing experimental results.

Figure 7 Expression and co-expression of NG2 and PDGFRα in the CC1 of neonatal rats under normal and hypoxic conditions.Images show red labeled NG2+ cells, green labeled PDGFRα+ cells, and yellow labeled NG2+/PDGFRα+ cells. The time points of the control group are 3 h (Aa–Ad), 24 h (Ba–Bd), 3 d (Ca–Cd), 7 d (Da–Dd) and 14 d (Ea–Ed), and of the hypoxic group are 3 h (Ae–Ah), 24 h (Be–Bh), 3 d (Ce–Ch), 7 d (De–Dh) and 14 d (Ee–Eh). The enlarged images of NG2+ cells (thin arrows), PDGFRα+ cells (short arrows) and NG2+/PDGFRα+ cells (thick arrows) seen in the fourth column are from the yellow box in the third column. Nuclei are counterstained with DAPI (blue). The number of NG2 cells in the CC1 following hypoxic exposure was significantly higher than in the corresponding controls.Scale bars: 30 μm (first, second, and third columns); 20 μm (fourth column). PDGFRα: Platelet-derived growth factor receptor α; DAPI:4′,6-diamidino-2-phenylindole; CC1: corpus callosum 1; C: control; H:hypoxia; h: hours; d: days.
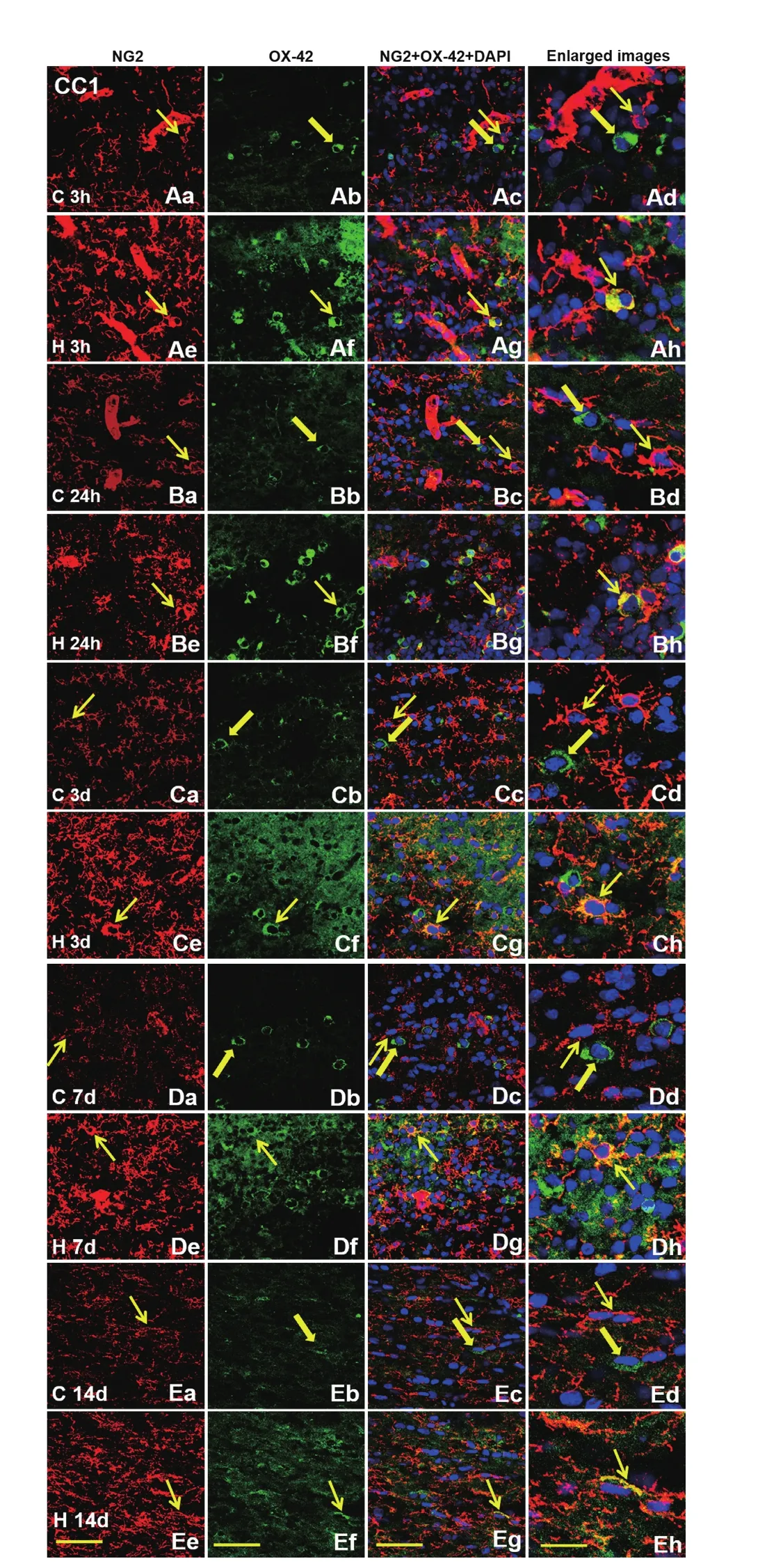
Figure 9 Colocalization of NG2 and OX-42 in amoeboid microglial cells in the CC1 of postnatal rats by double fluorescence labeling.In normal postnatal rat brain at 3 h (Aa–Ad), 24 h (Ba–Bd), 3 d (Ca–Cd), 7 d (Da–Dd) and 14 d (Ea–Ed), co-expression of NG2 (red, thin arrows) with OX-42-positive amoeboid microglial cells (green, thick arrows) is undetected. However, NG2 expression is induced in some amoeboid microglial cells (thin arrows) at 3 h (Ae–Ah), 24 h (Be–Bh),3 d (Ce–Ch), 7 d (De–Dh) and 14 d (Ee–Eh) after hypoxic insult. DAPI(blue) is used to counterstain nuclei. Scale bars: 30 μm (first, second,and third columns); 20 μm (fourth column). DAPI: 4′,6-Diamidino-2-phenylindole; CC1: corpus callosum 1; C: control; H: hypoxia; 1; h:hours; d: days.
Author contributions:PL and JHL designed this study. HXL, HYJ and LZ performed most experiments. HYW and JTL participated in immunostaining and the establishment of animal model. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Research ethics:The project was approved by the Institutional Animal Care and Use Committee, National University of Singapore (IACUC number 122/08).
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer review report:
Reviewer:Ben Waldau, University of California Davis, USA.
Comments to authors:The authors have done a nice job characterizing the relationship of NG2 and PDGFR during brain development and hypoxia. They also shed new light on the expression of NG2 in microglia under hypoxic conditions.

Figure 10 Colocalization of PDGFRα and OX-42 in amoeboid microglial cells in the CC1 of postnatal rats.In normal postnatal rat brain at 3 h (Aa–Ad), 24 h (Ba–Bd), 3 d (Ca–Cd), 7 d (Da–Dd) and 14 d (Ea–Ed), co-expression of PDGFRα (red,thin arrows) and OX-42 in amoeboid microglial cells (green, thick arrows) is absent. The expression of PDGFRα is not induced in amoeboid microglial cells at 3 h (Ae–Ah), 24 h (Be–Bh), 3 d (Ce–Ch), 7 d (De–Dh) and 14 d (Ee–Eh) after hypoxic insult. DAPI (blue) was used to counterstain nuclei. Scale bars: 30 μm (first, second, and third columns); 20 μm (fourth column). PDGFRα: Platelet-derived growth factor receptor α; DAPI: 4′,6-diamidino-2-phenylindole; CC1: corpus callosum 1; C: control; H: hypoxia; h: hours; d: days.
Arner LS, Stallcup WB (1981) Rubidium efflux from neural cell lines through voltage-dependent potassium channels. Dev Biol 83:138-145.
Ataliotis P, Mercola M (1997) Distribution and functions of platelet-derived growth factors and their receptors during embryogenesis. Int Rev Cytol 172:95-127.
Berry M, Hubbard P, Butt AM (2002) Cytology and lineage of NG2-positive glia. J Neurocytol 31:457-467.
Bu J, Akhtar N, Nishiyama A (2001) Transient expression of the NG2 proteoglycan by a subpopulation of activated macrophages in an excitotoxic hippocampal lesion. Glia 34:296-310.
Butt AM, Duncan A, Hornby MF, Kirvell SL, Hunter A, Levine JM, Berry M (1999) Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia 26:84-91.
Cameron B, Landreth GE (2010) Inflammation, microglia, and Alzheimer’s disease. Neurobiol Dis 37:503-509.
Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD (2000) NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci 20:6404-6412.
Chittajallu R, Aguirre A, Gallo V (2004) NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol 561:109-122.
Dawson MR, Levine JM, Reynolds R (2000) NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J Neurosci Res 61:471-479.
Dawson MR, Polito A, Levine JM, Reynolds R (2003) NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci 24:476-488.
Dheen ST, Kaur C, Ling EA (2007) Microglial activation and its implications in the brain diseases. Curr Med Chem 14:1189-1197.
Ffrench-Constant C, RaffMC (1986) Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature 319:499-502.
Fiedorowicz A, Figiel I, Zaremba M, Dzwonek K, Schliebs R, Oderfeld-Nowak B (2008) Trimethyltin-evoked apoptosis of murine hippocampal granule neurons is accompanied by the expression of interleukin-1beta and interleukin-1 receptor antagonist in cells of ameboid phenotype, the majority of which are NG2-positive. Brain Res Bull 77:19-26.
Gao Q, Lu J, Huo Y, Baby N, Ling EA, Dheen ST (2010) NG2, a member of chondroitin sulfate proteoglycans family mediates the inflammatory response of activated microglia. Neuroscience 165:386-394.
Gehrmann J, Matsumoto Y, Kreutzberg GW (1995) Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev 20:269-287.
Greenwood K, Butt AM (2003) Evidence that perinatal and adult NG2-glia are not conventional oligodendrocyte progenitors and do not depend on axons for their survival. Mol Cell Neurosci 23:544-558.
Hall A, Giese NA, Richardson WD (1996) Spinal cord oligodendrocytes develop from ventrally derived progenitor cells that express PDGF alpha-receptors. Development 122:4085-4094.
He Y, Cai W, Wang L, Chen P (2009) A developmental study on the expression of PDGFalphaR immunoreactive cells in the brain of postnatal rats. Neurosci Res 65:272-279.
Heldin CH, Westermark B (1999) Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79:1283-1316.
Horner PJ, Thallmair M, Gage FH (2002) Defining the NG2-expressing cell of the adult CNS. J Neurocytol 31:469-480.
Jakovcevski I, Zecevic N (2005) Sequence of oligodendrocyte development in the human fetal telencephalon. Glia 49:480-491.
Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH (2002) NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci 22:2792-2803.
Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ,Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, Kolodkin AL (2004)Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron 44:961-975.
Keirstead HS, Levine JM, Blakemore WF (1998) Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia 22:161-170.
Komitova M, Zhu X, Serwanski DR, Nishiyama A (2009) NG2 cells are distinct from neurogenic cells in the postnatal mouse subventricular zone. J Comp Neurol 512:702-716.
Kucharova K, Stallcup WB (2010) The NG2 proteoglycan promotes oligodendrocyte progenitor proliferation and developmental myelination.Neuroscience 166:185-194.
Levine JM, Card JP (1987) Light and electron microscopic localization of a cell surface antigen (NG2) in the rat cerebellum: association with smooth protoplasmic astrocytes. J Neurosci 7:2711-2720.
Levine JM, Stallcup WB (1987) Plasticity of developing cerebellar cells in vitro studied with antibodies against the NG2 antigen. J Neurosci 7:2721-2731.
Levine JM, Stincone F, Lee YS (1993) Development and differentiation of glial precursor cells in the rat cerebellum. Glia 7:307-321.
Levine JM, Reynolds R, Fawcett JW (2001) The oligodendrocyte precursor cell in health and disease. Trends Neurosci 24:39-47.
Li JJ, Lu J, Kaur C, Sivakumar V, Wu CY, Ling EA (2008) Effects of hypoxia on expression of transforming growth factor-beta1 and its receptors I and II in the amoeboid microglial cells and murine BV-2 cells.Neuroscience 156:662-672.
Li P, Lu J, Kaur C, Sivakumar V, Tan KL, Ling EA (2009) Expression of cyclooxygenase-1/-2, microsomal prostaglandin-E synthase-1 and E-prostanoid receptor 2 and regulation of inflammatory mediators by PGE(2) in the amoeboid microglia in hypoxic postnatal rats and murine BV-2 cells. Neuroscience 164:948-962.
Li X, Pontén A, Aase K, Karlsson L, Abramsson A, Uutela M, Backstrom G, Hellstrom M, Bostrom H, Li H, Soriano P, Betsholtz C, Heldin CH,Alitalo K, Ostman A, Eriksson U (2000) PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol 2:302-309.
Ling EA, Ng YK, Wu CH, Kaur C (2001) Microglia: its development and role as a neuropathology sensor. Prog Brain Res 132:61-79.
Nishiyama A (2007) Polydendrocytes: NG2 cells with many roles in development and repair of the CNS. Neuroscientist 13:62-76.
Nishiyama A, Chang A, Trapp BD (1999) NG2+ glial cells: a novel glial cell population in the adult brain. J Neuropathol Exp Neurol 58:1113-1124.
Nishiyama A, Yu M, Drazba JA, Tuohy VK (1997) Normal and reactive NG2+ glial cells are distinct from resting and activated microglia. J Neurosci Res 48:299-312.
Nishiyama A, Watanabe M, Yang Z, Bu J (2002) Identity, distribution,and development of polydendrocytes: NG2-expressing glial cells. J Neurocytol 31:437-455.
Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB (1996a) Interaction between NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells is required for optimal response to PDGF. J Neurosci Res 43:315-330.
Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB (1996b) Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res 43:299-314.Ong WY, Levine JM (1999) A light and electron microscopic study of NG2 chondroitin sulfate proteoglycan-positive oligodendrocyte precursor cells in the normal and kainate-lesioned rat hippocampus. Neuroscience 92:83-95.
Psachoulia K, Jamen F, Young KM, Richardson WD (2009) Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol 5:57-67.
RaffMC, Miller RH, Noble M (1983) A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature 303:390-396.
Reynolds R, Hardy R (1997) Oligodendroglial progenitors labeled with the O4 antibody persist in the adult rat cerebral cortex in vivo. J Neurosci Res 47:455-470.
Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD (2008) PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci 11:1392-1401.
Sedgwick JD, Schwender S, Imrich H, Dorries R, Butcher GW, ter Meulen V (1991) Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci U S A 88:7438-7442.
Stallcup WB, Beasley L (1987) Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci 7:2737-2744.
Stallcup WB, Arner LS, Levine JM (1983) An antiserum against the PC12 cell line defines cell surface antigens specific for neurons and Schwann cells. J Neurosci 3:53-68.
Staugaitis SM, Trapp BD (2009) NG2-positive glia in the human central nervous system. Neuron Glia Biol 5:35-44.
Trapp BD, Nishiyama A, Cheng D, Macklin W (1997) Differentiation and death of premyelinating oligodendrocytes in developing rodent brain.J Cell Biol 137:459-468.
Trotter J, Karram K, Nishiyama A (2010) NG2 cells: Properties, progeny and origin. Brain Res Rev 63:72-82.
Wilson HC, Scolding NJ, Raine CS (2006) Co-expression of PDGF alpha receptor and NG2 by oligodendrocyte precursors in human CNS and multiple sclerosis lesions. J Neuroimmunol 176:162-173.
Wilson SS, Baetge EE, Stallcup WB (1981) Antisera specific for cell lines with mixed neuronal and glial properties. Dev Biol 83:146-153.
Wolswijk G, Noble M (1989) Identification of an adult-specific glial progenitor cell. Development 105:387-400.
Yeh HJ, Silos-Santiago I, Wang YX, George RJ, Snider WD, Deuel TF(1993) Developmental expression of the platelet-derived growth factor alpha-receptor gene in mammalian central nervous system. Proc Natl Acad Sci U S A 90:1952-1956.
Yu XJ, Li Y, Tai LW, Chen X (2013) Glucocorticoid effects on adult hippocampal neural progenitor cells. Zhongguo Zuzhi Gongcheng Yanjiu 17:3521-3526.
Zhong Y, Bellamkonda RV (2007) Dexamethasone-coated neural probes elicit attenuated inflammatory response and neuronal loss compared to uncoated neural probes. Brain Res 1148:15-27.
Zhu L, Lu J, Tay SS, Jiang H, He BP (2010) Induced NG2 expressing microglia in the facial motor nucleus after facial nerve axotomy. Neuroscience 166:842-851.
- 中国神经再生研究(英文版)的其它文章
- The role of general anesthetics and the mechanisms of hippocampal and extra-hippocampal dysfunctions in the genesis of postoperative cognitive dysfunction
- Saponins from Panax japonicus attenuate age-related neuroinflammation via regulation of the mitogenactivated protein kinase and nuclear factor kappa B signaling pathways
- Taking out the garbage: cathepsin D and calcineurin in neurodegeneration
- MicroRNAs as diagnostic markers and therapeutic targets for traumatic brain injury
- Interferon regulatory factor 2 binding protein 2: a new player of the innate immune response for stroke recovery
- Endogenous retinal neural stem cell reprogramming for neuronal regeneration

